Identification of ultrastructural and biochemical cuticular markers influencing temperature of ice nucleation in selected genotypes of corn
Abstract
Corn is an economically important yet frost-sensitive crop, injured at the moment of ice nucleation. However, the influence of autumn temperatures on subsequent ice nucleation temperature is unknown. A 10-day chilling treatment under phytotron conditions (“mild”, 18/6°C) or (“extreme”, 10/5°C) generated no-visible damage but induced changes in the cuticle of the four genotypes in this study. The putatively more cold hardy Genotypes 884 and 959 leaves nucleated at colder temperatures compared to the more sensitive Genotypes 675 and 275. After chilling treatment, all four genotypes displayed warmer ice nucleation temperatures, with Genotype 884 expressing the largest shift to warmer nucleation temperatures. Cuticular hydrophobicity reduced while cuticular thickness remained unchanged under the chilling treatment. By contrast, under five-week field conditions, cuticle thickness increased in all genotypes, with Genotype 256 expressing a significantly thinner cuticle. FTIR spectroscopy revealed increases in the spectral regions of cuticular lipids in all genotypes after phytotron chilling treatment, while those spectral regions decreased under field conditions. A total of 142 molecular compounds were detected, with 28 compounds significantly induced under either phytotron or field conditions. Of these, seven compounds were induced under both conditions (Alkanes C31–C33, Ester C44, C46, β-amyrin, and triterpene). While clear differential responses were observed, chilling conditions preceding a frost modified physical and biochemical properties of the leaf cuticle under both phytotron and field conditions indicating this response is dynamic and could be a factor in selecting corn genotypes better adapted to avoiding frost with lower ice nucleation temperature.
1 INTRODUCTION
Due to the widespread impact and need for corn, there is strong interest and investment to move corn production into the Canadian prairies (DuPont Pioneer, 2017; Monsanto Canada, 2013; Saskatchewan Ministry of Agriculture, 2010). This geographic shift would provide new opportunities to meet production needs as well as diversify cropping options and locally produced products for the producers and consumers, respectively. The primary obstacle limiting production in the Canadian prairies is the cooler climate and early frost events in both spring and fall. In addition, continuing global warming will likely facilitate increased frost damage (Gu et al., 2008; Storey & Tanino, 2012).
Corn is generally considered sensitive to cold (Agelet et al., 2012; Chichester, 1979; Zeng et al., 2021), and it is recognized as both a chilling and frost-sensitive crop. Chilling injury occurs in sensitive crops at low temperatures in the absence of ice nucleation. Frost injury in these tender annual plants occurs upon ice formation, and intracellular freezing occurs in the plant tissue (Gusta et al., 2004). However, frost-sensitive crops can avoid nucleation by supercooling. Cold acclimation is a process by which, after exposure to non-freezing cold temperatures, frost-resistant plants are able to tolerate or supercool to colder temperatures in subsequent exposures (Catalá et al., 2011; Fujikawa et al., 1997; Hincha & Zuther, 2014, 2020). While our study is not a cold acclimation experiment, the impact of the cooler temperatures, which precede the first fall frost, has not been investigated on their subsequent influence on the ice nucleation temperature in various tender annual crops and their genotypes.
Corn has long been identified as a useful model system due to its prevalence worldwide, genetic diversity, and long-term comprehensive use as a model organism in agronomy (Smith et al., 2004; Strable & Scanlon, 2009). As the climate continues to change, it is significant to note that even now, frost injury continues to be one of the key factors in limiting production as it was 40 years ago (Lindow, 1983). This is reinforced by more recent examples of frost damage due to early spring frost causing $2 billion of damage over one weekend in the U.S. (Gu et al., 2008) and an 80% reduction in the 2012 spring apple blossoms in Ontario (Leung, 2012). Locally produced Canadian prairie grain corn is often unable to reach its full production potential due to its sensitivity to early fall frost. The first frost typically ends growth in the fall before the grain reaches physiological maturity. By improving frost avoidance (lowering the temperature of ice nucleation) in corn to avoid damage from the first frost, plants would gain additional time to reach physiological maturity. Since corn is not primarily grown in cooler climates, its interaction with cool temperatures and ability to avoid the first ice nucleation event is poorly understood.
The cuticle can serve as a hydrophobic barrier to ice propagation in leaves (Wisniewski & Fuller, 1999). The composition of the cuticle is primarily a combination of cutin and epicuticular waxes. Cutin is composed of a combination of hydroxy, epoxy fatty acids, and esterified aliphatic acids (Kolattukudy, 1980). The complementary cuticular waxes are long-chain and very long-chain fatty acids. The purpose of these waxes is to act as a hydrophobic defense barrier. With hydrophobic waxes and cutin, the cuticle is the first line of defense and acts as an interaction site of the plant to the environment (Bird & Gray, 2003). It has long been known to be a barrier to both biotic and abiotic stresses, including ice crystal formation (Thomas & Barber, 1974). Both the physical and biochemical compositional properties of the cuticle are of interest. The cuticle has been identified as an effective physical barrier to ice propagation as well as enabling avoidance of freezing through increased hydrophobicity (Wisniewski & Fuller, 1999). In a previous study, we observed that the wax-overproducing dewax mutant line of Arabidopsis thaliana can avoid ice nucleation on the leaf surface, freezing at a lower temperature compared to the parental line and wax-deficient mutants cer3 and cer1 (Rahman et al., 2021).
The purpose of this study is to understand the cuticular and epidermal surface contributions to reducing temperatures of ice nucleation, determine the effect of typical fall temperatures preceding the first fall frost (chilling treatment) on the subsequent response of the cuticular layer and identify the specific traits that will either physically or through compositional alteration, reduce the temperature at which ice nucleation occurs and subsequently improve frost avoidance in corn. These traits can then be used as selection criteria in corn breeding programs to expedite the selection and release of more stress-resistant varieties into colder temperature regions. Moreover, reducing transpirational water loss through the cuticle will be increasingly important as drought stress becomes more prevalent. Therefore, the study of the cuticular layer has potential impact on alleviating multiple stresses. We hypothesized that chilling treatment alters cuticular characteristics linked to reducing or increasing the temperature at which ice nucleation occurs in corn. To explore this hypothesis, we first studied if chilling treatment (“mild” = based on the average temperatures typical of the first 2 weeks of September preceding the first fall frost in Saskatoon, Saskatchewan, Canada, or “extreme” = lower chilling temperatures) can change leaf hydrophobicity and influence the subsequent temperature of ice nucleation in corn. Subsequently, we examined how chilling treatment induced chemical modification to the cuticle and identified specific classes of waxes and cutin esters that differentially accumulated among corn genotypes and treatment conditions.
