Physiological and biochemical regulation of tobacco by oxathiapiprolin under Phytophthora nicotianae infection
Abstract
As a fungicide, oxathiapiprolin has excellent effects on diseases caused by oomycetes. Fungicides generally protect crops by inhibiting pathogens, but little research has addressed the effects of fungicides on crops. This study combined transcriptomic and metabolomic analyses to systematically analyze the physiological regulatory mechanisms of oxathiapiprolin on tobacco under Phytophthora nicotianae infection. The results showed that under P. nicotianae infection, tobacco's photosynthetic rate and antioxidant enzyme activity increased after the application of oxathiapiprolin. Omics results showed that the genes related to carbon metabolism, disease-resistant proteins, and amino acid synthesis were highly expressed, and the amino acid content increased in tobacco leaves. This study is the first comprehensive investigation of the physiological regulatory effects of oxathiapiprolin on tobacco in response to P. nicotianae infection. These findings provide a basis for the balance between regulating tobacco growth and development and enhancing disease resistance under the stimulation of oxathiapiprolin and provide new research and development opportunities for identifying new disease-resistance genes and the development of high-yielding disease-resistant crop varieties.
1 INTRODUCTION
In agricultural production, chemicals, such as insecticides, fungicides, and herbicides, are widely used to control crop pests. Chemical pesticides play an important role in ensuring good food harvests. However, there are several problems associated with the use of pesticides. Although high doses of chemical pesticides can cause crop damage, ranging from reduced yields to crop death (Shahid et al., 2021), at the recommended dosage, pesticides can increase the yield per unit of the crop. After the foliar application of pesticides, they penetrate the epidermis and into the plant tissues, protecting the crop from pests and diseases while affecting the normal physiological and metabolic activities of the crop. Studies have found that glyphosate at 5%–10% of the recommended field rate stimulates barley growth (Cedergreen et al., 2006); when used in sugarcane fields, it can promote sugarcane maturation and increase sucrose content in sugarcane (Belz & Duke, 2014).
Neonicotinoid insecticides imidacloprid and thiamethoxam can induce disease resistance in Arabidopsis, thereby reducing the damage caused by powdery mildew (Ford et al., 2010). Spraying imidacloprid on cotton reduces the activity of antioxidant enzymes, thus reducing heat stress and increasing the photosynthetic rate to promote organic matter accumulation (Gonias et al., 2008). Jaleel et al. (2007) used triazole fungicides (paclobutrazol, triadimefon, and hexaconazole) to treat a variety of plants and found that the three fungicides increased photosynthetic pigment content, net photosynthetic efficiency, intercellular content, and increased chloroplast volume. Treatment of wood beans with 10 mg L−1 of dimethoate for 10 days significantly increased photosynthesis and promoted growth (Pandey et al., 2015). The effect of pesticides on key crop nutrients varies according to the pesticide species and crop type, and the stomatal conductivity and leaf flesh conductivity of lemons were reduced by treatment with Propargite NR440 oil (Abdel-Reheem et al., 1991). Using pesticides during the nutritional growth period of rice can lead to a decrease in photosynthesis (Boerjan et al., 1992).
Tobacco black shank is a rootstock disease caused by Phytophthora nicotianae (P. nicotianae), which affects all growth stages of the tobacco plant and has become one of the most persistent diseases of tobacco (Blaya et al., 2015; Jing et al., 2017). Tobacco black shank seriously affects the quality of tobacco products and is detrimental to the sustainable development of the tobacco industry (Wen et al., 2014). Due to their efficiency and convenience, chemical methods are still preferred to control tobacco black shank (Antonopoulos et al., 2010).
Oxathiapiprolin is a new piperidine fungicide with excellent efficacy against oomycete diseases, especially those caused by pathogenic blight, and is slightly toxic at very low dosages (Pasteris et al., 2016; Peng et al., 2020). It can control oomycete diseases in grapes, potatoes, and cucumbers, among others (D'Arcangelo et al., 2021; Miao et al., 2018). Ji et al. (2014) found that oxathiapiprolin had high efficacy against tobacco black shank. Pasteris et al. (2016) showed that oxathiapiprolin was effective against several stages of P. nicotianae, such as mycelial growth, sporulation, cystospore germination, and zoospore movement, with sporulation being the most sensitive stage tested. Oxathiapiprolin has excellent efficacy against oomycete diseases and can be used to control related diseases, but we do not know how oxathiapiprolin might affect tobacco. At the recommended dosage, there is no apparent damage to the crop and no significant change in crop phenotype, but little research has been done on the microscopic physiological and biochemical parameters of the crop.
In this study, we investigated the changes in various aspects of crop physiology and biochemistry induced by oxathiapiprolin under P. nicotianae infection. Combining transcriptomic and metabolomic analyses of tobacco leaves, we initially explored the regulatory mechanisms of oxathiapiprolin application on the growth and development of tobacco itself. The data gathered here also provide a theoretical basis for the safe application of oxathiapiprolin and provide new opportunities for the development of disease-resistant and high-yielding crop varieties.
