Transcriptome analysis of drought-responsive and drought-tolerant mechanisms in maize leaves under drought stress
Funding information: Major Science and Technology Project of Jilin Province, China, Grant/Award Number: 20210302003NC; National Key Research and Development Program of China, Grant/Award Number: 2016YFD0101203-4; Scientific and Technologic Development Program of Jilin Province, Grant/Award Number: 20200402028NC
Abstract
Maize is a major crop essential for food and feed, but its production is threatened by various biotic and abiotic stresses. Drought is one of the most common abiotic stresses, causing severe crop yield reduction. Although several studies have been devoted to selecting drought-tolerant maize lines and detecting the drought-responsive mechanism of maize, the transcriptomic differences between drought-tolerant and drought-susceptible maize lines are still largely unknown. In our study, RNA-seq was performed on leaves of the drought-tolerant line W9706 and the drought-susceptible line B73 after drought treatment. We identified 3147 differentially expressed genes (DEGs) between these two lines. The upregulated DEGs in W9706 were enriched in specific processes, including ABA signaling, wax biosynthesis, CHO metabolism, signal transduction and brassinosteroid biosynthesis-related processes, while the downregulated DEGs were enriched in specific processes, such as stomatal movement. Altogether, transcriptomic analysis suggests that the different drought resistances were correlated with the differential expression of genes, while the drought tolerance of W9706 is due to the more rapid response to stimulus, higher water retention capacity and stable cellular environment under water deficit conditions.
1 INTRODUCTION
Plants are exposed to various environmental stresses that include abiotic and biotic stress (Atkinson & Urwin, 2012; Chavez-Arias et al., 2021), such as temperature (extreme low and high air temperature), drought (water deficit), radiation (high light and UV), pests and diseases (Chavez-Arias et al., 2021; Pandey et al., 2017). In turn, these adverse environmental stresses lead to a defect on plant growth and yield production (Leng & Zhao, 2020). With global warming, the effect of drought on plants, especially crops, could be more aggravated (Bailey-Serres et al., 2019). Drought, as a severe environmental stress, can affect plant growth and development, decrease yield production and reduce production quality (Cao et al., 2021). Under increasing drought stress around the world, crop production has suffered loss equivalent to approximately $30 billion during the past decades (Gupta et al., 2020). Moreover, a high proportion of the population lives in regions that are suffering from drought stress.
Maize is not only a human grain and animal feed crop worldwide, but it is also becoming a major raw material for renewable energy production and many other industrial applications (Badr et al., 2020; Cairns & Prasanna, 2018; Chavez-Arias et al., 2021; Leng & Zhao, 2020). Its growth and development are seriously affected by various abiotic and biotic stresses, especially drought, which could cause defects in annual maize yield production of approximately 15% (Badr et al., 2020). As a sessile organism, maize has evolved an incredible capacity for its architecture and various mechanisms to adapt to and survive in drought environments. Of note, water deficit is the main factor that could lead to drought in maize. Maize has established various strategies in response to moisture changes in the air and soil to promote drought resistance (Gupta et al., 2020). A highly efficient root system can not only detect soil moisture rapidly and absorb a large amount of water immediately but also accelerate water uptake and expand the available region from the water limit soil (Ma et al., 2018; Potocka & Szymanowska-Pulka, 2018; Siddiqui et al., 2021; Zeng et al., 2019). Leaves are the crucial nutritive organ for the whole plant and play an essential role in gas and moisture exchange. Stomata function in regulating the response to drought; they consist of two guard cells surrounding a central pore (Bertolino et al., 2019). The closure/aperture of stomata, the primary index against drought, is adjusted by guard cell movement according to studies in maize. The stomata could be essential for moderating the gas exchange between leaves and the atmosphere (Bertolino et al., 2019; Gupta et al., 2020). Thus, maize could increase tolerance to drought stress by controlling