2 MATERIALS AND METHODS
2.1 Plant material and growth conditions
Four genotypes (256, 675, 884, and 959) of hybrid grain corn (Zea mays L. subsp. mays) donated by Corteva Agriscience (previously known as DuPont Pioneer; USA) were used in the study. The genotypes were from older breeding material of interest to the company for chilling and frost-related evaluation. The plants were grown in the greenhouse at about 28/22°C (day/night) under a 16-h photoperiod. Four presoaked corn seeds per 10 cm2 pot were planted at a depth of 3 cm in Sunshine Mix No. 4 (Sungro Horticulture Canada Limited) potting media. During the V2 seedling growth stage (Figure S1), at approximately 14 days, the seedlings were transplanted into standard 4 L pots. Nutricote 18-6-8 (Floracan), a 100-day slow-release fertilizer, was incorporated into the lower half of the substrate at a rate of 3.5 kg m−3 at the time of transplanting. The 3.5 kg m−3 is a half rate for a heavy feeding crop and was used with a regular application (twice a week during vegetative stages and daily during reproductive stages of the plants) of water-soluble fertilizers (N:P:K 20:20:20 + micronutrients, at 1 g L−1 for 100 ppm of nitrogen). For field production, untreated corn seeds were planted into Sutherland Series clay soil prepared by rotovating to a depth of 15 cm. They were planted using a hand seeder at approximately 4 cm depth with a seed spacing of 10 cm. The planting design was in paired rows, 2 m in length, with 1 m on center spacing between all rows. A solid seeded guard row was planted around the entire plot to normalize the microenvironment and limit edge effects. The experimental design of the field was a completely randomized design with 12 replicates. The pH of the soil was 7.8, electrical conductivity 1.1 dS cm−1, and had greater than 784.5 kg ha−1 of available potassium and greater than 560.32 kg ha−1 of available phosphorous. The residual nitrogen levels were less than 22.41 kg ha−1 in the 0–12″ soil profile. 112.06 kg ha−1 of nitrogen was added as 46-0-0 as part of the soil preparation process. The previous crop represented a mix of plots of various field crops.
2.2 Chilling treatments
In the initial experiments, corn plants were subjected to more “extreme” chilling conditions (“chilling treatment (extreme)”). This was conducted in a controlled environmental chamber (phytotron) to simulate more severe fall field conditions under parameters of 10/5°C, with a 12-h photoperiod for a period of 10 days at the VT stage (tassel emergence) with the V6 leaf sampled at the end of this period. Subsequent phytotron experiments exposed mature corn plants at the VT stage to conditions of 18/6°C under a 12-h photoperiod for 10 days (“mild” “chilling treatment (mild)”). The “mild” chilling parameter was selected to represent the 30-year average of Saskatoon, Saskatchewan, environmental conditions during the first 2 weeks of September, which occurs just prior to the first fall frost.
Under field conditions, the majority of sampling was conducted at six-time points (F) between August 16 (“Early”) and September 14. Specifically, F1 = August 16; F2 = August 24; F3 = August 31; F4 = September 7; F5 = September 12 (“Late”); F6 = September 14 (after 0°C conditions but no frost damage). Corn plants were in the mature reproductive stage over the sampling period.
2.3 Freezing treatment and thermal imaging
Whole plants were exposed to temperature conditions in a programmable controlled environment chamber at a linear ramp from 2°C to −10°C at a rate of −2°C per h to the final temperature of −10°C. The freezing response of the plants was measured using infrared thermal imaging captured using a FLIR T640BX (Manufactured in 2013, FLIR Systems Inc) as per Wisniewski et al. (2015). The camera was set using a continuous auto adjustment of the temperature span, which was corrected as the temperature was reduced in the programmable freezing chamber. Temperature measurements during cold exposure were captured at 30-s intervals across the four plants in the frame. Each pixel within the image served as a thermal sensor and measured the specific temperature at that location of the leaf or stem. As an indicator of latent heat released during freezing, resultant temperature readings were used to determine freezing temperature. To avoid sub-zero ice nucleation temperatures in the soil within the pot, whole plants at anthesis (age 7–10 weeks) were protected at the base with a thermal blanket. This simulated the natural effect of soil to act as a large heat sink and prevented ice nucleation in the roots. The absence of ice nucleation originating from the soil in the pot was verified through thermal imaging. Plants were allowed to nucleate naturally without the addition of ice nucleating substances. Based on the IR camera readings, the temperature at which ice nucleation was initiated was indicated by an exotherm or increased plant temperature at the time of freezing. Freezing point measurement was determined by plotting the plant spot temperature against the chamber reference temperature over time, using the second derivative of the curve to identify the exotherm. The measured exotherm corresponded to the temperature at which the leaf and stem freezing occurred. The two-way analysis of variance (2-way-ANOVA) of the freezing temperatures was conducted for each treatment, with a post hoc Tukey test, using R-Studio.
2.4 Measurement of leaf hydrophobicity
Fresh leaf tissue was excised from the collared leaf # 5–7 (Figure S1) within 30 cm of the tip of the leaf. This location was selected based on thermal imaging demonstrating the initial environmental impact of freezing affecting the leaf tip (Pearce & Fuller, 2001), as well as large-scale commercial reports of chilling and frost damage having occurred in this region (Nielsen & Christmas, 2001). Approximately 5 × 5 cm leaf samples were mounted flat onto a standard glass slide securing all outer edges with transparent satin tape. The control material used was a sheet (4.76 mm × 30.5 cm × 30.5 cm) of polytetrafluoroethylene (PTFE; WD Plastics Ltd.), which has known hydrophobic properties (ASTM Compass C813, 2014). Plants were sampled immediately following chilling treatment. The contact angle of chilled and non-chilled leaves was evaluated for hydrophobicity (Figure S2) using a standard contact angle measurement technique (ASTM Compass C813, 2014) to determine the leaf surface interaction with water. The contact angle was measured based on dH2O droplets (3–5 μL) from a syringe mounted directly perpendicularly above the surface of the leaf. Topographical images of each droplet were collected using a tripod-mounted iPhone 5S with a focal length of 4.10 mm. Images were analyzed using the ImageJ protractor function to determine the contact angle (Figure S2). The image describes the hydrophobic assumptions where a surface with a contact angle greater than 90° is considered hydrophobic, and surfaces with a smaller contact angle are more hydrophilic.