2 MATERIALS AND METHODS
2.1 Plant materials and growth conditions
In this study, tobacco seeds were washed in 75% ethanol for 45 s, soaked in 5% sodium hypochlorite for 8 min, and then rinsed three times in sterilized water. Seeds were sown in floating trays in the seedling shed, and the seedling substrate was autoclaved with high humidity (121°C, 30 min). The seedling substrate was then placed in a greenhouse at a temperature of 27°C, a light/dark time ratio of 16/8 h and a humidity of 70% for germination and growth. When the tobacco reached the five to seven true leaf stage, it was transplanted and transferred to a greenhouse with day and night temperatures of 28 and 25°C, a light/dark time ratio of 12/8 h, and 60% humidity for acclimatization.
On the 10th day after transplanting, the spore suspension (1 × 105 cfu ml−1) was inoculated on tobacco by root irrigation, 10 mL per tobacco. The spore suspension is prepared according to Han et al.'s (2016) method. Twenty-four hours after inoculation of the spore suspension, oxathiapiprolin was applied by spraying at a concentration of 10 mg L−1, 20 mL per tobacco. Three days after dosing, the third leaf of tobacco was taken, and the sample was washed in sterile water, then dried on sterilized paper, quickly frozen in liquid nitrogen, and stored at −80°C.
2.2 Phenotype measurement
Fresh weight was measured using a 1/10 electronic balance, maximum leaf length, leaf width, plant height, and all phenotypic measurements, including ten biological replicates.
2.3 Enzyme determination
The activities of antioxidant enzymes superoxide dismutase (SOD), peroxidase (POD), catalase (CAT) and the malondialdehyde (MDA) content were measured. SOD activity was measured by the photochemical reduction of p-nitro blue tetrazolium chloride, and POD activity was measured by the guaiacol method (Donahue et al., 1997). CAT activity was determined by UltraViolet absorption spectrometry (Feierabend & Engel, 1986). The thiobarbituric acid coloration method was employed to determine malonaldehydic acid (MDA) content (Heath & Packer, 1968). Including three biological replicates.
2.4 Photosynthetic pigment content
The photosynthetic pigment content in tobacco leaves was determined by the direct extraction method (Lichtenthaler, 1987; Thayer & Björkman, 1990). After removing the veins, weigh 0.1 g (± 0.01 g), 80% acetone was added to a constant volume of 10 mL. For chlorophyll fluorescence parameter measurements, the supernatant was extracted after 72 h in the dark. Using a UltraViolet spectrophotometer (JINGHUA Instruments 752, Shanghai JINGHUA Technology Instruments Co., Ltd), absorbance values were measured at wavelengths of 445, 644, and 662 nm. Each sample included three biological replicates.
2.5 Chlorophyll fluorescence
Chlorophyll fluorescence parameters were measured from the top in expanded leaves after 30 min of dark adaptation using a chlorophyll fluorometer (Fluor Cam FC 800-C/1010). The maximum photochemical efficiency (Fv/Fm), initial fluorescence (Fo), and maximum fluorescence (Fm) of photosystem II were described using Tambussi et al.'s (2002) method. Each sample included three biological replicates.
2.6 Transcriptomics
Third tobacco leaves were inoculated with P. nicotianae and applied with oxathiapiprolin after inoculation with P. nicotianae, and a blank control was collected for total RNA extraction, with three replicates of each treatment, for a total of nine samples.
2.7 Library preparation for transcriptome sequencing
Total RNA was used as input material for the RNA sample preparations. Briefly, mRNA was purified from total RNA using poly-T oligo-attached magnetic beads. Fragmentation was performed using divalent cations under elevated temperature in First Strand Synthesis Reaction Buffer (5×). First-strand cDNA was synthesized using a random hexamer primer and M-MuLV Reverse Transcriptase (RNase H-). Second-strand cDNA synthesis was performed using DNA Polymerase I, and RNase H. Remaining overhangs were converted into blunt ends via exonuclease/polymerase activities. After adenylation of the 3′ ends of DNA fragments, an adaptor with a hairpin loop structure was ligated to prepare for hybridization. To select cDNA fragments of preferentially 370–420 bp in length, the library fragments were purified with the AMPure XP system (Beckman Coulter).
PCR was then performed with Phusion High-Fidelity DNA polymerase, Universal PCR primers, and the Index (X) Primer. At last, PCR products were purified (AMPure XP system), and library quality was assessed on the Agilent Bioanalyzer 2100 system.
2.8 Quality control
In this step, clean data (clean reads) were obtained by removing reads containing adapters, reads containing ploy-Ns, and low-quality reads from raw data. At the same time, Q20, Q30, and GC content of the clean data were calculated. All the downstream analyses were based on clean data with high quality.
