Abscisic acid-mediated sugar responses are essential for vegetative desiccation tolerance in the liverwort Marchantia polymorpha
Abstract
Low-molecular-weight sugars serve as protectants for cellular membranes and macromolecules under the condition of dehydration caused by environmental stress such as desiccation and freezing. These sugars also affect plant growth and development by provoking internal signaling pathways. We previously showed that both sugars and the stress hormone abscisic acid (ABA) enhance desiccation tolerance of gemma, a dormant propagule of the liverwort Marchantia polymorpha. To determine the role of ABA in sugar responses in liverworts, we generated genome-editing lines of M. polymorpha ABA DEFICIENT 1 (MpABA1) encoding zeaxanthin epoxidase, which catalyzes the initial reaction toward ABA biosynthesis. The generated Mpaba1 lines that accumulated only a trace amount of endogenous ABA showed reduced desiccation tolerance and reduced sugar responses. RNA-seq analysis of sucrose-treated gemmalings of M. polymorpha revealed that expression of a large part of sucrose-induced genes was reduced in Mpaba1 compared to the wild-type. Furthermore, Mpaba1 accumulated smaller amounts of low-molecular-weight sugars in tissues upon sucrose treatment than the wild-type, with reduced expression of genes for sucrose synthesis, sugar transporters, and starch-catabolizing enzymes. These results indicate that endogenous ABA plays a role in the regulation of the positive feedback loop for sugar-induced sugar accumulation in liverworts, enabling the tissue to have desiccation tolerance.
1 INTRODUCTION
Low molecular-weight sugars such as sucrose are fundamental compounds serving as energy and carbon sources to support plant growth and development. Plants also accumulate sugars as compatible solutes in response to changes in environmental conditions, such as decreases in outer temperature and soil water potential and provoke internal mechanisms to protect cells from forthcoming severe stress such as drought and freezing (Sami et al., 2016). Soluble sugars serve as protectants of cells but also function as signalling molecules to control the expression of genes involved in plant growth and stress tolerance (Moore & Sheen, 1999; Rolland et al., 2006; Saddhe et al., 2021). Such “sugar signals” are generated by signalling networks mediated by trehalose 6-phosphate, the target of rapamycin kinase and Snf1-related kinase 1 and also affect other signalling pathways, including those for phytohormones such as gibberellins, ethylene, and abscisic acid (ABA) (Gibson, 2004; Lastdrager et al., 2014; Leon & Sheen, 2003; Sakr et al., 2018; Zhou et al., 1998).
Analysis of Arabidopsis mutants such as glucose insensitive (gin), sugar insensitive (sis), and impaired sucrose induction (isi) highlighted the role of ABA in physiological responses to sugars in angiosperm seeds. It was found that gin1, sis4, and isi4 were allelic to the ABA biosynthetic mutant aba2 and that gin5 was allelic to another ABA biosynthetic mutant, aba3. Furthermore, gin6, isi3, and sis5 were allelic to abi4, which has a mutation in a gene encoding an APETALA2-type transcription factor involved in ABA-induced gene expression (Arenas-Huertero et al., 2000; Laby et al., 2000; Rook et al., 2001). Among various ABA-insensitive (abi) mutants, abi4-1 is very resistant to sugars, while the abi5-1 mutant with a defect in the ABA-responsive element binding (AREB) transcription factor is slightly resistant; on the other hand, abi1-1 and abi2-1, mutants of protein phosphatase 2C show little or no resistance to sugars (Laby et al., 2000). Mutants of ABI3, encoding a B3 domain transcription factor, show various levels of sugar insensitivity depending on the different abi3 alleles (Dekkers et al., 2008). These results indicated that a specific ABA signalling pathway might mediate the sugar responses. ABA is responsible for the physiological control of seed maturation and germination, dormancy, and desiccation tolerance (Cutler et al., 2010; Fujii & Zhu, 2012; Vishwakarma et al., 2017). The signalling network that integrates sugar and ABA signals are thought to enable seeds to have phenotypic plasticity for a switch between growth and tolerance under ever-changing environmental conditions.