2.5 Analysis of leaf cuticle thickness by confocal laser scanning microscopy
Fresh leaf samples from mature corn plants were mounted on cover glasses and inserted into the TCS SP5 by Leica (Leica Microsystems GmbH) confocal laser scanning microscopy (CLSM) with a 20× air objective (modified from Celler et al., 2016). Microscope settings were adjusted to 1089 gain, −0.3 offset, line average of 4, format 1024 × 512, 300 Hz with emission capture 500–545 nm of excitation with a 488 nm Argon laser set at 25%. Three-dimensional views of the z-stack were generated using the orthogonal view function in ImageJ Fiji (Schindelin et al., 2012). The image contrast range was adjusted to a greyscale level of 350–15,000 (16-bit). Two replicates were selected for analysis from a total of six replicates for each sample treatment, and 10 measurements were taken across each still image in the X and Y direction. Three images of each in the X direction and Y direction were captured for each sample. The total data captured for each treatment by genotype was 180 measurements (two reps by three X-directions by three Y-directions by 10 measurements). Each image was preprocessed by using the smooth and sharpen function before making the image binary and zooming to a perspective of 600× to take the 10 measurements of cuticle thickness. Two CLSM experiments were performed: “mild” chilling treated (phytotron) and field produced. The field study was conducted at two-time points of mature corn at the reproductive stage (early = 2016-08-03, late = 2016-09-12) with sampling of leaf V6 [Figure S1 (adaxial side) to simulate chilling treated (Condition 2 “mild” and Condition 1 “severe”), respectively].
2.6 ATR-FTIR analysis of the cuticular leaf surfaces
The adaxial leaf surfaces of corn were evaluated in a non-tissue destructive method using spectroscopy with Attenuated Total Internal Reflection (ATR) at facilities of Canadian Light Source (Canadian Light Source Inc., Saskatoon, Canada). The ATR endstation was a Pike MiracIATR with a 45° Ge ATR Crystal equipped with a deuterated-triglycine sulfate (DTGS) detector at room temperature. The Pike MiracIATR probe accessory was secured onto the leaf sample with standard pressure. The infrared light is generated through a globar equipped with a silicon carbide source. Spectra were collected in the range of 800–4000 cm−1 with a spectral resolution of 4 cm−1. The spectra from both the ATR-FTIR (IFS 66 V) and FPA-FTIR (Vertex 70 V) were evaluated using OPUS Software (v. 7.0 Pro, Bruker Optics) and R-Studio to generate compositional maps to distinguish the compositional contribution of cuticular wax through peak area integration across the lipid fingerprint region. All spectra resultant from ATR-FTIR measurements were ATR corrected and averaged by genotype by treatment in OPUS. Resultant spectra were preprocessed in Orange (V3.3.10 Open Source) with a rubber band correction with positive peak direction and background action set at subtract. Integrations were performed at CH3 (region 1: 2960–3040 cm−1), asymmetrical CH2 (region 2: 2910–2960 cm−1), and symmetrical CH2 (region 3: 2840–2880 cm−1).
2.7 Cuticular wax extraction and GC/MS analysis
Analysis was conducted on fresh leaf tissue of mature corn at the reproductive growth stage with sampling within 30 cm of the leaf tip of the adaxial side of leaf V6. The samples were triple rinsed with distilled water and surface air dried prior to cuticular wax extraction. The wax extraction protocol was modified from Dietrich et al. (2005) for adaxial cuticular leaf wax extraction. The cuticular wax extraction was performed on the adaxial surface using ACS-grade Chloroform (BDH VWR Analytical). Plant material weighing approximately 300 mg was washed with 10 mL chloroform over the adaxial surface of the tissue 10 times using a 1 mL pipette. 1 μg of n-tetracosane as an external standard was added to each extract and was vortexed for 30 s. Lastly, samples were dried under a gentle stream of nitrogen gas. Extracted leaf material was recovered, dried, and weighed. GC–MS was performed on prepared samples using an Agilent 7890A GC instrument coupled to an Agilent 5975 inert mass selective detector (MSD) with a triple-axis detector. Sample extracts were loaded (injection volume 1 μL) with an Agilent G2614A auto-sampler. The column was a DB-1MS fused silica column (15 m × 250 μm; 0.25 μm film thickness). The GC oven, Agilent 7890A, was programmed with a thermal gradient starting at 80°C, first ramp to 220°C at 15°C min−1, then ramp to 340°C at 7.5°C min−1, hold at 340°C for 15 min. Helium was used as a carrier gas with a flow rate of 1.2 mL min−1. The injector temperature was set at 280°C MSD temperature. The derivatization for GC–MS analysis was performed with the addition of 200 μL HPLC-Grade acetonitrile (ACN) and 75 μL BSTFA+TCMS to the dried wax extract and was incubated at 60°C for 20 min. In preparation for the GC–MS analysis, the sample was reduced with a gentle stream of nitrogen to approximately 50 μL. The sample was placed in an insert run on the GC–MS with the following protocol (Dietrich et al., 2005). GC–MS Chromatograms were converted to Peak areas using ChemStation (Agilent Technologies) at WM Keck Metabolomics Laboratory at Iowa State University. Heatmaps were generated using the corrplot package in R. Z scores were calculated using the mean and standard deviation of all observations for each cuticular wax component. The master dendrogram was generated in Orange (3.3.7 Open Source), with Z scores calculated according to the same procedure.
3 RESULTS
3.1 Chilling-treated corn plants ice nucleate at significantly warmer temperatures
The freezing point in chilling treated samples occurred at significantly warmer temperatures than their non-chilled controls (Figure 1). This result suggests that pre-exposure to even very “mild” chilling conditions (18/6°C) can increase the temperature at which ice nucleation occurs. The temperature differential indicated there were both treatment and genotypic differences. The treatment effect was present in all samples where warmer freezing temperatures followed exposure to chilling treatment (Figure 1). The genotype effect was present in non-chilled samples where genotypes 256 and 675 (generally more sensitive) froze at warmer temperatures than genotypes 884 and 959 (generally more resistant).