2.9 Reads mapping to the reference genome
Reference genome and gene model annotation files were downloaded from NCBI directly. An index of the reference genome was built using Hisat2 v2.0.5 (USA), and paired-end clean reads were aligned to the reference genome using Hisat2 v2.0.5
2.10 Differential expression analysis
The DESeq2 software (1.20.0) was used to carry out two comparative combinations between the three samples tested and identify differentially expressed genes. DESeq2 provides statistical routines for determining differential expression in digital gene expression data using a model based on the negative binomial distribution. The resulting p-values were adjusted using Benjamini and Hochberg's approach for controlling the false discovery rate. Genes with an adjusted p-value ≤0.05 found by DESeq2 were assigned as differentially expressed. We used the clusterProfiler R package to test the statistical enrichment of differential expression genes in Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways and Gene Ontology (GO) pathways.
2.11 Metabolomics
Tissues (100 mg) were individually grounded with liquid nitrogen, and the homogenate was resuspended with prechilled 80% methanol by vortexing. The samples were incubated on ice for 5 min and then centrifuged at 15,000 g, 4°C for 20 min. Some supernatants were diluted to a final concentration containing 53% methanol by LC–MS grade water. The samples were transferred to a fresh Eppendorf tube and then centrifuged at 15000 g, 4°C for 20 min. Finally, the supernatant was injected into the LC–MS/MS system analysis (Want et al., 2013). The detection of the experimental samples using multiple reaction monitoring (MRM) was based on a NovoGene in-house database. The Q3 was used for metabolite quantification. The Q1, Q3, RT (retention time), DP (declustering potential), and CE (collision energy) were used for the metabolite identification. These metabolites were annotated using the KEGG database (http://www.genome.jp/kegg/), HMDB database (http://www.hmdb.ca/), and the Lipidmaps database (http://www.lipid maps.org/).
2.12 qRT-PCR
To validate the sequenced gene expression, tobacco leaves were used to extract RNA using the test kit (AG RNA AexPro reagent AG21101). The cDNA synthesis was performed using a kit (Evo M-MLV AG11728). Fluorescence quantification was performed using a real-time quantitative PCR instrument (ABI, StepOnePlus) and a kit (qPCR SYBR Green Master Mix): pre-denatured at 95°C for 3 min; 95°C for 5 s; annealed at 10 s and 75°C for 15 s. Forty cycles were performed with three biological replicates for each biological replicate. The primers used are shown in Table S3.
2.13 Statistical analysis
All experiments were repeated with at least three biological replicates, and all data are expressed as means and SDs. All presented data were statistically analyzed by ANOVA and Tukey test, and different letters indicate a significant difference (p < 0.05).
3 RESULTS
3.1 Tobacco phenotype shape
Oxathiapiprolin not only controls tobacco blight but also promotes tobacco growth and development. The application of oxathiapiprolin resulted in 91.5% protection against tobacco black shank. Compared to XP (P. nicotianae-infected tobacco), the effective leaf number of OP (tobacco treated with oxathiapiprolin after P. nicotianae infection) was 7.8, an increase of 22.3%; plant height was 22.4 cm, an increase of 48.8%; fresh weight was 24.7 g, an increase of 61.5%; maximum leaf length and width were 27.2 and 19.2 cm, an increase of 29.0% and 35.9%, respectively. Compared to X (Untreated tobacco), the effective leaf number of OP increased by 6.8%; plant height increased by 14.8%; fresh weight increased by 13.3%; maximum leaf length and width increased by 7.1% and 10.8%, respectively (Figure 1A–E). The application of oxathiapiprolin can effectively control tobacco black shank and increase the yield to a certain extent.
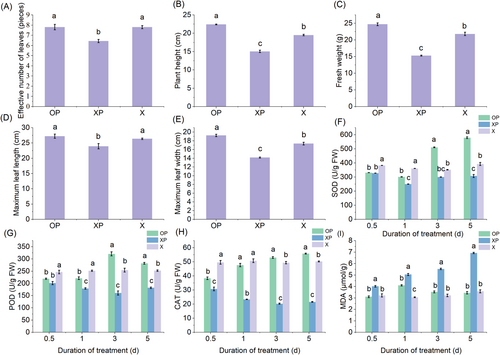
3.2 Antioxidant enzyme analysis
Under normal conditions, the reactive oxygen species (ROS) and antioxidant systems in plants are in a low-level dynamic equilibrium, but when induced by adversity, this equilibrium can be disrupted. In this study, the antioxidant enzymes SOD, POD, and CAT showed a decrease at first and then increased on the third day, while the MDA content increased at first and then decreased on the third day in OP. Compared to X, the SOD activity after 1 day was reduced by 19.8% in OP and 44.2% in XP; POD activity was reduced by 12.1% in untreated plants and 44.2% in XP; CAT activity was reduced by 6.5% in XP and 126.1% in OP; MDA content was increased by 34% in untreated tobacco and the MDA content increased by 34% compared to 43.3% for XP.
After 5 days, SOD, POD, and CAT activities increased by 13.4%, 11.8%, and 11.2%, respectively, and MDA content decreased by 3.8% in OP compared to X, while SOD/POD/CAT activities decreased by 32.2%, 27.8%, 57.1% and MDA content increased by 48.4% in X (Figure 1F–I). Under disease stress, the antioxidant enzyme activity of tobacco decreased for a short period in the initial stage but increased significantly after the application of the drug, indicating that oxathiapiprolin stimulated a more pronounced defense response and alleviated the stress caused by the disease.