stomatal closure to reduce water loss and enhance drought tolerance by reducing the density of stomata (Avramova et al., 2015; Ding et al., 2019; Zhang et al., 2020). In addition, the cuticular waxes of maize leaves could be a primary and essential barrier to prevent various environmental stresses, especially drought, by reducing water loss under conditions of low water content (Li, Du, et al., 2019; Xue et al., 2017). On the other hand, phytohormones in maize also play critical roles in growth and development and could be essential regulators in response to drought stress. Abscisic acid (ABA) can modulate stomatal closure to reduce water loss; hence increasing drought tolerance. ABA can also enhance drought resistance by its comprehensive crosstalk with cytokinin, auxin, brassinosteroids and other plant hormones (Cortleven et al., 2019; Hsu et al., 2021; Kurepa & Smalle, 2022; Wang et al., 2020). Recently, several studies have attempted to uncover the inherent mechanisms of maize in response to drought. The maize ZmRAFS gene family, which encodes raffinose synthase, is involved in raffinose synthesis and could accelerate the biosynthesis of raffinose and hydrolyses of galactinol, leading to enhanced drought tolerance in maize (Li, Zhang, et al., 2020). In addition, overexpression of the TPP-encoded trehalose-6-phosphate phosphatase in maize could increase the sucrose content in the ear to enhance drought tolerance and improve both kernel set and yield production under drought conditions (Nuccio et al., 2015).
To date, with the development of high-throughput sequencing methods, transcriptome (RNA-seq) and genome-wide association analyses (GWASs) have been increasingly performed to detect drought-associated genes to reveal the molecular mechanisms in response to drought stress. Transcriptome analysis of the maize reproductive and leaf meristem showed an extensive decrease in the transcript abundance of cell division- and cycle-related genes, while starch- and sucrose-related genes revealed large changes in expression levels in the ovary and leaves (Kakumanu et al., 2012). In addition, an RNA-seq analysis of the drought-tolerant line RIL70 and drought-susceptible line RIL93 revealed that cell wall biosynthesis and transmembrane transport process-related genes had high expression in drought-tolerant lines, which could be essential in the signal transduction processes of maize under drought conditions and increase drought tolerance in the drought-tolerant line RIL70 (Min et al., 2016). GWAS also provided a strong tool to mine the functional genes contributing to drought stress. ZmVPP1 and ZmNAC111 identified from the GWA study of 367 and 368 natural maize accessions, respectively, could improve maize drought tolerance and confer high-yield production under water deficit conditions (Mao et al., 2015; Wang et al., 2016). In addition, the combined analysis of the transcriptome and GWAS could also identify the drought-associated genes involved in root growth in response to drought stress (Guo, Li, et al., 2020).
In our study, we identified a drought-tolerant line (W9706) and a drought-susceptible line (B73) by evaluating physical indices under normal and drought conditions. To explore the mechanisms of drought resistance of W9706, we compared transcriptomic differences between W9706 and B73 and identified several biological processes that play essential roles in drought stress. These data suggest that W9706 could facilitate the increase in secondary metabolites such as wax and brassinosteroids, which could reduce water loss in leaves during drought stress. In addition, we also found that W9706 could control stomatal movement by responding to ABA.
2 MATERIALS AND METHODS
2.1 Plant materials
Two elite maize inbred lines showing opposite performance to drought stress were used in this study. The maize drought-resistant inbred line W9706 was obtained during our breeding program. The drought-susceptible inbred line was B73 with a high-quality reference genome.
2.2 Phenotyping of maize drought tolerance
Maize seeds were soaked in water at room temperature for 1 day under dark conditions. Then, three seeds were sown into a 1000 mL plastic beaker filled with soil and transferred to the growth chamber with the following settings until the three-leaf stage: temperature 26°C, photoperiod 14 /10 h (light/dark) and relative humidity 50 ± 5%. For the whole-plant drought treatment, water limitation was applied to the seedlings at the three-leaf stage, maintaining the soil water content at 40–50%.