In contrast to the above studies in angiosperms, information on the sugar-induced response and its relationships with environmental and hormonal factors in non-seed plants, including bryophytes, is very limited. It was previously reported that some bryophyte species in a natural environment accumulate more soluble sugars in winter than in summer and that the levels of soluble sugars is associated with freezing tolerance (Rütten & Santarius, 1992). Under an experimental condition, treatment of cultured protonema cells of the moss Physcomitrium patens with ABA and cold enhanced freezing tolerance, which was associated with accumulation of low-molecular-weight sugars and Late embryogenesis abundant (LEA)-like transcripts (Nagao et al., 2005, 2006). Sugars might serve as protectants that mitigate dehydration damage caused by freezing stress together with the action of LEA-like proteins. The liverwort Marchantia polymorpha can develop a high degree of dehydration tolerance in response to external sucrose (Akter et al., 2014; Hatanaka & Sugawara, 2010). ABA-induced accumulation of low-molecular-weight sugars and LEA-like transcripts is associated with reduced growth and enhanced desiccation and freezing tolerance in gemmae, dormant propagules for asexual reproduction (Akter et al., 2014). These studies indicated that there might be signal crosstalk between sugar and ABA for the control of dormancy and stress tolerance in liverworts similar to that in seed plants.
To determine the role of endogenous ABA in sugar and stress responses in bryophytes, we generated ABA-deficient lines of M. polymorpha by disrupting MpABA1, an ortholog of ABA-DEFICIENT1 (ABA1) encoding zeaxanthin epoxidase, which catalyzes the initial reaction toward ABA biosynthesis. The generated Mpaba1 lines accumulated only trace amounts of endogenous ABA. We expected the Mpaba1 lines to show not only reduced desiccation tolerance, but also reduced sugar responses. Thus, we analyzed the osmotic response to sugars, desiccation tolerance, dormancy, and sugar-induced gene expression in the Mpaba1 lines to determine whether the lack of endogenous ABA alters sugar-induced cellular responses.
2 MATERIALS AND METHODS
2.1 Plant materials and sugar treatment
Gemmae and thalli of M. polymorpha wild-type (WT) (accession Takaragaike-1) were cultured at 22°C on half-strength B5 agar medium containing 1% (w/v) sucrose under continuous light, unless otherwise stated (Jahan et al., 2022). Sugar treatment of gemmae was carried out using half-strength B5 liquid medium without sugars or with 0.1, 0.2, and 0.4 M of sucrose or mannitol.
2.2 Generation of genome editing lines
To make genome-editing lines of MpABA1, double-strand oligonucleotides corresponding to the target guide RNA (5′-GAGGAGGTATCGGAGGTC-3′) were fused downstream to the MpU6 promoter in the pMpGE010 vector containing the Cas9 gene cassette in T-DNA (Sugano et al., 2018). Agrobacterium (Rhizobium radiobacter) C58 strain harboring the above construct was used for infection of 2 week-cultured gemmalings of M. polymorpha as described previously (Kubota et al., 2013). After selection on a medium containing 10 mg L−1 hygromycin, the generated lines were individually cultured on a fresh selection medium for further propagation and DNA sequencing.
2.3 Gemma dormancy scoring
Thalli of WT and mutants grown for 4 weeks were used to score the germination rates and the number of gemma per gemma cup. Gemmae isolated from gemma cups located at the most basal position in thalli were observed under a dissecting microscope, and germination rates were determined by counting the numbers of gemmae with rhizoids and those without rhizoids.
2.4 Measurement of ABA
Extraction, purification and gas chromatography with mass spectrometry (GC–MS) of thalli cultured for 4 weeks were carried out according to protocols described previously (Kobayashi et al., 2010; Yamashita et al., 2016).