3.3 Effect on photosynthesis
Figure 2A–C showed the effect of oxathiapiprolin application on the photosynthetic pigment content of tobacco to determine the effect of drug application on photosynthesis. OP showed an initial decrease and then increasing in photosynthetic pigment content. After 1 day, the photosynthetic pigment content reached its lowest value, with chlorophyll a, chlorophyll b, and carotene content reduced by 6.7%, 16.8%, and 32.5%, respectively, compared to X; after 5 days, the photosynthetic pigment content reached its highest value, with chlorophyll a, chlorophyll b, and carotene content increased by 12.2%, 25.2%, and 16.7%, respectively, compared to X.
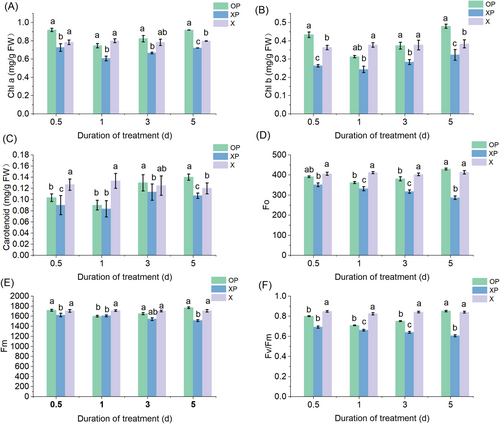
OP showed an initial decrease and then an increase in photosynthetic pigment content; after 1 day, chlorophyll a, chlorophyll b, and carotene contents of XP were reduced by 24.2%, 35.4%, and 37.5%, respectively, compared to X (Figure 2A–C); after 5 days, the photosynthetic pigment content reached its highest value, with chlorophyll a, chlorophyll b, and carotene content increased by 9.5%, 15.7%, and 11.1%, respectively, compared to X. These results showed that the photosynthetic pigment content of the tobacco was increased after the treatment with oxathiapiprolin, thereby affecting photosynthesis.
We carried out chlorophyll fluorescence measurements to investigate changes in the combined pigment content and to further investigate the effect on photosynthesis. The chlorophyll fluorescence kinetic parameters Fo, Fm, and Fv/Fm showed a decrease and then increasing in OP. After 1 day, Fo, Fm, and Fv/Fm reached their lowest value, with Fo, Fm, and Fv/Fm reduced by 11.9%, 6.5%, and 14.0%, respectively, compared to X. After 5 days, Fo increased by 3.8%, Fm by 3.6%, and Fv/Fm by 1.38% compared to X. Fo, Fm, and Fv/Fm of XP showed a decreasing trend with a 44.2% decrease in Fo, 12.9% decrease in Fm, and 38.9% decrease in Fv/Fm (Figure 2D–F).
The photosynthetic rate of tobacco infected with the P. nicotianae was significantly reduced, and oxathiapiprolin treatment effectively alleviated the damage caused by the P. nicotianae infection and increased the photosynthetic rate to some extent.
3.4 Genome sequencing, assembly, and annotation
To further investigate the physiological effects of oxathiapiprolin on tobacco under P. nicotianae infection, we performed a comparative transcriptome analysis by high-throughput digital gene expression sequencing. The raw data obtained were filtered, the sequencing error rate and GC content distribution checked, and clean reads were obtained for subsequent analysis; the overall sequencing error rate was 3%; all G + C content >42%; all Q20 > 97%; all Q30 > 92% (Table S1). Overall, the three sequenced groups expressed a total of 16,982 genes, and a total of 3849 differential genes (DEGs) were detected in different combinations of OP and XP: 1896 significantly upregulated and 1953 significantly down-regulated; 254 DEGs were detected for OP and X, 177 significantly upregulated and 77 significantly down-regulated; 4174 DEGs were detected for XP and X, 2260 significantly upregulated and 1914 significantly down-regulated (Figure 3).
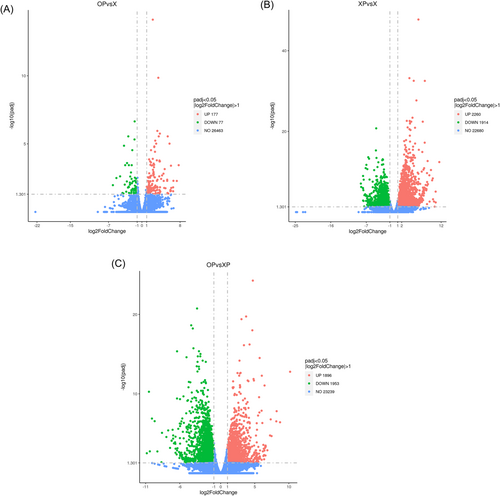
3.5 Transcriptome analysis
3.5.1 GO analysis
To further understand the effect of oxathiapiprolin on tobacco, we sequenced the DEG for GO functional enrichment in XP, OP, and X (Figure 4). For the DEG between XP and X, the redox-related and those molecular functional categories associated with iron-binding, oxidoreductase activity, carbohydrate phosphatase activity, calmodulin-binding, and ADP binding in the GO functional analysis were significantly enriched under P. nicotianae infection conditions (Figure 5B). For DEG of tobacco in response to black tibia infection, carbohydrate metabolic processes, tetrapyrrole binding, POD activity, oxidoreductase activity in the biological processes category, and iron ion binding in the molecular functions category were enriched in XP. In addition, the cellular composition is significantly enriched in the extracellular zone, the plasma extracellular body, and the cell periphery (Figure 5B).
