Integrative multi-omics analysis of three early diverged rosid species reveals an ancient hierarchical cold-responsive regulatory network
Abstract
Elucidating regulators, including transcription factors (TFs) and RNA-binding proteins (RBPs), underlying gene transcriptional and post-transcriptional co-regulatory network is key to understand plant cold responses. Previous studies were mainly conducted on single species, and whether the regulators are conserved across different species remains elusive. Here, we selected three species that diverged at the early evolution of rosids (~99–113 million years ago), performed cold-responsive phylotranscriptome experiments, and integrated chromatin immunoprecipitation- and DNA affinity purification-sequencing (ChIP/DAP-seq) analysis to explore cold-responsive regulators and their regulatory networks. First, we detected over 10,000 cold-induced differentially expressed genes (DEGs) and alternative splicing genes (DASGs) in each species. Among the DEGs, a set of TFs and RBPs were conserved in rosid cold response. Compared to TFs, RBPs displayed a delayed cold-responsive pattern, implying a hierarchical regulation of DEGs and DASGs. By integrating DEGs and DASGs, we identified 259 overlapping DE-DASG orthogroups (closely-related homologs) that were cold-regulated at both transcriptional and post-transcriptional levels in all three studied species. Notably, pathway analysis on each of the DEGs, DASGs, and DE-DASGs in the three species showed a common enrichment connected to the circadian rhythm. Evidently, 226 cold-responsive genes were directly targeted by at least two circadian rhythm components (CCA1, LHY, RV4, RVE7, and RVE8). Finally, we revealed an ancient hierarchy of cold-responsive regulatory networks at transcriptional and post-transcriptional levels launched by circadian components in rosids. Altogether, this study sheds light on conserved regulators underlying cold-responsive regulatory networks across rosid species, despite a long evolutionary history after their divergence.
1 INTRODUCTION
For adaptation to adverse environmental conditions, angiosperms have evolved sophisticated and advanced mechanisms and become the largest land plant lineage, with more than 350,000 known species widely distributed from tropical to polar terrestrial zones (Wu et al., 2020). Among the adverse environmental conditions, seasonal and daily variation in temperature is a fundamental component that affects plant growth, productivity, and geographical distribution (Kidokoro et al., 2022). Under cold stress, plants undergo physiological and molecular changes that increase the levels of specific proteins, metabolites, and phytohormones, which are generally regulated by transcription factors (TFs) and RNA-binding proteins (RBPs) at transcriptional and post-transcriptional levels (Caldana et al., 2011; Calixto et al., 2018; Ding et al., 2019; Kidokoro et al., 2022).
Thousands of differentially expressed genes (DEGs) were identified as involved in cold stress (Calixto et al., 2018; Hannah et al., 2005; Zheng et al., 2022) and many TFs have been suggested to regulate and manage their expression (Chinnusamy et al., 2007; Ding et al., 2019; Kidokoro et al., 2022). The best-characterized TFs are C-repeat binding factor (CBF) genes that are rapidly and transiently induced by cold stress and can enhance the freezing tolerance of plants through binding to cis-elements (CCGAC) in the promoters of cold-responsive (COR) genes (Jaglo-Ottosen et al., 1998; Stockinger et al., 1997). RNA-seq analysis of Arabidopsis cbf123 triple mutant showed that hundreds of CORs are regulated through a CBF-dependent pathway (Jia et al., 2016; Zhao et al., 2016). Activation of CBF target CORs, such as COR15A, COR47, KIN1, and RD29A, leads to an increase in plant freezing tolerance (Jaglo-Ottosen et al., 1998; Novillo et al., 2007; Wang & Hua, 2009). In addition, the cold responsiveness of plants is regulated by the circadian clock system, which is involved in plant stress responses by controlling daily energy expenditure and enabling plants to grow and reproduce under seasonal and daily temperature variations (Greenham & McClung, 2015; Harmer, 2009; Hsu & Harmer, 2014; Markham & Greenham, 2021; Oravec & Greenham, 2022). In the clock system, some circadian clock-related TFs, such as CIRCADIAN CLOCK ASSOCIATED 1 (CCA1), LATE ELONGATED HYPOCOTYL (LHY), and REVEILLES (RVEs), have been proven involved in cold tolerance by directly regulating the expression of downstream CORs, such as CBFs, COR15A/15B, COR28, and COR27 (Creux & Harmer, 2019; Dong et al., 2011; Kidokoro et al., 2021; Wang et al., 2017).