2.5 Desiccation tolerance tests
Gemmae were cultured with or without 0.1, 0.2, and 0.4 M sucrose for 3 days and transferred onto a cellophane sheet set on water-wet filter paper in a petri dish. For desiccation treatment, the petri dish was placed in a container containing silica gel and kept at 23°C for 2 days in the dark to avoid any light-enhanced desiccation injury. The dried gemmae on the cellophane were rehydrated by adding half-strength B5 liquid medium and kept for 1 h at room temperature. The gemmae with the cellophane were then transferred to a fresh half-strength B5 agar medium and cultured for 2 weeks. The survival rate was determined by counting the number of regenerated green gemmalings relative to the number of used gemmae. Experiments using the same concentrations of mannitol as osmotic controls were also carried out.
2.6 Sugar analysis
Gemmalings rinsed in water were weighed, frozen, and about 20 mg of the material was crushed using a mortar and a pestle in a 10 volume of 80% (v/v) ethanol. Insoluble materials were removed by centrifugation at 14,000g for 5 min at 4°C, and the supernatant was dried and suspended in water. After the removal of water-insoluble material by centrifugation, the supernatant was used as a sugar sample. The amount of total soluble sugars was determined by the anthrone-sulfuric acid assay (Akter et al., 2014; Yemm & Willis, 1954). The sugar sample was mixed with 2% (w/v) anthrone in 75% (v/v) sulfuric acid and boiled for 10 min, and absorbance at 620 nm was measured with glucose as a standard.
2.7 Statistical analysis
Experiments carried out with three biological replicates were analyzed by Student's t-test for two-group comparison and by ANOVA with Tukey's HSD (honestly significant difference) test for the comparisons of multiple sample means.
2.8 RNA extraction and reverse transcription-PCR analysis
Total RNA was extracted from sugar-treated gemmalings and used for first-strand cDNA synthesis as described previously (Tougane et al., 2010). PCR reactions were conducted as described by Ghosh et al. (2016). Primer sets used for the analysis are shown in Table S1.
2.9 RNA sequencing library construction, sequencing, and data analysis
RNA sequencing (RNA-seq) was carried out by Beijing Genomics Institute (BGI). The RNA-seq library construction was carried out using TruSeq RNA sample prep kit v2 (Illumina) for sequencing by BGIseq-500, generating a single read of 50 bp (Zhu et al., 2018). Sequence data are available from DDBJ (accession number DRA016056). The CLC genomics workbench software version 22 (Qiagen) was used for quality filtering of raw read data, mapping to the M. polymorpha genome v3.1 (Phytozome 13; https://phytozome-next.jgi.doe.gov/) and extraction of differentially expressed genes (DEGs). DEGs have an FDR of less than 0.05 and an expression value change of greater than 2-fold.
3 RESULTS
3.1 Growth, dormancy, and sugar responses in MpABA1 genome-editing lines
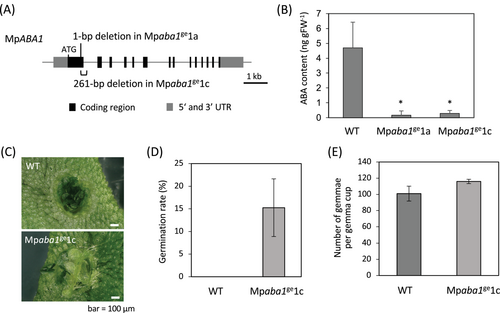
In order to obtain ABA-deficient lines of M. polymorpha, we disrupted the MpABA1 gene encoding zeaxanthin epoxidase homologous to Arabidopsis ABA1 by CRISPR-Cas9-mediated genome editing (Sugano et al., 2018). An 18-nucleotide target sequence was designed for the first exon of the MpABA1 gene, and the sequence was fused with that of the gRNA backbone in the T-DNA of the pMpGE010 vector for Agrobacterium-mediated transformation. By sequence analysis of the generated transformants, we obtained Mpaba1ge1a with a deletion of 1 bp causing a frameshift and Mpaba1ge1c with a deletion of 261 bp in the MpABA1 locus (Figure 1A). GC–MS analysis of cultured thalli revealed that the amount of endogenous ABA in both Mpaba1ge1a and Mpaba1ge1c lines was very small in comparison with that in WT (Figure 1B). There was