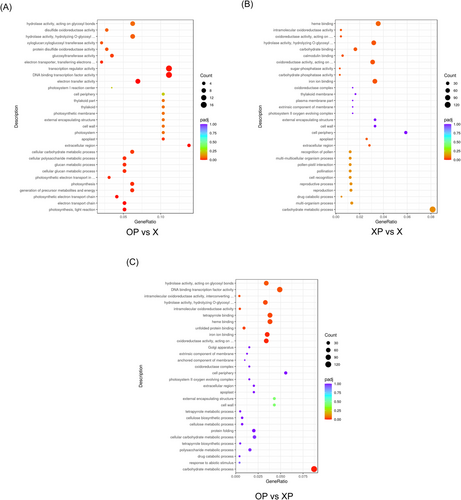
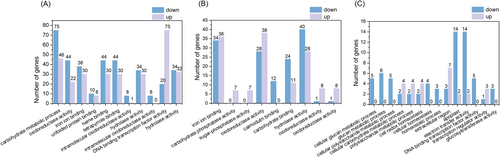
Between the treatment of OP and X, in biological processes, DEGs were significantly enriched in the pathways of cellular polysaccharide metabolism, carbohydrate metabolism, and redox homeostasis; the extraplasmic and extracellular regions in the cellular composition category are unique terms for OP, as well as the molecular function category of electron transporter and DNA transcription factor activity (Figure 5C).
Between OP and XP, DEGs in OP were significantly enriched in the biological processes of carbohydrate metabolism, response to abiotic stimuli, drug catabolic metabolism, and polysaccharide metabolism; in cell composition, cell wall, outer encapsulated structures and plastid extracellular bodies responded to DEGs in oxathiapiprolin treated tobacco; molecularly, DEGs were significantly enriched in oxidoreductase activity, iron ion binding, unfolded protein binding, heme binding, and tetrapyrrole binding, and hydrolase activity, which, were overexpressed in response to oxathiapiprolin treatment (Figure 5A). Under P. nicotianae infection, oxathiapiprolin acts as a stressor that affects tobacco metabolism, and oxathiapiprolin promotes growth by regulating carbohydrate synthesis, transportation, and distribution.
3.5.2 KEGG analysis
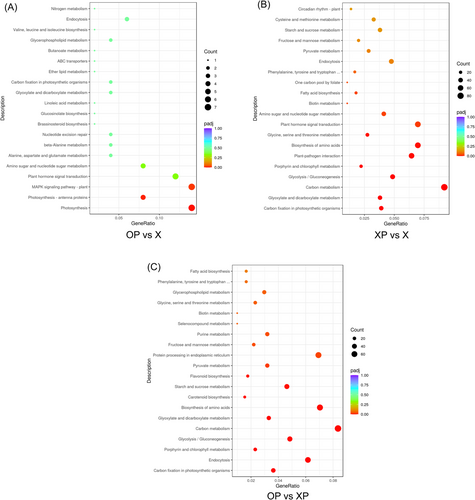
We performed a KEGG analysis (Figure 6). For DEGs between X, OP, and XP, pathways of plant-pathogen interactions, phytohormone signaling, endocytosis, and amino acid metabolism were enriched in both OP and XP (Figure 7D). Between OP and XP, DEGs were significantly enriched in the functions of carbon fixation, endocytosis, porphyrin and chlorophyll metabolism, glycolysis/gluconeogenesis, and carbon metabolism in photosynthetic organisms; in addition, flavonoid biosynthesis, pyruvate metabolism, and protein processing in the endoplasmic reticulum were highly expressed in oxathiapiprolin treated tobacco (Figure 7A).
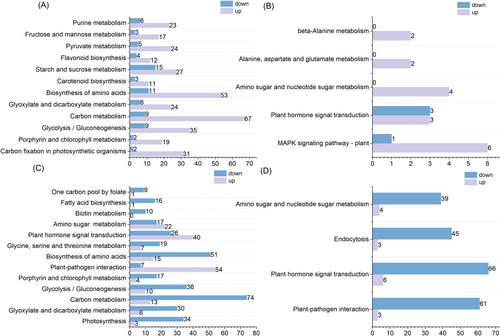
The genes encoding key enzymes, such as phosphoglycerate kinase, phosphoenolpyruvate carboxylase, and transketolase, which are highly expressed under oxathiapiprolin treatment, play an important role in regulating carbon metabolism products.