On the other hand, post-transcriptional regulation is also a key process in reprogramming gene expression for plants to tolerate or survive constant changes in temperature (Ding et al., 2019; Kidokoro et al., 2022). Like TFs in transcriptional gene regulation, RBPs are essential in controlling post-transcriptional RNA processing, including capping, polyadenylation, alternative splicing (AS), RNA export, translation, and turnover (Bach-Pages et al., 2020; Calixto et al., 2018). Under cold stress, upregulation and/or downregulation of RBP genes may cause differentially post-transcriptional RNA processing. AS is an important post-transcriptional mechanism during RNA processing by producing different transcripts and possibly protein variants from a single gene for increasing transcriptome and proteome diversity (Wu et al., 2018). The concentration, localization, and activity of RBPs determine AS events to produce different transcripts (Fu & Ares, 2014; Lee & Rio, 2015). In plants, extensive AS events have been implicated in various abiotic stresses, including cold, salt, heat, and other environmental stresses (Calixto et al., 2018; Liu et al., 2018; Zhao et al., 2021; Zheng et al., 2022). For example, genome-wide AS profiling in Arabidopsis showed a large number of differentially alternative splicing genes (DASGs) after cold treatment, suggesting that plants may fine–tune AS events of gene transcripts for adapting to cold stress (Calixto et al., 2018). Among DASGs, CCA1, a core component of the circadian clock, has two splice variants: CCA1α and CCA1β. CCA1β competitively inhibits the DNA-binding activity of CCA1α and LHY by forming nonfunctional heterodimers of CCA1β-CCA1α and CCA1β-LHY (Seo et al., 2012). Under cold stress, CCA1β production is repressed to release the activity of CCA1α and LHY, whereby CCA1α and LHY promote the expression of downstream CORs for improving plant cold tolerance (Seo et al., 2012).
In the past years, numerous cold-induced DEGs and DASGs were identified in different plant species by high-throughput methods (Calixto et al., 2018; Liu et al., 2018; Wang et al., 2021; Zhao et al., 2021; Zheng et al., 2022). Identifying core regulators (e.g., TFs and RBPs) and elucidating the molecular mechanisms underlying the outcome of DEGs and DASGs are key to understanding the reprogramming of gene regulatory networks in plant response to cold stress. Previous studies were generally performed on single plant species, especially the reference plant Arabidopsis thaliana, and whether the regulators and mechanisms underlying transcriptional and post-transcriptional changes are conserved in different plant lineages remains elusive. In this study, we focused on rosids, a large group of eudicots in angiosperms, and selected three plant species: Carya illinoinensis and Populus trichocarpa from fabids (rosid I), and Arabidopsis thaliana from malvids (rosid II). Fabids and malvids are two main clades of rosids, comprising >40,000 and ~ 15,000 species, respectively (Chase & Reveal, 2009). The three plant species diverged during the early evolution of rosids and have evolved independently for over 100 million years (Kumar et al., 2017). The long evolutionary history caused great differences in their growth habits and geographic distributions, and thus, we asked whether the regulators and mechanisms underlying gene transcriptional and post-transcriptional regulation are conserved across rosids in response to cold stress.
To solve the question, we performed omics analysis of the genomes, transcriptomes, and regulomes and identified common core regulators in regulating cold responses across the three plant species. First, by RNA-seq experiments, we investigated global expression profiles of cold-induced DEGs and DASGs in each of the three studied species. Subsequently, we analyzed cold responses of TFs and RBPs and identified those TF and RBP families that are conserved in cold responses, which likely contribute to DEGs and DASGs in rosids. Moreover, we explored overlapping genes (i.e., DE-DASGs) of DEGs and DASGs and performed homologous analysis to identify conserved DE-DASG orthogroups in rosids. Finally, we selected five circadian components and integrated transcriptomes and regulome maps to identify their target CORs for constructing a hierarchical cold-responsive regulatory network.
2 MATERIALS AND METHODS
2.1 Genome data and divergence times of the studied species
The proteome sequences and gene annotations of A. thaliana, C. illinoinensis, and P. trichocarpa were obtained from Huang et al. (2019), Rhee et al. (2003) and Tuskan et al. (2006). Their divergence times were extracted and assessed from the TimeTree website (Kumar et al., 2017).
2.2 The design of cold-treated RNA-seq experiments
To explore molecular changes of rosids under cold stress, we performed RNA-seq experiments in the 3 selected plant species after being cold treated at 4°C for 0, 2, 24, and 168 h. Before cold treatment, all the plant seedlings were cultured in an climate chamber under 16/8 h light/dark regime at 25°C. For A. thaliana, 3-week-old seedlings were used for the cold-stress treatments. For the other 2 species, young seedlings with a height of ~30 cm were used, including 2-month-old P. trichocarpa and 6-month-old C. illinoinensis. To ensure that the leaves of the four samples (0, 2, 24, and 168 h) would be harvested at similar developmental stages at the same time on the same day, we designed the experiment carefully to eliminate the influence of differences in circadian time and/or development stages on cold-affected transcriptional changes. In brief, the 168 h cold-treated seedlings were first cultured in the chilling climate chamber (4°C) for a week, then the 24, 2 h cold-treated seedlings were transferred into the chamber at the appropriate times. The control samples (0 h) were consistently cultured under 25°C. After the treatments, the plants of A. thaliana, C. illinoinensis, and P. trichocarpa generally had ~10–12, ~8–9, and ~10–12 leaves, respectively. To ensure that the seedling leaves of the samples (0, 2, 24, and 168 h) were in similar developmental stages, only the fourth expanded leaves were collected at the same time on the same day. Total RNA of the collected leaves was isolated by TRIzol reagent and quantified by NanoDrop ND-1000, and the RNA integrity was assessed by Agilent 2100. Finally, the cDNA libraries of the isolated RNA were constructed for Illumina RNA sequencing at LC-Bio Technology Co., Ltd. (Hangzhou, China). During the experiment, three biological replicates were performed for each sample.