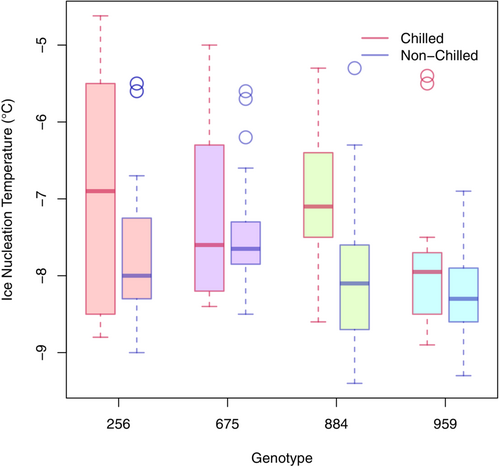
Post hoc analysis (Table 1) indicated genotype 959 froze at significantly colder temperatures than all other genotypes. There were no measurable differences among genotypes or treatments on the temperature of ice nucleation following the exposure to “extreme” chilling treatment (10/5°C Condition 1; data are not shown). Through visual observation of the IR camera, ice nucleation was initiated mainly on the leaves in all genotypes tested after either “mild” or “extreme” chilling conditions and, within a few seconds, quickly spread to the rest of the plant.
| Tukey HSD multiple comparison of means | ||||
|---|---|---|---|---|
| diff | Lower | Upper | p-value | |
| 675–256 | −0.0668 | −0.5880 | 0.4545 | 0.9873 |
| 884–256 | −0.3611 | −0.8613 | 0.1390 | 0.2437 |
| 959–256 | −0.8527 | −1.3627 | −0.3428 | 0.0001 |
| 884–675 | −0.2944 | −0.8034 | 0.2146 | 0.4400 |
| 959–675 | −0.7860 | −1.3046 | −0.2673 | 0.0007 |
| 959–884 | −0.4916 | −0.9890 | 0.0058 | 0.0541 |
| NC-CT | −0.6006 | −0.8802 | −0.3210 | <0.0005 |
3.2 Hydrophobicity on corn leaf surfaces is significantly reduced following chilling exposure
Exposure to chilling also caused a decrease in hydrophobicity in all corn genotypes (Figure 2) in both “extreme” and “mild” chilling conditions. Extreme chilling conditions caused a greater shift in hydrophobicity with a median around 80° (“extreme”) compared with 75° (“mild”; Figure 2). Following the extreme chilling treatment, corn genotypes exhibited a mean contact angle of 74.56° (hydrophilic; F0.05 (3,140) = 15.57, p ≤ 0.0005, μ = 74.556°, σ = 19.68°), while non-chilled plants had a mean contact angle of 96.87° (hydrophobic; F0.05 (3,140) = 1.72, p = 0.1649, μ = 96.868°, σ = 10.514°). The main effect of the “extreme” chilling treatment was to decrease the contact angle by 30°.
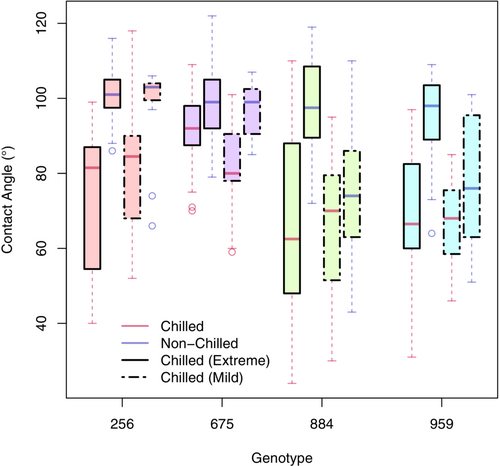
However, genotype 675 demonstrated a different main effect with a contact angle reduction of 7.5° following chilling treatment. The hydrophobicity of genotype 675 was not modified to the same degree as the remaining genotypes. Following the “mild” chilling treatment, there was a clear grouping for the resistant and sensitive genotypes, which clustered together. The main effect of “mild” chilling treatment in sensitive genotypes 256 and 675 was a contact angle reduction of 15°. By contrast, in the putative-resistant genotypes 884 and 959, the average reduction in contact angle was 7°. By combining genotypes to evaluate the treatment effect under both conditions, the chilling treatment significantly reduced hydrophobicity despite a large variation in the contact angle mean (Figures S3 and S4).
3.3 Thickness of the corn cuticle increases over time under field but not under phytotron conditions
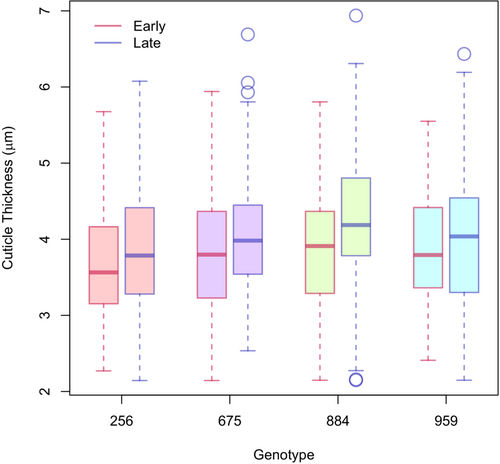
Under field growing conditions, there was an increase in cuticle thickness across all the corn genotypes used in the study. The increase in thickness was observed across all genotypes (including 884 and 959) and between early (August 3) and late (September 12) measurements over 5 weeks (Figure 3). The main effect of the sample period yielded an F-ratio of F0.05(1,955) = 25.50, p < 0.001, indicating a highly significant difference between early (μ = 3.80 μm, σ = 0.73 μm) and late (μ = 4.05 μm, σ = 0.81 μm) sampling periods. There was a differential in cuticle thickness of 0.25 μm between early and late, with a narrow upper and lower limit ranging from 0.15 and 0.35 μm, respectively (Table S1). These narrow limits indicated low variability and stability among the traits. The main effect for genotypes yielded an F-ratio of F0.05(3,955) = 5.15, p < 0.001, indicating a significant difference in cuticle width between genotypes. A post hoc analysis (Table S1) showed sensitive genotype 256 is significantly (p < 0.05) different than each of the three other genotypes with a considerably thinner cuticle. Comparison of means of genotypes 884 and 959 under field conditions also indicates significant differences (p < 0.10). Under field conditions, all genotypes increased cuticle thickness over time, with genotypes 884 and 959 expressing the largest and smallest increase in thickness between “early” and “late” samplings, respectively. Under “mild” Phytotron chilling conditions, these two resistant genotypes were selected (885, 959) to evaluate cuticle thickness response. However, there were no significant effects of either treatment or genotype (884, 959) on the adaxial cuticle thickness (p < 0.05; Table S2 and Figure S5) under the “mild” 10-day phytotron chilling treatment.