Various enzymatic activities play an important role in regulating carbon metabolites, and under P. nicotianae infection, oxathiapiprolin affected the carbon metabolism of tobacco through the regulation of enzymatic activities, thus affecting the growth and development of tobacco. In addition, among (heat shock transcription factor) HSFs, 18 were upregulated, seven were down-regulated, and this change was specific to OP. As a heat stress transcription factor, HSF can effectively mitigate heat stress in plants and has important functions in regulating plant disease resistance (Tejedor-Cano et al., 2010). In contrast, between XP and X, DEGs were highly expressed in photosynthetic carbon fixation, carbon metabolism, phytopathogenic interactions, phytohormone signaling, and endocytosis pathways, with upregulation of DEGs mainly assigned to phytopathogenic interactions and phytohormone signaling pathways (Figure 7C).
To further explore the physiological and biochemical effects of oxathiapiprolin treatment on tobacco, OP and X were analyzed; KEGG enrichment results showed that DEGs were significantly enriched in Mitogen-activatde protein kinase (MAPK) signaling pathways, amino and nucleotide sugar metabolism, alanine, aspartate and glutamate metabolism, and endocytosis (Figure 7B). Among these, the upregulation of DEGs was mainly assigned to the MAPK signaling pathway, and the MAPK cascade pathway mediates pathogen-induced plant disease defense responses through phosphorylation of WRKY transcription factors associated with disease resistance (Peng et al., 2018). The above results suggest that oxathiapiprolin can improve defense by affecting MAPK signaling. Qrt-PCR analysis also confirmed the physiological effects of oxathiapiprolin on tobacco (Figure S1).
3.6 Metabolite analysis
To systematically characterize the changes in tobacco leaves, we performed metabolite analyses for the three treatments. A total of 939 metabolites were detected, including amino acids, carbohydrates, lipids, nucleotides, secondary metabolites, and hormones (Figure 8A); KEGG analysis was carried out based on the coordinated exercise of the biological functions of the different metabolites (Figure S2). Between OP and XP, 125 differential metabolites were detected (Figure 8A), mainly including amino acids and their derivatives, sugars and their derivatives, nucleotides and their derivatives, lipids and their amines, and other metabolites; these metabolites had significant changes in carbon metabolism, gluconeogenesis, and other pathways (Figure 8D).
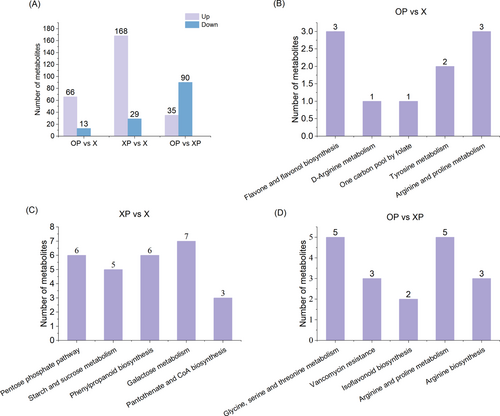
Metabolites such as benzenes, organic acids and amino acids accumulate to varying degrees in diseased plants. Infection of plants by pathogens rapidly triggers signal transduction processes at the plasma membrane and cytoplasm of plant cells (Devanna et al., 2021). The signal transduction involved in these reactions encompasses several critical pathways that can set up subsequent reactions at multiple levels of gene expression patterns (Bolouri Moghaddam et al., 2016).
A total of 79 differential metabolites were detected in the OP compared to X (Figure 8A); metabolites such as organic acids, amino acids, flavonoids, and their vitamins in the OP are differentially expressed in tobacco; these metabolites were significantly enriched in flavonol biosynthesis, phenylpropanoid biosynthesis and amino acid metabolic pathway (Figure 8B). 197 differential metabolites were detected between XP and X (Figure 8A) and were significantly enriched in metabolic pathways such as gluconeogenesis and amino acid synthesis (Figure 8C). XP was more enriched in differential metabolites than OP and X, suggesting that tobacco enhances disease resistance through its metabolic regulation.
3.7 Transcriptional metabolic co-analysis
A combination of transcriptomic and metabolomic data showed that genes encoding amino acid metabolism-related proteins were highly expressed in OP compared to X and XP, and metabolites, such as serine, citrulline, sarcosine and alanine accumulated; the mutual response of transcription and metabolism was reflected in the amino acid metabolic pathway (Figure 9). The unique enrichment of carbohydrate metabolic pathways in OP compared to X and XP suggested that oxathiapiprolin affects tobacco growth and development by influencing carbon metabolism in tobacco.
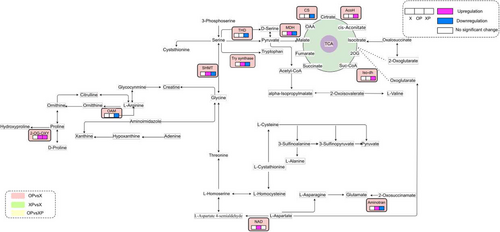
Besides, the genes encoding key enzymes such as isomerase, aldolase, and glycerate kinase were significantly upregulated, and intermediates (such as glycine and alanine for synthesizing related enzymes) were accumulated to varying degrees. Phytopathogen-related pathways and related metabolites are abundantly expressed in XP compared to OP and X. In summary, XP could enhance its resistance to pathogenic fungi by regulating metabolic reactions in the body to produce a range of metabolites in response to oomycete infection; OP not only has a suppressive effect on the P. nicotianae that improves tobacco resistance but also promotes its growth and development by influencing carbon metabolism, amino acid pathways, and secondary metabolites.