2.3 Genome-wide analysis of cold-induced DEGs and DASEs
We performed RNA-seq analysis as described by Nie et al. (2022). In brief, clean reads of cold-treated RNA-seq samples were aligned to the reference genome and genes using the hisat2-2.1.0 software (Kim et al., 2015) with the parameters (–min-intronlen 20–max-intronlen 5000–rna-strandness RF). Then, the gene expression under control (0 h) and cold treatment (2, 24, and 168 h) conditions were calculated by StringTie v2.0.3 (Pertea et al., 2015) with default parameters. Subsequently, DEGs between normal (0 h) and each of the cold treatments (2, 24, and 168 h) were analyzed by DESeq2 (Love et al., 2014) based on three criteria: at least one sample with TMM > 5, more than two-fold change in expression levels before and after cold treatment, and a statistical difference with p-value <0.01 (Table S1). When a gene is upregulated at one time point and downregulated at another, the most contrasting expression value of the gene at 2, 24, and 168 h compared to control conditions (0 h) was selected. In addition, 5 types of AS events, including intron retention (IR), alternative 3′ splice site (A3SS), alternative 5′ splice site (A5SS), skipping exon (SE), and mutually exclusive exons (MXE), were identified by rMATS v4.0.2 (Shen et al., 2014). A false discovery rate (FDR) ≤ 0.05 was set as the criterion for identifying cold-induced DASEs between control and cold treatments (Table S2).
2.4 Gene Ontology term enrichments
The gene ontology (GO) term annotations for the A. thaliana genes were directly acquired from The Arabidopsis Information Resource (TAIR) database (Rhee et al., 2003). According to the method described by the previous reports (Guo et al., 2022; Salojarvi et al., 2017), the P. trichocarpa, and C. illinoinensis genes were annotated with the highest alignment score matches against A. thaliana proteins from BLASTP searches (E-value < 1e−5) (Altschul et al., 1990). The GO term enrichment analysis was performed using the OmicShare tools (www.omicshare.com/tools).
2.5 Identification and characterization of TFs and RBPs
The TFs and RBPs were obtained through the following strategies. For TFs, we downloaded A. thaliana and P. trichocarpa TFs from the plantTFDB 5.0 (Jin et al., 2017) and in C. illinoinensis we identified TFs according to the method described by (Jin et al., 2017) (Table S3). For RBPs, we extracted experimentally identified A. thaliana RBPs from a supplementary table of the previous publication (Bach-Pages et al., 2020). By using A. thaliana RBPs as queries, we employed BLASTP 2.5.0 (Altschul et al., 1990) to retrieve RBP homologs in P. trichocarpa and C. illinoinensis (E-value < 1e−10, identity > 60%) (Table S4).
To identify protein families of TFs and RBPs, we downloaded all Hidden Markov Model (HMM) profiles (>19,000) of protein domains from the Pfam database (Finn et al., 2016) and searched them against the proteome sequences of the three plant species by HMMER 3.1b2 with an E-value < 1e−5 (Eddy, 1998). Accordingly, we obtained gene families for each TF and RBP (Tables S3 and S4).
2.6 Identification of DE-DASG ortho-groups
To explore whether DE-DASGs across the rosids are homologous, we classified the up- and down-DE-DASGs of the three rosids into different ortho-groups using Orthofinder v2.3.8 (Emms & Kelly, 2019) with the parameters -M dendroblast; -S diamond; −A mafft; -I 1.5 (Table S5 and S6).
2.7 Identification of conserved COR genes targeted by circadian clock components
We developed the following pipeline to determine conserved COR genes (CoCORs) regulated by circadian clock components in rosids. First, we obtained A. thaliana ChIP-seq (CCA1) and DAP-seq (LHY, RVE7, RVE4, and RVE8) datasets (Kamioka et al., 2016; O'Malley et al., 2016) and performed ChIP/DAP-seq analysis as described (Nie et al., 2022). The genes with at least one binding peak of the five TFs in the 1-Kb upstream of the translation initiation site (TIS) were considered direct target genes of the corresponding TFs in A. thaliana. Second, we collected cis-regulatory binding motifs of CCA1, LHY, RVE7, RVE4, and RVE8 (Kamioka et al., 2016; O'Malley et al., 2016) and searched them against the 1-Kb promoter sequences of P. trichocarpa and C. illinoinensis genes using FIMO (E-value < 1e−3) (Grant et al., 2011). The genes with at least one cis-regulatory binding site for the five TFs were identified as potential targets of the TFs in P. trichocarpa and C. illinoinensis. Subsequently, all coding genes in the three species were classified into ortho-groups according to their homology. The ortho-group genes that have binding sites for the five TFs and are differentially regulated after cold stress in all three rosids are defined as CoCORs (Table S7).