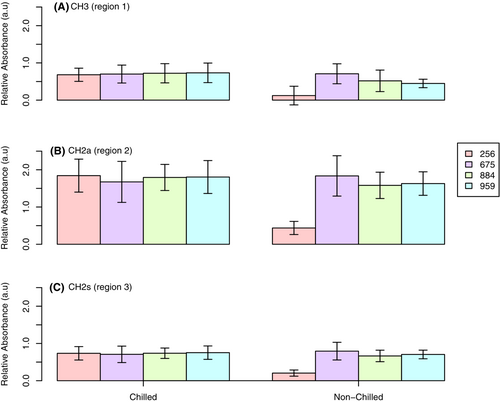
3.4 ATR-FTIR analysis of leaf epidermal surfaces identify differential changes in lipid accumulation among the corn genotypes and between phytotron growth chamber and field conditions
Unlike cuticular thickness, lipid fingerprint regions (2800–3000 cm−1) revealed by ATIR-FTIR showed under “mild” chilling conditions, the CH3 (region 1) increased in the chilling treated samples (higher integration areas), which is consistent across all genotypes tested except for 675 (Figure 4A). 675 showed no change in response to chilling treatment. Genotype 256 was the only genotype to express a significant (p < 0.05) increase in CH3 following chilling treatment. The lack of responsiveness of genotype 675 is consistent with the previous findings in both thermal and hydrophobic measurements. Although not significant, the asymmetrical CH2 (region 2; Figure 4B) and symmetrical CH2 (region 3; Figure 4C) broadband integration have the same trend in genotype 675, showing an inverse and limited response compared with the other genotypes. Genotypes 884 and 959 showed a stable and slight reduction in integration areas of both regions 2 and 3. By contrast, genotype 256 had a greater increase in regions 2 and 3 than the other genotypes. In all regions, the results indicated 884 and 959 have a consistent stability of response to chilling treatment with a slight reduction of integration areas following exposure. We observed a treatment effect across the asymmetrical CH2 bending groups, with a strong effect (p < 0.05) across the CH3 demonstrated by an increase in peak area intensity following chilling treatment. A moderate effect (p < 0.10) of chilling treatment, indicated by peak area increase, across the asymmetrical bend. There was no effect of chilling treatment on the symmetrical bend.
Under field-grown conditions, spectra for the lipid fingerprint region indicated a decrease in intensity over 5 weeks of field conditions from August 16 to September 14 (Figures S6–S8). All genotypes expressed a general depreciation of intensity in the lipid fingerprint regions over time (Figures S6–S11), indicating a shift in cuticular wax composition. In genotypes 256 and 675, the early measurement period had the highest peak intensity. By contrast, genotypes 884 and 959 showed increasing intensity between August 24 and September 7. Genotype 675 had the highest initial (relative absorbance) intensity of 0.06 (a.u.) at the asymmetrical CH2 bend (region 2). Genotypes 256, 884, and 959 had lower initial intensities of approximately 0.035 (a.u.). Under field conditions, according to the ATR-FTIR findings, the asymmetrical and symmetrical CH2 bending groups show a trend in overall wax reduction measured by peak area integrations. The final reporting period of September 14 has approximately half the intensity of the initial reporting period of August 16 in all regions following natural outdoor conditions (Figures S6–S8). This is additionally verified with a comparison of means of peak area integration between the measurement periods where early and late are significantly reduced over time (p < 0.05; Table S3).
3.5 The composition of waxes in corn cuticles is different between mild chilling in growth chamber and field conditions
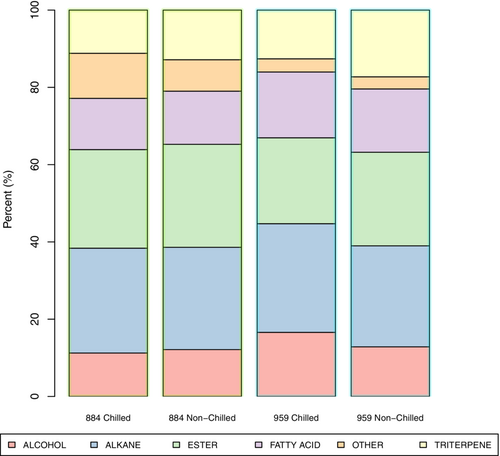
Within the growth chamber treatments, the highest abundance across the cuticular components was found within large grouped clades in both resistant types “mild” condition 884 (chilling treated and non-chilled) and 959 (non-chilled) (Figure 5). The dendrogram, produced using a divisive hierarchal cluster analysis (HCA), demonstrates many small clusters, inferring a large degree of dissimilarity among the groups (Figure 5). Twenty-five out of 142 cuticular compounds were found to significantly (p < 0.05) contribute to the differential between chilling treated and non-chilled samples in genotypes 884 and 959 under “mild” chilling conditions. Under field conditions, the cuticular compositions of 10 compounds were found to significantly (p < 0.05) contribute to the differential between early (August 22) and late (September 30) samples in genotypes 675, 884, and 959. Of the cuticular wax compounds identified, seven (i.e., alkane C29, C31, C33; ester C44, C46; β-amyrin; triterpene; Table 2) were common between the “mild” phytotron condition and field produced sets across all sampling dates, therefore, they were amalgamated to form a superset of 28 cuticular wax compounds (Table 2). The composition of 28 cuticular wax compounds was identified with significant variation of concentrations between chilling treated and non-chilled samples. There is a strong genotype effect based on metabolic signatures across the heat map over the composition of the 28 identified cuticular wax compounds between resistant genotypes 884 and 959 in response to chilling treatment (Figure S12). There was an increase in the concentration of cuticular waxes in 26 of the 28 compounds in genotype 884 in response to chilling, with the exceptions of the C32OH isomer and the methacrylic acid tetradecyl ester. In genotype 884, the largest increase occurred in the dendrogram cluster containing: C28OH, Alkane C35, FA.C18, Oleamide, C30OH, and Alkane C33. The percent abundance of each chemical classification for resistant genotype 884 was not modified by chilling treatment (Figure 6). Chilling treatment induced a reduction of 24 of the 28 compounds in genotype 959; four compounds increased (n-dodecyl methacrylate, FA.C16, α-Tocopherol, and methacrylic acid tetradecyl ester), and one remained unchanged (C26OH). The percent abundance of each chemical class showed changes in alcohols modified by chilling treatment from 17% (chilling treated) to 13% (non-chilled) and in triterpenes from 13% (chilling treated) to 17% (non-chilled). The only notable change in abundance between resistant genotypes 884 and 959 was in the “other” group class containing α-Tocopherol, Oleamide, and X.Z.2.Methylglutaconic acid 10%(884) and 3%(959). Across all genotypes (675, 884, 959), the dendrogram cluster containing very long chain Fatty Acids (VLCFA; C26–C32) increased in response to late (September 30) field season conditions (Figure S13), with the most significant VLCFA response in genotype 959. The dendrogram cluster (rows 22–27) showed a decrease in the prevalence of compounds for genotypes 675 and 884 in response to late-season conditions. By contrast, genotype 959 increased in all compounds except Ester C46 Mixture, which remained constant. Genotypes 884 and 959 had a large (>4×) spike in two ester groups (methacrylic acid tetradecyl ester and n-dodecyl methacrylate) at the late season measurement (and 675 had a very low prevalence of these compounds). Sensitive genotype 675 had a large decrease in the concentration of alkanes C28 (−0.018 μm/g), C31 (−0.03 μm/g), C33 (−0.038 μm/g), and C35 (−0.02 μm/g).