4 DISCUSSION
Studying how physiological and biochemical indicators change in crops under pesticide stress is important for promoting crop growth and material production. In this experiment, we investigated the physiological and biochemical effects of oxathiapiprolin on tobacco in the presence of P. nicotianae with the help of transcriptomics and metabolomics. We found that in addition to the effect of oxathiapiprolin on tobacco black shank, it significantly affects amino acid metabolism, carbon metabolism, and other related pathways in tobacco (Figure 9). At the same time, high expression of resistance genes increased the resistance of tobacco to P. nicotianae. Combined with morphophysiology, we identified mechanisms related to growth regulation and disease resistance of tobacco by oxathiapiprolin under P. nicotianae infection.
4.1 Promoting tobacco growth and development through carbon metabolism
In our experiment, the photosynthetic pigment content of tobacco was increased, and chlorophyll fluorescence parameters were all increased to varying degrees, reflecting the increased photosynthetic rate of tobacco treated with oxathiapiprolin. Photosynthesis is the most important basic physiological activity, providing the material and energy needed for plant growth and development, and is a major factor in determining yield and quality (Högy et al., 2009). Photosynthesis is one of the important carbon supply routes in plants.
Under oxathiapiprolin treatment, differentially expressed genes were highly enriched in the Calvin cycle, and genes encoding key enzymes such as isomerase, aldolase, and glycerate kinase were significantly upregulated. Cingulum heptulose-1,7-bisphosphatase controls carbon influx and regeneration during the Calvin cycle of plant photosynthesis and plays an important role in the basic pathway of carbon fixation (Olçer et al., 2001). Seuter et al. (2002) found that transferring the FBP/SBPase gene from Synechococcus sp. PCC7942 into tobacco significantly improved the efficiency of photosynthetic CO2 fixation and photosynthetic capacity of the transgenic tobacco and promoted the accumulation of sugars in plant and plant growth. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) plays a central role in the Calvin cycle, glycolysis, and other pathways, influencing the flow of carbon sources and oxidative phosphorylation in mitochondria, which in turn affects the development of reproductive organs that provide energy mainly through respiration (Rius et al., 2008). The differential expression of the genes encoding these key enzymes promotes carbon fixation by the photosynthetic pathway and, thus, carbohydrate accumulation.
Differential genes were also highly expressed in the glycolytic pathway in tobacco treated with oxathiapiprolin. The glycolytic pathway is an important process in sugar metabolism, and its main role in higher plants is the oxidation of sucrose to produce ATP, NADH, and pyruvate (Millar et al., 2011); this pathway is associated with plant responses to abiotic stresses such as temperature, drought, and salinity (Zhang et al., 2011; Zhang et al., 2011). Some key enzymes in the glycolytic pathway (phosphoglycerate kinase, phosphoenolpyruvate carboxylase, and phosphofructokinase) were upregulated. Oxathiapiprolin regulates the carbon metabolism of tobacco based on the control of tobacco black tibiasis and affects the growth and development of tobacco by influencing the Calvin cycle and glycolysis pathways.
4.2 Enhanced amino acid metabolism promotes tobacco growth and disease resistance
Only ammonia can be incorporated directly into organic matter among the nitrogen compounds available to plants. The carbon backbone of protein amino acids is derived from photosynthetically fixed carbon, glycolysis, and several intermediates in the tricarboxylic acid cycle (Miret & Munné-Bosch, 2014). In this experiment, the amino acid synthesis and metabolic pathways were highly expressed under induction in oxathiapiprolin-treated tobacco, with varying glycine, cysteine, and alanine content increases. As the carbon backbone for amino acid synthesis, intermediates in the photosynthetic carbon fixation and glycolytic pathways, such as erythrose 4 phosphate, glycerol 3 phosphate, and phosphoenolpyruvate, are highly expressed and provide precursors for amino acid synthesis. Glycine plays a catalytic role in plant tissue growth (Mangeon et al., 2009); it has been shown that the balance of cysteine levels in plants is important for plant resistance to external pathogens and plant immunity (Álvarez et al., 2012). Glutamate is an important amino acid involved in synthesizing many metabolic substances related to the enhancement of plant stress resistance, but it also plays an important role in response to abiotic stresses (Cañas et al., 2008; Kan et al., 2017). In this study, glutamate accumulated to large amounts in OP and promoted tobacco growth, development, and disease resistance. Oxathiapiprolin-induced amino acid involvement alters certain physiological metabolism in plants and regulates related gene expression and key enzyme activities to enhance the adaptive response of tobacco to stress. In addition to the effective control of P. nicotianae, oxathiapiprolin acts as an activator, inducing changes in the physiological aspects of tobacco and promoting its growth and development.