2.3 Measurement of physiological indices
For the measurement of physiological indices, two-thirds of the third leaf from the base was collected, and major veins were removed at 0, 7, and 10 days after drought conditions had begun. The relative water content (RWC) was measured as previously described (Jones, 1978) with some modifications: the leaves were floated on distilled H2O for 12 h. The relative water content was calculated with the following formula: RWC (%) = [(FW − DW)/(TW − DW)] × 100; FW is the fresh weight; TW is the total saturation weight; DW is the dry weight.
The relative electrolytic leakage (REL) was measured as described by Liu et al. (2009) with some modifications: the leaves were boiled in water for 15 min. The relative electrolytic leakage was calculated with the following formula: REL (%) = R1/R2 × 100; R1 is the electrolytic leakage before boiling; R2 is the electrolytic leakage after boiling.
The hydrogen peroxide content (H2O2), malondialdehyde (MDA) concentration, free proline (PRO) and catalase (CAT) activities were determined by using a commercial hydrogen peroxide assay kit, MDA assay kit, proline assay kit and Catalase assay kit (Nanjing Jiancheng Bioengineering Institute), respectively, according to the manufacturer's instructions. These indices were measured by using the spectrophotometer.
2.4 RNA extraction and detection
For RNA extraction and RNA-seq, the leaves of the seedlings were immediately frozen in liquid nitrogen and stored at −80°C. Total RNA of the third leaf samples was isolated using TRIzol reagent and the UItrapure RNA kit (Cowin Biotech) following the manufacturer's protocols. The RNA was treated with DNase I (RNase Free) to remove the DNA (Cowin Biotech). The RNA was tested using gel electrophoresis to check its integrity. The concentration of the RNA was determined by the Implen NanoPhotometer P360.
2.5 RNA-seq data analysis
Transcriptome analysis was managed by Biomarker Technologies. Sequencing libraries were generated using the NEBNext UltraTM RNA Library Prep Kit for Illumina following the manufacturer's instructions. The library fragments were selected to be 200–250 bp in length. Then, library preparations were sequenced on an Illumina HiSeq 2500 platform, and paired-end reads were generated.
Clean data (clean reads) were obtained by removing reads containing adapters, reads containing poly-N sequences and low-quality reads from the raw data according to the instructions. Then, the clean reads were mapped to the Zea mays B73 reference genome V4 (http://plants.ensembl.org/index.html) with STAR, and the mismatch limitation was set to 1. The differentially expressed genes (DEGs) were identified using the edgeR package in R (https://www.r-project.org/) (Robinson et al., 2010). Genes with FDR ≤0.05 and |log2fold change| ≥ 1 were identified as DEGs. The heatmap of all DEGs was drawn by using the pheatmap package in R (https://www.r-project.org/). Gene ontology (GO) enrichment analysis for all DEGs was performed on the BMKCloud platform (www.biocloud.net). To identify significantly enriched pathways (FDR < 0.05), a Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis for all DEGs was performed using the clusterprofiler package in the R program.
2.6 Functional enrichment analysis for upregulated and downregulated DEGs
AgriGOV2.0 (http://systemsbiology.cau.edu.cn/agriGOv2/index.php) was used for GO enrichment analysis for upregulated and downregulated DEGs (Tian et al., 2017). The GO terms with FDR ≤ 0.05 were determined to be significantly enriched. To reduce redundancy, the child GO terms were retained, and the parent terms with hierarchical relationships were removed. Enrichment dot bubbles were plotted using the “ggbubble” code of the ggplot2 package in R. Functional category enrichment for DEGs involved in pathways was evaluated with MapMan (v3.6.0) functional annotation (Thimm et al., 2004).
2.7 qRT-PCR analysis
First-strand cDNA synthesis was performed using the UEIris II RT-PCR System for First-Strand cDNA Synthesis (US EVERBRIGHT). ZmUBI2 was used as an internal reference gene. The primers used for the qRT-PCR are shown in Table S1. Each qRT-PCR system (10 μL) contained 5 μL of 2× BimakeTM SYBR Green Master Mix, primers with 0.5 μM and appropriately 5× diluted cDNA. qRT-PCR was performed using the PCRmax Eco 48 real-time PCR system. The reaction conditions were as follows: step 1 was holding for 10 min at 95°C; step 2 was denaturing for 15 s at 95°C, annealing for 30 s at 55°C and extension for 30 s at 72°C for 40 cycles; and step 3 was held for 15 s at 95°C, 60 s at 60°C and 15 s at 95°C.