2.8 Statistical analysis
The significance of enrichment analysis between gene groups was calculated by Fisher Exact test through R programming.
3 RESULTS
3.1 Global gene expression profiles in rosids under cold stress
Physiological and molecular changes in plants under cold stress are usually caused by up- or downregulated expression of genes (Kidokoro et al., 2022). To capture the landscape of DEGs under cold stress, we performed RNA-seq experiments in three rosid species before and after cold treatments (see Materials and Methods). Based on RNA-seq analysis, we found that the development-related genes are expressed at relatively similar levels at different time points of cold treatment, suggesting a common developmental stage of the collected leaves (Figure S1).
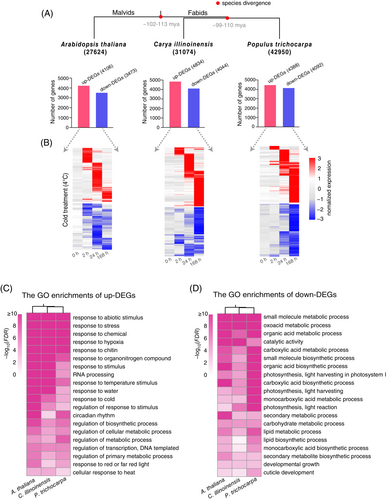
Subsequently, we employed the RNA-seq datasets to identify a large number of cold-induced DEGs: 7579, 8878, and 8480 in A. thaliana, C. illinoinensis, and P. trichocarpa, respectively (Table S1). Notably, more upregulated than downregulated DEGs were observed in all three plants (Figure 1A). Expression profiles show that these upregulated DEGs mainly clustered in three time points: 2, 24, and 168 h of cold treatments (Figure 1B). We found that the number of upregulated DEGs in A. thaliana was significantly higher after 2 h and lower after 168 h of cold stress treatment than the counterparts in the other two species. Likewise, there were significantly more downregulated DEGs at 2 h and less downregulated DEGs at 168 h in A. thaliana than in C. illinoinensis and P. trichocarpa (Figure 1B). These findings reveal different cold-responsive speeds: an early extensive response in A. thaliana but a delayed extensive response in C. illinoinensis and P. trichocarpa.
3.2 Common biological processes of cold responses in rosids
Despite different cold-responsive speeds between the three plant species, GO term enrichment analysis of the DEGs showed a very similar enrichment (Figure 1C, D). The upregulated DEGs are mainly enriched in abiotic stress processes, for example, response to hypoxia, water and cold, and circadian rhythm (Figure 1C). In addition, regulations of transcription and RNA processing are also among the most enriched GO terms, which suggests that the regulators (e.g., TFs and RBPs) at the transcriptional and post-transcriptional levels are likely to be more activated under cold stress (Figure 1C). The downregulated DEGs are mainly enriched in processes such as catalytic activity, photosynthesis, developmental growth, small molecular, and/or lipid biosynthetic/metabolic processes (Figure 1D). It is reasonable that to improve cold tolerance, plants need to reduce the expression of the genes involved in activities of plant development and growth (Kidokoro et al., 2022).
3.3 Conserved cold-responsive TF families in rosids
Differential changes in gene expression at the transcriptional level are likely caused by fluctuations in TFs (Calixto et al., 2018; Kidokoro et al., 2022). To detect the roles of TFs under cold stress, we first identified a total of 1717, 1899, and 2466 TFs in A. thaliana, C. illinoinensis, and P. trichocarpa genomes, respectively (Table S3, see Materials and Methods). Transcriptome analysis shows that over 500 TFs were differentially cold-regulated, covering a high proportion of the genome TFs, that is, 32%, 35%, and 22%, respectively, in A. thaliana, C. illinoinensis, and P. trichocarpa (Figure 2A, Table S3). Notably, there were more upregulated than downregulated TFs and their expression patterns in cold-responsive speeds agree well with the aforementioned global expression patterns of DEGs (Figures 1B, 2B). This finding suggests more upregulated TFs might be involved in modulating differentially regulated genes under cold stress.
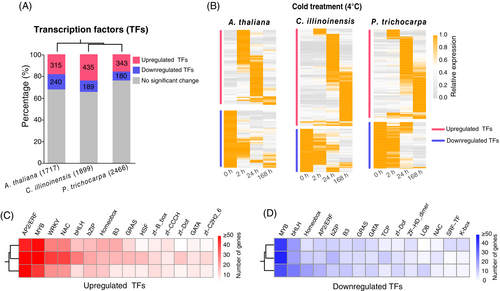
Moreover, we classified all TFs into gene families based on their protein domain architectures (Table S3, see Materials and Methods). Interestingly, the up- and downregulated TFs were clustered in common gene families in three plant species (Figure 2C, D). For example, the main families of upregulated TFs were AP2/ERF, MYB, WRKY, and NAC, the best-known TF families involved in abiotic stresses (Guo et al., 2022; Khan et al., 2018). The counterparts of downregulated TFs were MYB, bHLH, Homeobox, and AP2/ERF. Notably, AP2/ERF and MYB were the two most prominent TF families with many more upregulated members, but also a considerable number of downregulated members, in all three plants under cold stress.