4.3 High expression of disease-resistant protein-related genes improves disease resistance in tobacco
Between the three treatments, HSF-related coding genes of OP were highly expressed compared to X and XP, 15 related coding genes were significantly upregulated, and the genes encoding heat shock proteins (HSP) HSP70 and HSP90 were upregulated at the same time. As transcription factors, HSFs activate stress-related genes in response to abiotic and biotic stresses (Andrási et al., 2021; Zhang et al., 2011; Zhang et al., 2011). The HSF gene plays a key role in biological stress response; Yu et al. (2019) found that most HSF genes in cassava respond to biotic stress.
HSP70 and HSP90 play roles in forming signaling molecules, signal transduction, and transcriptional activation of transcription factors. The HSF interacts with heat stress components and participates in the heat stress response of organisms by regulating the expression of the HSP gene (Akerfelt et al., 2010). Recent studies have found that HSF plays an important role in regulating plant disease resistance (Yura & Nakahigashi, 1999). Bechtold et al. (2013) reported that in transgenic Arabidopsis thaliana plants overexpressing At HSF A1b when inoculated with pathogenic bacteria for 4 days, their disease resistance was significantly stronger than that of wild-type plants.
Under no-stress conditions, HSP90 has a high expression level during the development and germination of Brassica napus seeds, indicating that HSP90 plays an important role in seed development and germination (Reddy et al., 1998). Tobacco HSP90 interacts with Rar1 (Required for Mla12 resistance 1) and TIR-NB-LRR (Toll/interleukin 1 receptor-nucleotide binding-leucine rich repeat) proteins to confer resistance to tobacco mosaic virus (TMV) in plants (Liu et al., 2004).
Besides, the antioxidant enzyme activity of OP increased to varying degrees in a stable state. Enzymes such as POD, CAT, and APX constitute the antioxidant defense system in plants, maintain the balance of ROS metabolism, and resist and repair the damage to plants caused by adversity (Singh et al., 2017). In this study, the decrease in MDA content indicates that the agent reduces the membrane lipid peroxidation in the cells, resulting in less cell damage. At later stages, the difference in antioxidant enzyme activity was not significant in tobacco treated with oxathiapiprolin compared to the blank control, and untreated tobacco was more affected by blight and had significantly lower antioxidant enzyme activity. Chemical treatment can affect the metabolism of ROS in the crop, and the plant has an antioxidant system that can scavenge the ROS after ROS damage. Oxathiapiprolin use promotes the activity of the SOD-dominated cytoprotective enzyme system to some extent to improve disease resistance. In addition, oxathiapiprolin also affected phytohormone synthesis and transduction pathways, and the expression levels of SA-responsive genes (SnRK, ABF) were upregulated. Plant hormones often form complex interaction networks to regulate various physiological activities of plants, and they play a key role in plant resistance to abiotic and biotic stresses (e.g., drought, cold, high temperature, high salt, and pathogens) (Ullah et al., 2018; Verma et al., 2016; Xie et al., 2019). Studies have shown that jasmonates can regulate various physiological processes, such as stomatal movement and root development in plants, mediating plant stress resistance (Wasternack & Hause, 2013). The contents of gibberellin and jasmonic acid increased under oxathiapiprolin treatment, indicating that oxathiapiprolin mediates plant disease resistance through its effect on hormones.
The treatment of oxathiapiprolin can effectively alleviate the damage of Phytophthora to tobacco and improve the stress resistance of tobacco. This transcriptional regulation of agent-induced regulation increases tobacco resistance to P. nicotianae under conditions of P. nicotianae infection. Our results indicate that oxathiapiprolin can be used as a chemical inducer to enhance the resistance of tobacco to Phytophthora while preventing and controlling tobacco black shank, which was beneficial to the growth and development of tobacco.
5 CONCLUSION
This study shows that the effect of oxathiapiprolin on tobacco black shank is not limited to the inhibitory effect on P. nicotianae but that oxathiapiprolin also stimulates the regulation of tobacco itself. In this study, in the case of P. nicotianae infection, oxathiapiprolin acted as an activator to increase the activity of antioxidant enzymes in tobacco, induced carbon metabolism and amino acid synthesis and metabolic pathways, increased the amino acid content of tobacco and promoted the growth and development of tobacco. At the same time, the resistance of tobacco to P. nicotianae was enhanced by regulating the synthesis of plant hormones and the transcriptional expression of resistance genes. This study provides a basis for the effects of oxathiapiprolin on tobacco through various mechanisms and gives a theoretical reference for improving tobacco disease resistance and efficiency.
AUTHOR CONTRIBUTIONS
Qin Jiao designed the experiments and wrote the article with contributions of all the authors, Jiahui Deng and Xiaoyan Zhao analyzed the data; Xiangfeng Yao conceived the project; Min Li and Zhouyang Pei performed the experiments; Xiangdong Li and Xingyin Jiang supervised and completed the writing; Fengwen Zhang wrote the article.
ACKNOWLEDGMENTS
The author thanks the Shandong Province Tobacco Company project (KN275), the Science and Technology Project of Hebei (202313000004149), and Anhui (202034180004192) for their support.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