3 RESULTS
3.1 Differential drought responses of maize inbred lines W9706 and B73
Two maize inbred lines, the drought-tolerant line W9706 and the drought-susceptible line B73, were screened to study the drought-responsive mechanism of maize at the three-leaf stage. To detect the performance of W9706 and B73 under drought conditions, we collected morphological, physiological and biochemical indices (Figure 1A–C). Generally, the W9706 seedlings exhibited no significantly different performance on the plant architecture compared to B73 under normal water irrigation, while W9706 had a more advanced growth and development morphology compared to B73 after drought stress for 7 and 10 days (Figure 1A). In addition, W9706 still showed normal green and growth condition leaves, while the B73 exhibited yellow, dry and withered leaves (Figure 1B).
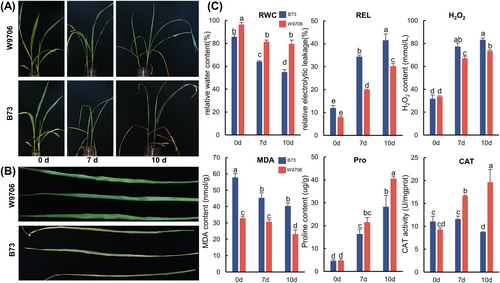
Under normal conditions (0 day drought treatment), these two lines showed no significant difference in REL, H2O2, proline content or CAT activity (Figure 1C). W9706 had a larger RWC than B73, and W9706 showed a lower MDA content than B73 (Figure 1C). Overall, the ROS content, osmotic species and antioxidant enzymes did not show any significant difference in the drought-tolerant line (W9706) and drought-susceptible line (B73) under normal conditions.
During drought stress conditions, all of the indices changed in both the tolerant and susceptible lines. Regardless of drought treatment for 7 or 10 days, B73 seedlings showed increasingly severe withering compared with W9706, which was reflected in the RWC. The RWC of both lines decreased, while B73 showed a more significant reduction than W9706 (Figure 1C). W9706 showed significantly lower REL, H2O2 content and MDA content than B73 (Figure 1C). The proline content in W9706 was significantly higher than that in B73. In addition, W9706 showed higher CAT activity than B73 (Figure 1C). Overall, these data showed that W9706 suffered less damage and had more stable cellular homeostasis under drought and showed higher drought resistance than B73.
3.2 RNA-seq analysis of W9706 and B73 under drought conditions
To compare the molecular basis and transcript differences involved in the drought response between the drought-tolerant and drought-susceptible lines, the leaf tissues of three-leaf stage W9706 and B73 after 7 days of drought treatment were collected and subjected to RNA-seq analysis. Though the 10 days treatment had more effect on the seedlings, seedlings were too withered to extract RNA. However, seedlings treated for 7 days still had a significant phenotype difference between W9706 and B73 and the leaves were suitable for RNA exaction. Notably, for each inbred line, three biological replicates were performed. The clean data of each sample reached 7.64 GB, and the percentage of Q30 bases was 88.41% or above. In total, approximately six million clean paired-end reads were obtained for each sample (Table S2). After trimming the adapters, low-quality sequences and ambiguous reads, approximately four million mapped paired-end reads were obtained, and approximately 70% of the purified reads from each sample could be perfectly aligned to the maize B73 reference genome (Table S2).
To assess the reliability of differentially expressed genes between the three replicates (from W9706 and B73), Pearson's correlation coefficient was used as the evaluation index of biological repeated correlation, and all coefficients exceeded 90%, indicating a high correlation between the three biological replicates from the two maize lines (Figure S1).