3.4 Global alternative splicing profiles in rosids under cold stress
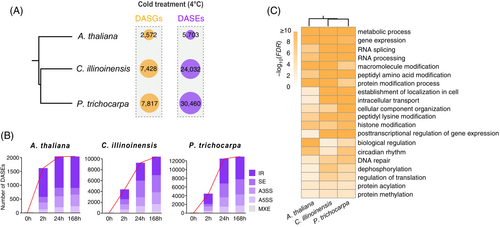
In addition to transcriptional regulation, we identified an enrichment of post-transcriptional regulation, such as RNA processing, in response to cold stress (Figure 1C). Based on transcriptome analysis, we detected 2572, 7428, and 7817 cold-induced DASGs, which occupied 10%, 24%, and 18% of the coding genes in A. thaliana, C. illinoinensis, and P. trichocarpa, respectively (Figure 3A, Table S2). Among DASGs, many have multiple AS sites, producing diverse numbers of 5703, 24,032, and 30,460 differential AS events (DASEs), respectively, in A. thaliana, C. illinoinensis, and P. trichocarpa (Figure 3A, Table S2). This is mainly caused by AS occurrences at the same or different exon/intron boundaries across a gene, leading to more RNA transcript diversities. Number distributions of DASEs in the three species show a similar pattern: more DASEs were observed at the middle and late stages of cold treatment (24 and 168 h) than at the early stage (2 h) (Figure 3B, Table S2). Consistent with previous studies (Calixto et al., 2018; Zhao et al., 2021), IR is the most common AS mode in the three plant species (Figure 3B). Despite a relatively small role of skipping exon (SE) in A. thaliana, SE provides a crucial role in the other two plants (C. illinoinensis and P. trichocarpa). Furthermore, GO term enrichment analysis shows that these DASGs from three different plant species were enriched in similar biological processes, such as metabolic processes, gene expression, RNA splicing, and circadian rhythm (Figure 3C). Given the key role of RBPs in RNA processing (Bach-Pages et al., 2020; Calixto et al., 2018), thousands of cold-induced DASEs might at least partially result from fluctuations in RBP genes.
3.5 A delayed cold responsivity of RBP genes
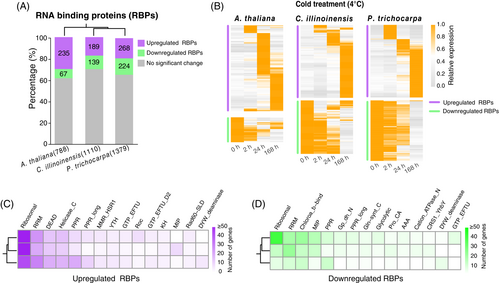
In A. thaliana, we found a total of 788 high-confident RBPs that were experimentally confirmed by RNA-binding assays (Bach-Pages et al., 2020). Using the RBPs for homologs searching, we identified 1110 and 1379 RBPs in C. illinoinensis and P. trichocarpa, respectively (Figure 4A, Table S4). Transcriptome analysis shows that 36%, 31%, and 35% of the RBPs were differentially regulated by cold stress in A. thaliana, C. illinoinensis, and P. trichocarpa, respectively (Table S4). Like TFs, we identified more upregulated RBPs than downregulated ones, especially in A. thaliana (Figure 4A). Interestingly, in contrast to TF genes, RBP genes show a delayed responsive pattern after cold treatment in all three plant species (Figures 2B, 4B). For example, only a relatively small number of RBP genes were observed to be differentially regulated at the early stage (2 h) of cold stress.
3.6 Conserved cold-responsive RBP families in rosids
Based on RNA-binding domains, we classified RBP genes into different gene families (Table S4). Among the top 15 cold-responsive RBP families, ribosomal and RRM were the most prominent families, with more genes being upregulated than downregulated under cold stress in all three plant species (Figures 4C,D). DEAD and Helicase_C families are mainly enriched in the upregulated RBPs (Figure 4C), while Chloroa_b-bind and MIP families clustered in the downregulated RBPs (Figure 4D).
3.7 Cold association of transcriptional and post-transcriptional activities in rosids
As described above, thousands of genes were identified as cold-induced DEGs and/or DASGs in the three plants (Figures 1 and 3). Furthermore, we saw that upregulated DEGs showed a significant overlapping association with DASGs, that is, 558, 1340, and 1419 up-DE-DASGs, in A. thaliana, C. illinoinensis, and P. trichocarpa, respectively (Figure 5A, Table S8). Similarly, downregulated DEGs also significantly correlate with DASGs in the three plant species. In the following sections, we determined whether these genes across the three studied plants are homologous and function in a conserved way.