no remarkable difference in thallus growth between WT and the Mpaba1 lines, but we observed a difference in dormancy of gemmae in gemma cups. Gemmae are propagules that are dormant when they exist in the gemma cup, and rhizoid formation is the first sign of breaking gemma dormancy. While there was no rhizoid growth of gemmae in gemma cups in WT, many gemmae growing rhizoid were observed in the Mpaba1ge1c line (Figure 1C), which was supported by data of the percentage of the rhizoid germination in gemmae (Figure 1D). When we counted the number of gemmae per gemma cup of WT and Mpaba1ge1c, there was no significant difference in the numbers between these lines (Figure 1E), suggesting that endogenous ABA has no effect on the total number of gemmae. These findings indicate that endogenous ABA plays a role in gemma dormancy of M. polymorpha. To determine the effects of sugars on WT and ABA-deficient lines, we analyzed the growth of gemmalings on media containing 0.1 and 0.2 M sucrose. We also analyzed the effects of the same concentrations of mannitol to determine whether the response is sucrose specific or due to an osmotic effect. It was found that both sucrose and mannitol inhibited the growth of gemmalings in all lines tested, but more inhibition was observed in both Mpaba1ge1a and Mpaba1ge1c lines than in WT (Figure S1).
3.2 Susceptibility to desiccation in the ABA-deficient line
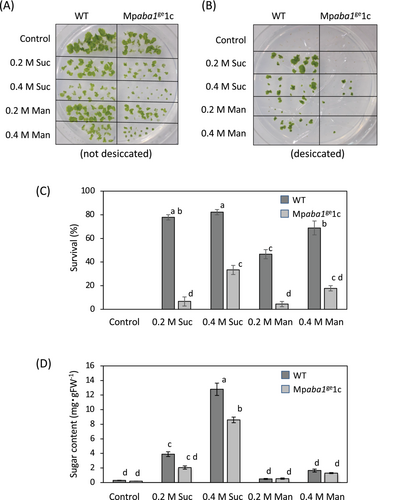
Next, we determined the effects of sucrose and mannitol on the survival of gemmalings after desiccation. We focused on Mpaba1ge1c with a larger deletion of DNA than that in Mpaba1ge1a, although there was no phenotypic difference between these two lines. Gemmae of these plants were preincubated with or without sucrose and mannitol for 3 days and then desiccated for 2 days in a closed chamber containing silica gel, and survival was determined after rehydration and regrowth. Without desiccation, all gemmae survived with or without sucrose and mannitol treatment in both WT and Mpaba1ge1c, but the size of gemmae was smaller in Mpaba1ge1c than in WT (Figure 2A), possibly because Mpaba1ge1c was more sensitive to osmostress (Figure S1). After desiccation, we found that sucrose- and mannitol-treated gemmae of WT showed a higher survival rate than those of Mpaba1ge1c (Figure 2B,C). Determination of levels of soluble sugars in tissues indicated that sucrose treatment causes accumulation of soluble sugars in tissues and that the levels of sugars were reduced in Mpaba1ge1c (Figure 2D).
3.3 Sucrose-induced gene expression in the ABA-deficient line
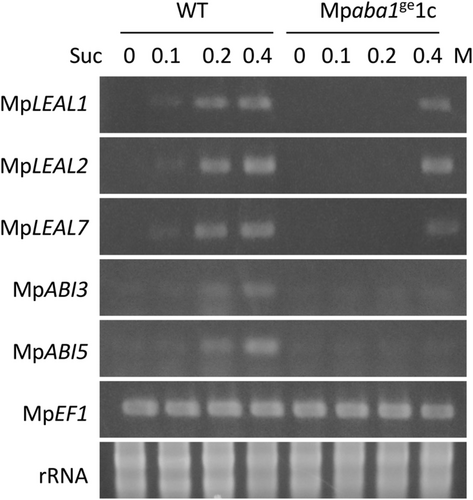
In our previous study, ABA treatment of M. polymorpha gemmalings increased the expression of LEA-like genes encoding hydrophilic proteins along with sugar accumulation and enhancement of desiccation tolerance (Akter et al., 2014; Jahan et al., 2019). Results of semi-quantitative RT-PCR analysis of representative LEA-like genes indicated that expression of these transcripts is upregulated by increasing the concentration of sucrose in WT, but the levels of expression were lower in Mpaba1ge1c (Figure 3). It is suggested that expression of ABA-induced genes is mediated by evolutionarily conserved promoter elements recognized by transcription factors related with ABI3 and AREB in M. polymorpha (Ghosh et al., 2016). Analysis of sucrose-induced expression of MpABI3 and MpABI5 encoding ABI3- and AREB-like transcription factors, respectively, revealed that expression of these transcripts was also increased by sucrose in WT but not in Mpaba1ge1c (Figure 3). Results of quantitative RT-PCR analysis indicated a similar tendency of sucrose-induced gene expression and that the level of induction is greater than that achieved by the same concentration of mannitol (Figure S2).