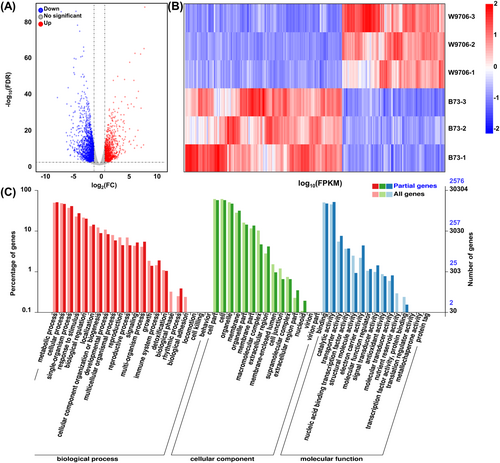
With a threshold of absolute value of logFC ≥ 1 and FDR <0.05, 3146 differentially expressed genes (DEGs) were identified in response to drought stress. Among them, 1244 DEGs were upregulated, and 1902 were downregulated (Figure 2A). Cluster analysis of the DEGs between control and drought-treated showed that DEGs were grouped into two clusters, and more DEGs were downregulated than upregulated (Figure 2B, Table S3). A total of 24 DEGs were selected to evaluate the reliability of the RNA-seq results. In agreement with our RNA-seq data, the real-time RT-PCR assay showed that drought stress strongly upregulated the expression of 12 candidate genes, while downregulating the expression of 12 candidate genes, showing the high correlation of the qRT-PCR and transcriptome results (Figure S2). These results further indicate that the sequencing data were reliable.
3.3 Different drought responses to metabolic processes between B73 and W9706
To determine the processes in which these DEGs could be involved in the drought response, we used MapMan software to reveal the difference in the canonical biosynthetic and metabolic pathways, signal transduction and other biological processes between W9706 and B73 (Figure S3). We found that most DEGs were involved in cell wall, lipid, starch and sucrose metabolism, secondary metabolism and light reactions, suggesting that these processes play essential roles in drought responses.
Previous studies on drought have revealed that a large number of carbohydrate metabolism-related genes exhibit significant expression level changes. Several genes involved in the sucrose and starch metabolism process, which encode sucrose phosphate synthase, sucrose synthase, starch synthases and amylases, showed significant difference in expression in W9706 compared to B73 (Figure S4A,B, Table S4). Two invertase genes exhibited decreased transcript abundance in W9706 compared to B73, while two sucrose synthase genes and one shrunken gene showed higher expression levels in W9706 than in B73, suggesting that the synthesis of sucrose would be reduced, and the degradation of sucrose would be induced (Figure S4A, Table S4). In addition, two starch synthase genes exhibited increased transcript abundance in W9706 compared to B73, while four amylase genes showed lower expression levels in W9706 than in B73, suggesting that the synthesis of sucrose would be induced, and the degradation of sucrose would be reduced (Figure S4B, Table S4).
Taken together, these data suggest that carbohydrate metabolism processes could have significant differences in maize after drought treatment. Additionally, the pathways related to cell walls, lipids, and secondary metabolite also showed enrichment of various DEGs, suggesting that these pathways could contribute to the response to drought stress and enhance drought tolerance.
3.4 Transcriptomic responses in drought-tolerant and drought-susceptible maize lines
In addition to the biological pathway analysis, we performed GO analysis on the DEGs by using the BMKCloud online tool to detect the essential function of these DEGs in the response to drought stress. The DEGs caused by drought treatment in W9706 and B73 were enriched for GO terms in the BP (biological process), CC (cellular component) and MF (molecular function) categories. For all of the DEGs, GO: 0008152 (metabolic process), GO: 0009987 (cellular process), GO: 0044699 (single organism process), GO: 0050896 (response to stimuli) and GO: 0065007 (biological regulation) were observed as the common and top five most significantly enriched GO terms in the BP category. Within the CC group, GO: 0044464 (cell part), GO: 0005623 (cell), GO: 0043226 (organelle), GO: 0016020 (membrane) and GO: 0044422 (organelle) were the most enriched CC terms. Within the MF group, GO: 005488 (binding), GO: 0003824 (catalytic activity) and GO: 0005218 (transporter activity) were most enriched MF terms (Figure 2C). These results indicate that the DEGs within these biological processes, cellular components and molecular functions could be essential regulators of the different drought responses between the two lines.