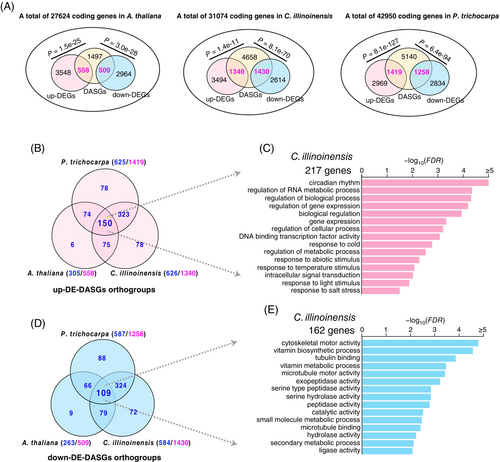
3.8 Identification of 259 conserved ortho-groups in rosids
Biased retention and loss of gene duplicates in plants after independent whole-genome and/or small-scale duplications lead to complex and intertwined evolution of co-orthologs and in-paralogs across different species (Guo et al., 2022; Koonin, 2005). To determine common cold-responsive genes between species, we classified genes that are differentially regulated at both transcriptional and post-transcriptional levels into ortho-groups: sets of homologous genes descended from the recent common ancestor of the plants (Emms & Kelly, 2019) (see Materials and Methods). For example, 558, 1340, and 1419 up-DE-DASGs are classified into 305, 626, and 625 ortho-groups, respectively in A. thaliana, C. illinoinensis, and P. trichocarpa (Table S5). Among these orthogroups, Venn diagram analysis shows a significant overlapping of 150 ortho-groups among the three plants (Figure 5B, Table S5). From the 150 ortho-groups, we extracted 217 C. illinoinensis genes and performed GO term enrichment analysis (Figure 5C). As expected, the genes are mainly enriched in abiotic stresses, such as response to abiotic stresses (salt and cold stress) and circadian rhythm (Figure 5C). The GO term enrichments of the counterparts in other two species have similar results (Figure S2). The same analysis was also conducted for down-DE-DASGs, and we identified 109 overlapping ortho-groups with many genes enriched in diverse enzymatic activities (Figure 5D,E, Figure S2, Table S6). The findings together show a total of 259 ortho-groups (150 + 109) that are regulated at both the transcriptional and post-transcriptional levels in a conserved manner in rosids under low temperatures.
3.9 Seven striking TF ortho-groups involved in circadian rhythms
Among the 259 ortho-groups, there were 27 upregulated TF ortho-groups (Table S9) and it is noteworthy that 7 of them encode known circadian clock components, including CCA1/LHY, RVE4/8, RVE7, PRR9, BBX19, COL9, and JMJD5 genes (Table 1). Our results are consistent with previous reports that CCA1, LHY, and PRR9 can be regulated at the transcriptional and post-transcriptional levels under cold stress in Arabidopsis (James et al., 2012; Seo et al., 2012). In our study, these circadian genes were also cold-regulated in C. illinoinensis, and P. trichocarpa and we found that additional circadian clock-related genes were differentially cold-regulated at both the transcriptional and post-transcriptional levels in rosids (Table 1). This explains the aforementioned repeated observations that the biological process of circadian rhythm is enriched (Figures 1C, 3C, and 5C). The seven ortho-groups are upregulated under cold stress in all three plant species, and in contrast, AS types of genes in each ortho-group show a great diversity, which suggest conservation of gene expression but a rapid evolution of AS events after rosid radiation (Table 1). Moreover, MYB is the most prominent TF family including three orthogroups (CCA1/LHY, RVE7, and RVE4/8).
| Family (orthogroup) | Cold regulation Ath/Cil/Ptr | Ath (AS-type) | Cil (AS-type) | Ptr (AS-type) |
|---|---|---|---|---|
| MYB (OG0000072) | UP/UP/UP | CCA1 (A5SS) LHY (A3SS,A5SS,IR,SE) |
CIL1205S0016 (A3SS, A5SS, MXE, IR, SE) | Potri.014G106800 (A3SS, A5SS, MXE, IR, SE) |
| MYB (OG0000110) | UP/UP/UP | RVE4 (A3SS, SE) RVE8 (A3SS, IR) |
CIL0982S0042 (A5SS) |
Potri.017G146800 (IR) |
| MYB (OG0000043) | UP/UP/UP | RVE7 (SE) | CIL1030S0151 (IR) CIL1245S0064 (IR) |
Potri.004G074300 (IR) Potri.012G038300 (IR) |
| PRR (OG0000018) | UP/UP/UP | PRR9 (A5SS) | CIL0897S0115 (IR) CIL1083S0010 (IR) CIL1194S0023 (IR) |
Potri.002G179800 (A3SS, IR, SE) Potri.014G106000 (A3SS, IR, SE) |
| BBX (OG0000101) | UP/UP/UP | COL9 (IR) | CIL1155S0003 (IR) | Potri.002G214500 (IR) Potri.014G170600 (IR) |
| BBX (OG0000088) | UP/UP/UP | BBX19 (IR, SE) | CIL0978S0047 (IR) | Potri.001G061800 (A3SS) |
| Cupin (OG0000219) | UP/UP/UP | JMJD5 (A3SS, IR) | CIL1465S0014 (IR) | Potri.001G016200 (IR) |
- Note: The short names (Ath, Cil, and Ptr) indicate the species (Arabidopsis thaliana,Carya illinoinensis, and Populus trichocarpa).