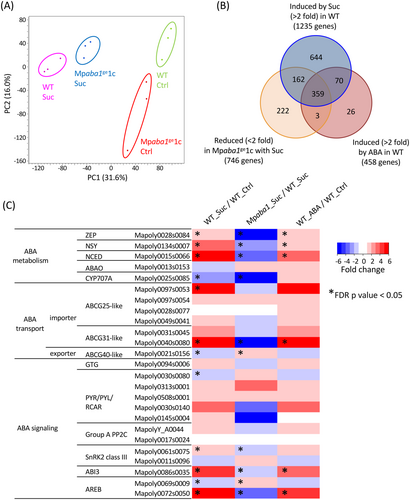
To get more insight into changes in gene expression in response to sucrose, we carried out RNA sequencing (RNA-seq) analysis using gemmalings of WT and Mpaba1ge1c treated or not treated with 0.2 M sucrose. Results of the principal component analysis of triplicate samples indicated a distinctive difference in sugar-induced gene expression between WT and Mpaba1ge1c (Figure 4A). Data analysis revealed that, of 17,788 genes examined, expression of 1235 genes was enhanced more than 2-fold by sucrose in WT. Of these 1235 genes, the expression of 521 genes (42.2%) was decreased (<2-fold) in Mpaba1ge1c with the same treatment, indicating that these sucrose-induced genes are under the control of endogenous ABA. Comparison with ABA-induced genes we previously identified in M. polymorpha (Jahan et al., 2022) revealed that, of 458 ABA-induced genes, 429 genes were those also induced by sucrose (Figure 4B). We analyzed the relative abundance of transcripts of representative genes for ABA metabolism, transport, and signalling. It was found that genes for ABA biosynthesis enzymes NSY and NCED, a putative ABA transporter ABCG31-like, and transcription factors MpABI3 and MpABI5 were among the 359 genes induced by sucrose, reduced in Mpaba1ge1c and induced by ABA as shown in Figure 4C. Analysis of the RNA-seq data also revealed that expression of all 36 identified ABA-induced LEA-like genes is increased by sucrose and reduced in Mpaba1ge1c (Figure S3), consistent with the results shown in Figure 3.
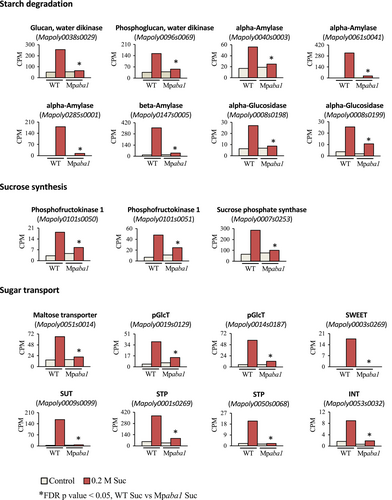
We also analyzed the expression of genes for sugar metabolism, which might account for the sucrose-induced sugar accumulation. Analysis of RNA-seq data revealed that the expression of genes for specific isoforms of enzymes such as alpha-amylase and beta-amylase for starch catabolism, genes for sucrose synthesis such as sucrose phosphate synthase, and genes for sugar transporters such as SUT, STP, and SWEET orthologs is induced by sucrose (Figure 5). Expression of the majority of these sucrose-induced genes was reduced in Mpaba1ge1c, indicating that endogenous ABA is critical for upregulation of these genes by sucrose.