Moreover, to investigate the drought responses at the molecular level, we analyzed the BP category terms under drought treatment by using the online tool agriGOV2 (Table S5). To reduce redundancy, we kept child GO terms and removed the parent terms with hierarchical relationships in this study. A total of 87 BP GO terms (37 enriched by upregulated DEGs and 50 enriched by downregulated DEGs) were enriched by drought treatment in W9706 (Figure 3A,B, Table S5). For the GO terms enriched by the upregulated DEGs, the 37 BP terms could be classified into three groups. The first was related to biosynthetic and metabolic processes, including the “wax biosynthetic process,” “chitin catabolic process” and “brassinosteroid biosynthetic process.” The second was related to responses to stimulus, including “response to desiccation” and “response to abscisic acid.” The third was related to growth and development, including “cell tip growth” and “fruit development” (Figure 3A, Table S5). These results suggest that maize could enhance the secondary metabolism processes, which could protect plants from water loss, while accelerating the responses to external stimuli under drought conditions. In addition, the GO terms enriched by the downregulated DEGs showed that the drought-tolerant maize line W9706 could repress many biological processes compared with the susceptible maize line for defense against drought treatment. “Water transport” and “stomatal movement” both showed downregulation in W9706 compared with B73, indicating that the drought-tolerant line could decrease water loss by controlling stomatal closure and transpiration under drought treatment (Figure 3B, Table S5).

3.5 Transcriptomic responses to drought in different maize cultivars
To identify the universal and specific drought response mechanisms in different maize, we compared drought transcriptome results between two different maize cultivars. two public RNA-seq analyses were selected for comparison. Both had similar drought treatment than our study and sampled leaves of maize subjected to drought for 5 days (named 5D-seq hereafter) (Waititu et al., 2021) or 7 days (named 7D-seq hereafter) (Zhang et al., 2020).
Next, the upregulated DEGs of these two transcriptomes data and this study were used for GO analysis. 129 and 168 BP terms were enriched in the 5D-seq analysis and 7D-seq analysis, respectively, while 138 BP terms were enriched in this study (Figure S5). Venn diagram analysis showed a total of 48 shared BP terms in these three analyses (Figure S5A). The 48 BP terms could be classified into two groups. The first was related to biosynthetic and metabolic processes, including the “lipid biosynthetic process,” “carbohydrate metabolic process” and “glucan metabolic process.” The second was related to responses to external stimulus, including “response to water deprivation,” “response to abscisic acid” and “response to metal ion” (Figure S5B). These results suggested that the different maize cultivars could all enhance the expression level of genes related to the biosynthetic and metabolic processes, like carbohydrate and lipid, to increase drought tolerance. In addition, the terms related to response to the external stimulus, like hormones and ions, were also enriched in all three transcriptome analysis, indicating that maize could accelerate stress signal responses.
In addition, 46, 78 and 57 BP GO terms were unique in 5D-seq, 7D-seq and this analysis (Figure S5A). For the 57 unique GO terms in this study, these terms were divided into two parts. The first was related to the secondary metabolite biosynthesis process, while the second belonged to the growth and development process. These results were similar with the previous analysis in this study. The 46 unique GO terms in the 5D-seq were also related to the biosynthesis and metabolic process, which could be child or parent terms of the shared terms from the three analyses. The 78 unique GO terms in the 7D-seq were more complex and were related to secondary metabolite biosynthesis process, like the “coumarin biosynthetic process” (Table S6).
In total, all these results indicated that the biosynthesis and metabolism process as well as response to stimulus signal could be universal regulatory mechanism in maize under drought condition for enhancing drought tolerance. Moreover, the W9706 could specifically increase the content of secondary metabolites, especially wax to enhance drought tolerance. Though we have identified many commonly functional genes and drought-related terms in response to drought stress, we have not done the function analysis on these genes; this will be subject to another manuscript.