3.10 Cold-responsive genes regulated by circadian clock components
Through a stringent pipeline, we identified the CoCORs targeted by the above identified 5 clock components CCA1, LHY, RVE4, RVE7, and/or RVE8 in rosids (Table S7) (see Materials and Methods). As shown in Figure 6A, the 5 circadian components regulate different numbers of CoCORs but with a common pattern of dominant upregulation in the 3 rosids. For instance, as compared to only 17 downregulated CoCORs, there are 101 CoCORs identified to be upregulated by CCA1 (Figure 6A, Table S7). GO term analysis further shows that these CoCORs are enriched in environmental stresses, such as response to light, cold, and circadian rhythm (Figure 6B). Moreover, the Venn diagram shows a significance of overlapping CoCORs regulated by the 5 TFs (Figure 6C), and among the overlapping CoCORs, 16 are regulated by all 5 TFs and 226 are regulated by at least 2 of the 5 TFs. The intertwined DNA binding of CoCORs by the five circadian TFs suggests a complex regulatory network launched by circadian clock components.
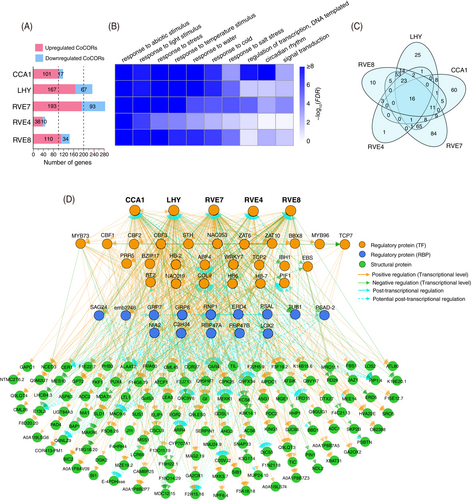
3.11 A conserved hierarchy of cold-responsive regulatory network in rosids
To construct the network, we employed the CoCORs involved in abiotic stresses that were directly regulated by the five circadian components. Taking into account the cold-responsive speeds of the genes under cold treatments at 2, 24, and 168 h and also their regulatory relationships, we constructed a hierarchical regulatory network with 3 layers (Figure 6D).
The first layer is composed of 30 TFs including many well-known cold-regulated genes, such as CBFs, PRR5, ZAT6, ZAT10, COL9, BT2, etc. In this layer, the 5 clock components play a dominant role to upregulate the expression of 21 TFs and in turn work together with these upregulated TFs to further regulate the expression of downstream genes (e.g., RBPs and structural genes in the second and third layers). The second layer is composed of 14 RBPs. Among the 14 RBPs, 12 are upregulated by the first layer TFs. These 12 RBPs function in a feedback mechanism by potentially regulating AS of TFs in the first layer and function in regulating AS of the downstream layer genes. For example, by analyzing individual nucleotide resolution crosslinking and immunoprecipitation (iCLIP)-seq experimental data (Meyer et al., 2017), we found that GRP7 can directly bind to transcripts of BT2 and HB6 in the first layer and COR27, KCS6, BAP1, COR413-PM1, ERD15, LTL1, PSBTN, and MEE14 in the third layer, and many of these genes undergo differentially AS events under cold stress. The third layer is composed of 140 CORs mainly encoding structural proteins. These genes are differentially regulated in expression and/or alternative splicing by the first and/or second layer regulators.
4 DISCUSSION
When exposed to cold stress, plants undergo a series of molecular changes to protect themselves from chilling and/or freezing damage (Ding et al., 2019; Kidokoro et al., 2022). By high-throughput methods, previous studies provided insights into the reprogramming of gene regulation in response to cold stress (Calixto et al., 2018; Liu et al., 2018; Wang et al., 2021; Zhao et al., 2021; Zheng et al., 2022). However, the studies were generally performed with single plant species and focused on the best-known CBF-dependent pathway genes. Here, we used 3 plant species (A. thaliana, C. illinoinensis, and P. trichocarpa) form different rosid lineages, which diverged early during the evolution of rosids (~100 million years ago) and performed RNA-seq experiments with plants subjected to cold stress to investigate conserved regulators and mechanisms underlying rosids in response to cold stress. Over 7000 DEGs were identified in each species and these show common responses of biological processes, such as cold stress process and circadian rhythm (Figure 1C). Interestingly, the three plant species show different cold-responsive speeds: an early response in A. thaliana but a delayed and extensive cold-response in C. illinoinensis and P. trichocarpa (Figures 1B and 2B). The difference in cold-responsive speeds maybe due to the different growth characteristics between annual herbaceous plants (e.g., A. thaliana) and perennial woody plants (e.g., C. illinoinensis and P. trichocarpa). Compared to woody plants, herbaceous plants are more sensitive to temperature changes (Cornelius et al., 2013), which might cause an early extensive cold response.