| Cuticular wax composition of adaxial leaf surface | Union set |
|---|---|
| α-Tocopherol | “mild” Condition |
| Alkane.C29 | BOTH |
| Alkane.C31 | BOTH |
| Alkane.C33 | BOTH |
| Alkane.C35 | “mild” Condition |
| C26.OH | “mild” Condition |
| C28.OH | “mild” Condition |
| C30.OH | “mild” Condition |
| C32.OH.isomer | “mild” Condition |
| Cholestane | “mild” Condition |
| Dicyclohexyl phthalate | “mild” Condition |
| Ester.C42.mixtures.C16.22.FA.C26.20.OH | “mild” Condition |
| Ester.C44.mixtures.C16.C22.FA.C22.C28.OH | BOTH |
| Ester.C46.mixtures.C16.C24.FA.C22.C30.OH | BOTH |
| Ester.C48.mixtures | “mild” Condition |
| FA.C16 | “mild” Condition |
| FA.C18 | “mild” Condition |
| FA.C26 | “mild” Condition |
| FA.C28 | “mild” Condition |
| FA.C30 | “mild” Condition |
| FA.C32 | “mild” Condition |
| Methacrylic acid tetradecyl ester | Field |
| n-Dodecyl methacrylate | Field |
| Oleamide | “mild” Condition |
| α-Amyrin | “mild” Condition |
| β-Amyrin | BOTH |
| Triterpene.3425.8.259.365.408 | BOTH |
| X.Z.2.Methylglutaconic.acid | Field |
- Note: Chemicals of significance (p < 0.05) from both conditions were combined to create a union set of compounds. Union set is the set of elements found in either “mild” or “field” or “both” identifying their degree of contribution (p < 0.05) to the set.
4 DISCUSSION
The first fall frost is typically expected within the first 2 weeks of September in Saskatoon, Saskatchewan. Once this event occurs, it is normally followed by 2–3 weeks of subsequent frost-free days. Like other frost-sensitive crops, corn survives by avoiding ice nucleation and not tolerating the presence of ice in its tissue (Levitt, 1980). The survival of corn into these frost-free days through avoidance of ice nucleation will allow for additional and critical maturation time. Physiological modifications of plants induced by chilling treatment (simulated fall temperature conditions prior to the first fall frost) are of interest as it represents an opportunity to select for critical physiological traits involved in freezing avoidance.
In order for ice formation to occur, three conditions must be met: (1) the presence of sub-zero temperatures; (2) an ice nucleator must be present (if temperatures are above the spontaneous ice nucleation point); and (3) water must be present (Ashworth et al., 1985). The temperature of ice nucleation in corn has been reported to be at a much higher temperature (e.g., 4°C; Lindow, 1983) compared to the results of our study (−6.8°C to −8.4°C). Since all plants were treated similarly, we assume extrinsic/intrinsic nucleators were similar in quantity and quality across the leaves and treatments between genotypes. Our studies did not spray the foliage with water since we had already determined significant differences in trichome number and type between the various corn genotypes (data not shown). Trichomes will hold water to varying extents which would introduce another variable into the experiments. Therefore, the corn leaves were subsequently supercooled, which is likely why the ice nucleation temperature of our leaves occurred at a lower temperature than what is reported elsewhere.
Although chilling injury is observed in corn seedlings at temperatures below 12°C (Stamp, 1984; Zeng et al., 2021), there is evidence that corn seedlings can be successfully chilling-acclimated (Anderson et al., 1994). In our study, exposure of corn genotypes to either mild or extreme chilling conditions did not reduce the subsequent temperature of ice nucleation, in fact, in some cases, ice nucleation temperature became less negative. While initially, this result may seem at odds with Anderson et al. (1994), chilling resistance and avoidance of ice nucleation are two completely separate mechanisms. Chilling resistance is dependent upon mechanisms within the symplasm, while frost or ice nucleation avoidance in tender annual plants is dependent upon nucleators and the hydrophobic characteristics of the external extracellular cuticular layer (Wisniewski et al., 2015). We emphasize the goal of our study was to simply determine the influence of the average temperatures 10 days before the first fall frost across corn genotypes on ice nucleation temperature. The goal was not to determine if cold acclimation altered ice nucleation temperature in corn genotypes. Our study shows that both mild and extreme chilling treatments negatively impact the overall ice nucleation temperature in corn. Moreover, corn has a large leaf area and biomass, which increases the probability of an ice nucleation event and is one of the primary factors in determining the freezing temperature (Ashworth & Kieft, 1995). In Pinus, increasing leaf size was found to be the reason for greater variability in freezing temperatures (Kaku, 1975). A decreasing range in freezing temperatures was also observed for Buxus as leaves matured (Kaku, 1971). Other studies using thermal imaging and evaluating freezing processes also identified that size, surface area, and weight are crucial in modifying the ice nucleation temperatures (Carter et al., 2001; Pearce & Fuller, 2001; Wisniewski et al., 2002; Workmaster & Palta, 1999). While a full discussion on extrinsic versus intrinsic nucleators is beyond the scope of this paper (see Charrier et al., 2017), in general, intrinsic nucleators are more active in freezing tolerant plants with preferred internal sites of ice nucleation (Ashworth et al., 1985; Brush et al., 1994; Sakai & Larcher, 1987). Extrinsic nucleators have been studied for quite some time in bacteria and largely appear in frost-sensitive plant material (e.g. Ashworth et al., 1985; Lindow, 1983). Since corn is a frost-sensitive, annual crop, we assume ice nucleation was based on extrinsic and not intrinsic nucleators. Our visual observations using the IR camera validated this assumption since ice nucleation appeared to first occur on the surface of the leaves/stem. The reduction in cuticular hydrophobicity following chilling treatment corresponds with the warmer ice nucleation temperatures indicating a reduced ability to withstand sub-zero temperatures. It has been widely demonstrated that higher cuticular hydrophobicity in the aerial section of plants should better avoid ice nucleation (Sharma et al., 2015; Wisniewski et al., 2002; Wisniewski & Fuller, 1999; Workmaster & Palta, 1999). These previous reports support our findings, where a link between the ability to withstand freezing and hydrophobicity was established. In a study with A. thaliana, we detected a sharp decrease of the hydrophobic very-long-chain waxes in the whole cuticle (both adaxial and abaxial) of the rosette leaves following a two-week of cold acclimation regime of 5/2°C under a 16/8 h light–dark cycle (Rahman et al., 2021). That study also found that cold-acclimated A. thaliana dewax mutant with higher content of hydrophobic waxes froze at a colder temperature than both cold-acclimated parental wild-type and cold-acclimated wax-deficient cer3 mutant lines (Rahman et al., 2021). The chilling treatment appeared to decrease the total hydrophobic waxes of the cuticle in all corn genotypes, which decreased their ability to avoid freezing under subzero freezing conditions. Measuring the change in both adaxial and abaxial cuticle surfaces of the corn leaf tissue following chilling treatment would be a good exploration in future research.