3.6 Identification of responses associated with drought in W9706 and B73
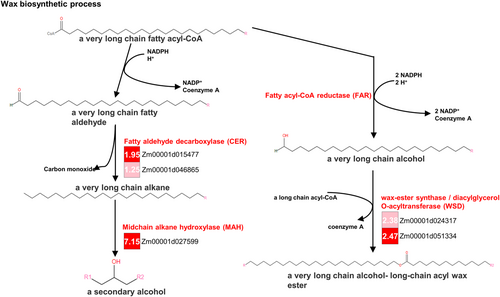
To further investigate the drought tolerance mechanism, we compared the expression patterns of genes under enriched terms in the drought-tolerant line W9706 and drought-susceptible line B73. First, the pathway related to the biosynthesis of wax was examined (Figure 4). The BP term “wax biosynthetic process” (GO: 0010025) included five DEGs in the agriGO database. These five DEGs, which products could be the key enzymes in the wax biosynthesis process, showed abundant expression in the drought-tolerant line W9706 compared to the drought-susceptible B73 (Figure 4, Table S4). Among them, CER, which encodes fatty aldehyde decarboxylase in maize, catalyzes the conversion of fatty aldehydes to alkanes, and one (Zm00001d046865) of two related genes were upregulated in the drought-tolerant line W9706 (Figure S6A, Table S4). In addition, 18 FAR genes (encoding fatty acyl-CoA reductase) were identified in maize, and five genes had altered expression. Three (Zm00001d024712, Zm0001d047859 and Zm0001d049950) out of five genes showed higher expression in W9706, while the other two genes (Zm00001d007714 and Zm00001d043909) showed higher expression in B73 (Figure S6B, Table S4). Moreover, two sets of genes (Glossy and KCS) related to wax biosynthesis were identified in maize. Six out of ten genes named Glossy showed abundant expression in W9706 compared with B73, while two genes showed higher expression in B73. Seven of 12 genes encoding ketoacyl-CoA synthase (KCS) showed higher expression in W9706 than in B73, while three genes were upregulated in B73 (Figure S6C,D, Table S4). These data suggest that the activated wax biosynthesis process in the drought-tolerant line under drought treatment was associated with drought tolerance in maize.
Second, the term “chitin catabolic process” (GO: 0006032) was significantly enriched with upregulated DEGs in the drought-tolerant line W9706 (Figure 5A, Table S4). All seven genes encoding chitinase, which can catalyze the conversion of chitin to chitodextrin, showed abundant expression in the drought-tolerant line W9706 (Figure 5A, Table S4). This suggests that more chitin metabolic processes showed drought induction in the tolerant lines than in the susceptible lines.
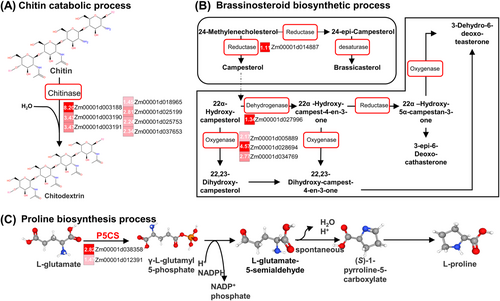
Third, the term “brassinosteroid biosynthetic process” (GO: 0006032) was enriched for the upregulated DEGs in W9706 but not from B73. Among the five upregulated DEGs under this term, one gene encoded reductase, three genes encoded oxygenase, and one gene encoded dehydrogenase; they were enriched for W9706 and showed lower expression in B73 (Figure 5B, Table S4). In addition, proline in plants could be an essential amino acid that plays a key role in maintaining osmosis homeostasis under drought stress. Therefore, we also examined the term “proline biosynthesis” to evaluate the genes under this term (Figure 5C, Table S4). We found that two genes (Zm00001d012391 and Zm00001d038358) encoding delta-pyrroline-5-carboxylate synthase, which is involved in the first step of proline biosynthesis and catalyzes glutamate to glutamyl-5-posphosphate, showed abundant expression in W9706 compared with B73 (Figure 5C). These results indicate that the drought-tolerant line W9706 could manufacture and maintain more proline than B73 under drought stress and promote tolerance to drought.