4 DISCUSSION
4.1 Role of ABA in sugar signalling is common in embryophytes
ABA has been postulated to play a role in the enhancement of freezing and desiccation tolerance in various species of angiosperms. Earlier works have demonstrated that the application of ABA to cultured plant cells can induce freezing tolerance, as reported in potato (Chen et al., 1983), bromegrass (Chen & Gusta, 1983), and Brassica napus (Orr et al., 1986). The ABA-deficient (aba1) mutant is less freezing tolerant (Mantyla et al., 1995), while ectopic expression of the ABI3 gene enhanced freezing tolerance in Arabidopsis (Tamminen et al., 2001). The importance of transcriptional networks and signal crosstalk between ABA and abiotic stress signals such as drought, cold, and heat for environmental stress tolerance has been proposed (Nakashima et al., 2014).
In this study, we described the importance of endogenous ABA in sugar-induced desiccation tolerance. Accumulation of sugars is commonly observed in overwintering plants during cold seasons and is thought to contribute to high freezing tolerance (Sakai & Yoshida, 1968; Siminovitch, 1981). Sugars protect cells by mitigating lesions of the plasma membrane caused by freeze-induced dehydration (Uemura & Steponkus, 2003). Sugars can replace water molecules associated with the hydrophilic surface of the lipid bilayer to avoid membrane damage under dehydration (Luzardo et al., 2000). The role of ABA in sugar response has been known for over two decades through analyses of sugar-insensitive Arabidopsis mutants having deficiencies in genes for ABA biosynthesis and ABA-dependent transcription factors (Rolland et al., 2006). Yet, little has been clarified about the important molecules that affect ABA signals during sugar responses. Previous studies revealed that ABA is sensed by the signalling complex with PYR/PYL/RCAR receptor (PYL), group A protein phosphatase 2C (PP2C-A) and subgroup III SNF1-related protein kinase 2 (SnRK2), where PYL inhibits PP2C-A upon ABA binding and causes activation of SnRK2, which in turn phosphorylates and activates various proteins such as ion channels and transcription factors (Cutler et al., 2010). Genes for PYL, PP2C-A, SnRK2, and ABA-activated transcription factors are conserved in bryophytes (Bowman et al., 2017; Komatsu et al., 2020), and the role of PYL, PP2C-A, and ABI3 orthologs in the ABA response in M. polymorpha has been demonstrated (Eklund et al., 2018; Jahan et al., 2019; Tougane et al., 2010). Our previous study showed that ABA treatment of M. polymorpha gemmae with increasing sucrose concentrations in media enhanced tolerance to freezing and desiccation, and that there was a positive correlation between tolerance levels and sucrose accumulation in tissues (Akter et al., 2014). These studies indicated that ABA might play a role in sugar signalling in liverworts, as reported in angiosperms, although profiles of sugar-regulated genes mediated by ABA in the liverwort have not been clarified.
This study using an ABA-deficient mutant of M. polymorpha for analyses of sugar-induced growth inhibition, desiccation tolerance, sugar accumulation, and global gene expression revealed that the cellular response in M. polymorpha to external sucrose is largely dependent on endogenous ABA. Considering that liverworts are evolutionarily among the earliest diverging lineages of land plants (Bowman et al., 2017), it is likely that the role of ABA in sugar signalling is not exclusive in angiosperms but is common in embryophytes. RNA-seq analysis of sucrose-treated gemmalings revealed that expression of genes for ABA biosynthesis, ABA-activated transcription, ABA transport, and LEA-like proteins is induced by sucrose, which should contribute to desiccation tolerance (Figures 2-4). The results shown in this study are consistent with those of our previous study showing that a liverwort mutant lacking one of the PYL genes has a reduced sugar response (Jahan et al., 2019). The effect of sucrose is likely in part due to its osmotic effect because the same concentrations of mannitol, which did not induce sugar accumulation, enhanced desiccation tolerance to a certain extent in WT (Figure 2). The mannitol-induced desiccation tolerance was reduced in Mpaba1ge1c, indicating that the osmotic signal is also mediated by endogenous ABA.