Our study also showed that the cuticular thickness remained unchanged in response to chilling treatment under controlled growth conditions for 10 days. In contrast, growing corn under field conditions for 5 weeks significantly increased the cuticle's thickness (p < 0.05). There were also detectable genotypic differences in cuticular thickness. Freezing-sensitive genotype 256 was found to have a significantly (p < 0.05) thinner cuticle compared with all other genotypes. Cuticular waxes in the cuticle are known to change under varied environmental parameters (Sherrick et al., 1986). Differences in cuticular wax thickness between the phytotron and field results could be explained by factors such as direct sunlight (Hull et al., 1975; Skoss, 1955), temperature and water stress (Skoss, 1955), relative humidity (Sutter, 1979), photoperiod (von Wettstein-Knowles, 1982), and wind under field conditions. Fluctuations in these environmental factors would be more extreme under field production compared with phytotron conditions, which may have contributed to the differences in our findings. In addition to environmental factors, there is potential for a duration effect to modify the cuticular thickness. For example, a 5-week period under field conditions was subject to a longer period to synthesize wax than the 10 days under phytotron-induced “mild” chilling treatment. In leek (Allium porrum L.), previous late-stage wax synthesis occurred beyond the cell expansion stage (Rhee et al., 1998), and the distribution of chemical compounds in the cuticle differed based on developmental age (Jetter & Schäffer, 2001). Aside from known environmental modifiers, we observed significant late-season field effects in terms of cuticular wax deposition and cuticular thickness with a comparable time period (2 weeks) between August 24 and September 12. However, there were no significant changes in the cuticular thickness under “mild” chilling treatment in the growth chamber. Here, it is notable that cuticle thickness has been shown in an array of species to not correlate with water permeability and surface interaction (Riederer & Schreiber, 2001) while in other studies, a thicker cuticle was shown to be effective in avoiding ice nucleation (Wisniewski & Fuller, 1999; Workmaster & Palta, 1999). But in other cases, the cuticle alone could not prevent ice nucleation (Wisniewski et al., 2002).
ATR-FTIR spectroscopy results in response to the chilling treatment revealed significant modifications in the CH3 and CH2 symmetrical and asymmetrical fingerprint regions. Change in peak area intensity of CH2 functional groups has been attributed to a change in the cuticular accumulation of cutin and waxes (España et al., 2014; Heredia-Guerrero et al., 2014). The CH3 group is indicative of methyl groups rather than methylene, as are found within the band. The methyl groups are an extension of aliphatic compounds and aromatic residuals most closely associated with cutan (Heredia-Guerrero et al., 2014). The aliphatic hydrocarbon functional group combined with its derivatives (alcohols, alkanes, ketones, and esters) comprise plant waxes and cutin and are hydrophobic (Koch, 2008). Aromatic residuals are known for their influence over protein regulation and stability, in addition to their hydrophobic characteristics (Dougherty, 2007; Howell et al., 1999). Adam and Elliott (1962) demonstrated the strong hydrophobic characteristics of methyl over methylene (~30% greater). In previous gas chromatography analyses of the cuticular wax layers in tomato, apple (Chen et al., 2008), corn, and A. thaliana (Kunst & Samuels, 2003), aliphatic carbon compounds were found to be an important component of the chemical makeup (Chen et al., 2008). Willick et al. (2018) reported that aliphatic lipid chains represented by CH2 symmetrical and asymmetrical vibrational peaks were greater in the flag leaf of drought-resistant wheat Stettler than in the drought-sensitive wheat Superb. However, under moderate drought stress conditions, there was a higher relative increase of CH2 vibrational peaks observed in the flag leaf of drought-sensitive wheat Superb. In A. thaliana, cold acclimation decreased the CH2 symmetrical vibrational peak in wax-deficient freezing-sensitive mutant cer3, whereas CH2 (both symmetrical and asymmetrical) and CH3 peaks were increased under cold acclimation in freezing-resistant mutant dewax and in parental genotype (Rahman et al., 2021). Our ATR-FTIR study indicated that chilling treatment altered the aliphatic lipid chains of the cuticle of most corn genotypes, in which the sensitive genotype 256 displayed the biggest changes in all lipid fingerprint regions. The exception is the sensitive genotype 675, which was once again found non-responsive to chilling treatment. Resistant genotypes 884 and 959 displayed stability with relatively little change in the lipid fingerprint regions in response to chilling treatment.