In addition to transcriptional regulation, post-transcriptional regulation is a key process in reprogramming gene expression under cold stress (Calixto et al., 2018; Zhao et al., 2021). Like DEGs, thousands of DASGs were identified in rosids under cold stress. Interestingly, the number of DASGs in A. thaliana is significantly less than the counterparts in C. illinoinensis and P. trichocarpa (Figure 3A). This difference may be due to shorter introns in Arabidopsis than in C. illinoinensis and P. trichocarpa (Figure S3), and to our knowledge, alternative splicing tends to occur around the exons interrupted by long introns (Fox-Walsh et al., 2005; Roy et al., 2008). Consistent with the DEGs, DASGs show an enrichment in components involved in the circadian rhythm (Figure 3C). Unexpectedly, enrichments of biological processes related to cold or abiotic stress were not observed for DASGs. This is probably because a limited knowledge of cold-induced DASGs and their genetic roles as compared to an extensive study of cold-induced DEGs. Regarding this, we provide a complete list of cold-induced DASGs in three rosid species (Table S2) and hope this list will be a potential reference for future research of splicing regulation under cold stress.
Moreover, association analysis of transcriptional and post-transcriptional activities revealed 259 conserved DE-DASG orthogroups in rosids. From DE-DASG orthogroup genes, circadian rhythm is again significantly enriched (Figure 5C). Circadian clock system can allocate stress-responsive processes to specific times of the day, which provides a control of over daily energy expenditure and enables plants to grow and reproduce under temperature changes (Bieniawska et al., 2008; Espinoza et al., 2008; Espinoza et al., 2010; Markham & Greenham, 2021). Here, we found that some conserved circadian clock components were reprogrammed at both transcriptional and post-transcriptional levels under cold stress in rosids (Figure 5C). Among them, MYB is the most prominent TF family, including three ortho-groups with five Arabidopsis genes (CCA1/LHY, RVE7, and RVE4/8), which likely contribute to the flexibility and controllability of the cold response network. Thus, by integrative omics of phylotranscriptomes and regulome maps (RNA-seq and ChIP/DAP-seq data), we identified the direct targets and constructed a conserved hierarchy of cold-responsive regulatory network launched by the five MYB TFs. The existence of many well-known cold-response genes, such as CBFs, RBG7/8, ZAT6/10, and COR27, in the network demonstrate a high confidence of the network but also imply an importance of circadian clock for plants in response to cold stress. In the first layer of the network, in addition to 21 upregulated TFs launched by circadian clock components, there are four downregulated TFs (TCP7, EBS, IBH1, and PIF1). This demonstrates a potential negative regulatory loop between differentially regulated TFs for controlling the balance between plant growth and cold tolerance. Evidentially, phytochrome-interacting factor 1 (PIF1) negatively regulates plant freezing tolerance likely through repressing the expression of CBFs (Jiang et al., 2020). In the second layer, the up- or down-regulation of RBPs may cause the more intense post-transcriptional regulation, such as RNA splicing. The third layer is composed of 140 downstream genes targeted by the first and second layer, and among the genes, some (e.g., COR47, TIL, SLD1, LOS2, and ADC1/2) have showed functions in plant freezing tolerance (Cao et al., 2005; Charron et al., 2008; Chen et al., 2012; Cuevas et al., 2008; Lee et al., 2002). Altogether, our research shed light on conserved hierarchical cold-responsive networks in rosids at the transcriptional and post-transcriptional levels via circadian clock components, which provide insights into conserved core regulators and ancestral mechanisms underlying cold-responsive gene regulatory network across rosids.
AUTHOR CONTRIBUTIONS
Wenwu Wu, Jianhua Zhu, and Xuejiao Jin designed the research; Liangyu Guo and Zhiming Xu performed the study; Shuo Wang did the RNA-seq experiments of the plants and Xiaoxue Ye performed the RNA-seq analysis; Liangyu Guo, Wenwu Wu and Jianhua Zhu wrote and edited the manuscript.
ACKNOWLEDGMENTS
We thank Dr. Lihong Xiao and the members from Dr. Wenwu Wu's laboratory in Zhejiang A&F University for suggestions to improve the quality of the study. This work was supported by the National Natural Science Foundation of China (grant number 31871233).
Open Research
DATA AVAILABILITY STATEMENT
The RNA-seq datasets of the three plant species including A. thaliana, C. illinoinensis, and P. trichocarpa under cold stress have been deposited to NCBI BioProject (Accession: PRJNA767196) and CNGBdb (Accession: CNP0002243).