4.2 Possible role of ABA in the positive feedback loop for sugar-induced sugar accumulation
Plant growth is continuously regulated by environmental cues such as light, temperature, nutrition, and soil water potential, which can affect levels of soluble sugars. Especially cold-induced accumulation of soluble sugars such as hexoses, sucrose, raffinose-family oligosaccharides, and fructans, and their possible roles in cold hardiness in winter are documented in a variety of plant species (Yuanyuan et al., 2009). Accumulation of sugars must be controlled by sugar-metabolizing enzymes and membrane transporters, which are readily modified by internal as well as environmental signals. Plant cells, therefore, have mechanisms to continuously sense sugar levels and control cellular metabolisms through sugar-triggered signal transduction processes. Studies on Arabidopsis indicate complex mechanisms underlying sugar sensing and signalling operated by Regulator of G-protein signalling (RGS1) (Urano et al., 2012), trehalose-6-phosphate (T6P) (Schluepmann et al., 2003), Snf1-related protein kinase-1 (SnRK1) (Tsai & Gazzarrini, 2014), hexokinase (Granot et al., 2013), and TOR kinase (Xiong & Sheen, 2012). Furthermore, experimental evidence suggested the presence of a sucrose-specific signalling mechanism (Chiou & Bush, 1998) and that sucrose transporters such as SUT2 and SUT4 have been proposed as sucrose sensors (Barker et al., 2000; Li et al., 2012; Vaughn et al., 2002). However, the mechanisms by which these putative sensors generate signals and modulate gene expression are largely unknown. RNA-seq analysis in this study revealed that sucrose treatment induces transcripts for starch degradation, sucrose synthesis and sugar transport in M. polymorpha. Typical examples are prominent increases in transcript levels of alpha- and beta-amylases and the SUT-related transporter by sucrose (Figure 5). Previous studies indicated that sucrose treatment alone can induce sugar accumulation and desiccation tolerance in gemmae, indicating that the sugar signal provoked both sucrose uptake and synthesis (Akter et al., 2014). In conjunction with the results of sugar analysis showing that sugar accumulation is reduced in Mpaba1ge1c (Figure 2D), it is possible that endogenous ABA plays a role in the regulation of the positive feedback loop for sugar-induced sugar accumulation, enabling the tissue to efficiently accumulate sugars for dormancy and desiccation tolerance. Our future goal is to clarify a transcriptional network controlling sucrose-triggered gene expression. Our studies will also focus on the analysis of ABA responses in mutants of sugar-induced transcription factors for clarification of the mechanism underlying the crosstalk between ABA- and sugar-signalling.
AUTHOR CONTRIBUTIONS
Experiments were conducted by Nobiza Khatun, Akihisa Shinozawa, Kosaku Takahashi, Hideyuki Matsuura, Akida Jahan, Mousona Islam, Md. Masudul Karim, Rahul Sk, and Mikako Yoshikawa. Data analysis was done by Akihisa Shinozawa and Kimitsune Ishizaki. Manuscript preparation was conducted by Nobiza Khatun, Akihisa Shinozawa, Yoichi Sakata, and Daisuke Takezawa.
ACKNOWLEDGEMENTS
We thank Takayuki Kohchi for providing pMpGE010, and Kei Saito and Mayuka Hiraide for technical assistance. We also thank Nodai Genome Research Center of Tokyo University of Agriculture for data analysis of RNA-seq. This work was supported by Grant-in-Aid for Scientific Research (No. 20K06680, 18H04774, JP15H05955, and 19H03247) of the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan.
CONFLICT OF INTEREST STATEMENT
There are no conflicts of interest in this study.
Open Research
DATA AVAILABILITY STATEMENT
Data Availability StatementThe datasets generated in study are available from the corresponding author on reasonable request.