Under field conditions, the ATR-FTIR findings across all three regions of interest demonstrated a tendency to decrease over the 5-week treatment period. This decrease could be attributed to the modification of the composition of wax constituents based on the GC–MS results for the field conditions. Longer chain fatty acids and larger biomolecules were preferentially synthesized over time. According to GC–MS results, the pathway for ester synthesis seems to have slowed greatly in the field grown 884 and 959. Ester-cutin is converted into both cutan and non-ester cutin after the cell expansion stages (Schreiber & Schonherr, 2009). Increased chain length (which increases hydrophobicity) in the accumulated waxes has been observed for Sorghum bicolor and A. thaliana (Atkin & Hamilton, 1982; Jenks et al., 1996). Riederer and Schneider (1990) found warm day and night temperatures can modify the wax composition. Most wax constituents increase under warm days. Esters were found to preferentially increase with warm night temperatures (Riederer & Schneider, 1990). In our studies, the night temperatures were markedly cooler during the late period in the field, and this may account for the drop in ester production under field conditions despite an increase in the primary alcohol content.
Changes in the wax composition in the cuticle are expected to change slowly over time as the plant ages (Baker et al., 1982; Jetter & Schäffer, 2001; Maier & Post-Beittenmiller, 1998; Prasad & Giilz, 1990; Wang et al., 2015). Due to a shorter time range, the differences detected in the cuticular composition according to ATR-FTIR analysis were subtle in most corn genotypes under 10 days of “mild” chilling treatment. ATR-FTIR spectra are basically describing the measurement of specific vibrational signatures of the atoms in a molecule (Türker-Kaya & Huck, 2017). A shift toward a change in the prevalence of alkanes and long-chain fatty acids, as was observed in the field samples, may, in part explain the reduction in the intensity in the ATR-FTIR spectra in the lipid fingerprint regions. Lastly, the limitation of the penetration of the ATR into the thicker field-produced cuticle could represent a biased sampling of the upper surface of the wax. The upper surface of the wax will degrade over time due to the environment, and new deposition occurs on the innermost side adjacent to the epidermis and may not have been adequately excited by the light to measure true accumulation.
Significant shifts (p < 0.05) in the percent abundance of compounds in the field samples induced by the chilling treatment were observed. Notable increases were identified in alkanes and fatty acids. These long-chain carbon compounds are known contributors to the hydrophobic properties of the cuticular surface (Nadiminti et al., 2015). In addition to these larger group percentage shifts, genotype 884 had a significant increase (p < 0.05) in oleamide. Oleamide is an extremely hydrophobic compound and has been reported in thermoregulation and sensitivity (Laws et al., 2001), as well as signaling systems and plant defense (Chatterjee et al., 2010). As for percent composition, we observed a strong decrease in triterpenes. Pentacyclic triterpenoids are found in many wax species (Jetter et al., 2006) and tend to accumulate in the intracuticular wax (Buschhaus & Jetter, 2012). In genotype 959, a significant decrease (p < 0.05) in amyrins was observed. The study by Buschhaus and Jetter (2012) discovered that the accumulation of β-amyrin, which takes place exclusively in the intra-cuticular waxes, can diminish the effectiveness of the water barrier imposed by the cuticle. The decrease in the accumulation of amyrins in genotype 959 may indicate a mechanism by the corn plants to maintain the barrier against ice propagation during freezing.
The characteristics linked to the cuticular layer as a barrier indicate the importance of freezing avoidance, such as a thinner cuticle associated with the more freezing-sensitive genotype. Chilling treatment in the field tends to change the total wax production and favors longer-chain carbon aliphatic compounds. Longer-chain fatty acids have low polarity compared with short and medium-chain fatty acids, making them well-suited to create a hydrophobic moisture barrier (Gunstone, 2006). A highly hydrophobic barrier, made of long-chain and unsaturated fatty acids (Ivanov, 2015), seems effective in freezing avoidance through the delay of ice nucleation into the intracellular space (Fuller et al., 2003; Wisniewski et al., 2009; Wisniewski & Fuller, 1999). This study reinforces that the cuticular layer is a critical region of interest to improve freezing avoidance to move corn into new geographies for increased acreage and consistent production.
One of the limitations of this study was the limited diversity within our material to respond to cold treatment. However, using mature corn plants with contrasting chilling resistant genotypes for understanding modifications to the adaxial cuticular layer was a useful model. This system enabled investigation of the physiological mechanisms within the cuticle, acting as a barrier between the plant and environment to control and prevent ice nucleation. Caution should be applied to studies only utilizing controlled environments or phytotron conditions since cuticular responses are clearly different from the field. Continued development of understanding the implications of the cuticular influence on freezing avoidance has widespread potential. It will impact the development and expansion of underutilized crops into temperate regions abundant in arable land. In order to meet the demand to move the corn belt northward, this crop will need to be more frost-avoidant. Identifying genotypes and cultivars that do not lose cuticular hydrophobicity under field conditions and also identifying the key cuticular components responsible for hydrophobicity will be important to enable corn to avoid frost and lower the temperature at which ice nucleation occurs on the surface of the leaf.
AUTHOR CONTRIBUTIONS
Kaila Hamilton planned the experiments, conducted the work, interpreted the data, and wrote the thesis upon which this paper is based; Tawhidur Rahman interpreted the data and wrote the paper; Jason Sadowski helped to analyze the data; Chithra Karunakaran helped with the synchrotron work; Karen Tanino received the funding, supervised Kaila Hamilton, planned the experiments and helped interpret the data; all co-authors reviewed the final draft.
ACKNOWLEDGMENTS
The authors acknowledge generous funding from the Natural Sciences and Engineering Research Council (NSERC) (Canada), Corteva Agriscience, Western Grains Research Foundation, Robert P. Knowles Scholarship, Mitacs through the Japanese Society for the Promotion of Science, Saskatchewan Ministry of Agriculture, the Saskatchewan Wheat Development Commission, Alberta Wheat Commission, and the Manitoba Crop Alliance. We thank Jackie Bantle, Eldon Siemens, Doug Waterer, and the phytotron team for plant care. Ann Perera at the W.M. Keck Metabolomics Research Laboratory was an immense help running and analyzing the GC–MS as was Eiko Kawamura at our local WCVM imaging center using the CLSM. Microscopy techniques were learned from the imaging team at NMBU with special recognition of YeonKyeong Lee and Jorunn Olsen. Part of the research described in this paper was performed at the Canadian Light Source (CLS), a national research facility of the University of Saskatchewan, which is supported by the Canada Foundation for Innovation (CFI), the Natural Sciences and Engineering Research Council (NSERC), the National Research Council (NRC), the Canadian Institutes of Health Research (CIHR), the Government of Saskatchewan, and the University of Saskatchewan. We gratefully acknowledge the CLS research facility staff, Scott Rosendahl and Stuart Reed.
Open Research
DATA AVAILABILITY STATEMENT
All experimental data that support the findings of this study are available upon reasonable request from the corresponding author.