Finally, we also examined several terms related to the “responses to the stimulus,” such as the “response to desiccation,” “response to abscisic acid” and “response to alcohol.” Under the “response to desiccation” term, 11 upregulated DEGs were found in W9706, and they could encode dehydrin, BURP and late embryogenesis abundant (LEA) protein, which are involved in maintaining water and protecting maize from osmotic stress (Figure 6A, Table S4). Among them, 3, 4 and 4 genes out of 8, 7 and 10 DEHYDRIN, LEA and BURP genes identified in maize, respectively, showed higher expression in W9706 than in B73, indicating that enrichment of these genes in W9706 could increase the water retention capacity (Figure 6B–D, Table S4). In addition, the “response to abscisic acid” was also enriched by the upregulated DEGs, suggesting that W9706 could respond stronger and faster to ABA than B73.
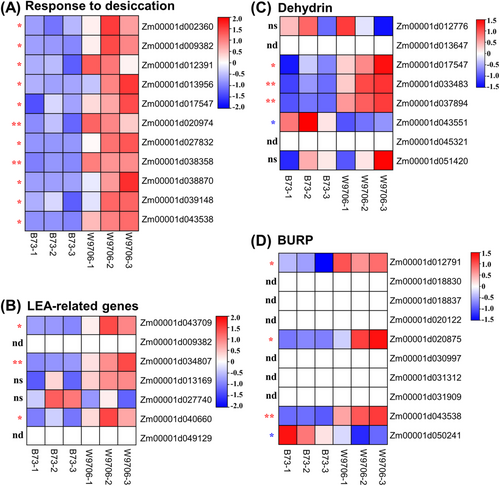
Taken together, these results suggest that the enrichment of biosynthesis- and metabolism-related processes, secondary metabolite-related processes and responses to external stimuli were associated with drought tolerance and play an essential role in maintaining cellular osmosis homeostasis and structure integrity as well as sustaining essential life activity and response signal transduction rapidly under drought stress.
3.7 Identification and analysis of transcription factors involved in drought stress
Transcription factors (TFs) are key regulators in plants that play an essential role in sensing and regulating plant responses to drought stress. In our study, various TF families were identified, including AP2/EREB, ARR, bHLH, ERF, HSF, MYB, NAC and WRKY (Table S7). Among these TFs, the number of bHLH DEGs was the highest under drought stress, reaching 22 DEGs, while the GRAS TF family had only one DEG. These TFs are not only involved in multiple phytohormone signal transduction in response to external stimuli, such as drought, but could also be an adjusted factor in the crosstalk of different plant hormones. Taken together, the different expression patterns of various TF families indicate that maize had a complex and enormous regulatory network in response to drought stress, and the various TFs could have performed key functions in drought stress.
3.8 KEGG pathway enrichment and annotation analysis of DEGs under drought stress
To further investigate the biological process pathways involved in drought stress, we analyzed the functional fate of these identified drought-responsive DEGs by mapping them to the KEGG online database. The DEGs were enriched in 14 KEGG pathways with a Q-value <0.05 (Figure S7). We focused on the biosynthesis and metabolism pathways that account for most pathways through KEGG annotation, such as carotenoid biosynthesis (zma00906) and starch and sucrose metabolism (zma00500). In the metabolism pathways, carbon metabolism (zma01200) had the largest number of annotated DEGs, with a total of 50 DEGs, while starch and sucrose metabolism, pyruvate metabolism, glycerophospholipid metabolism, alanine, aspartate and glutamate metabolism, alpha-linolenic acid metabolism and butanoate metabolism had 32, 21, 21, 16, 13 and 9 DEGs, respectively (Table S8). Among the biosynthesis pathways, biosynthesis of amino acids (zma01230) had the largest number of enriched DEGs, with a total of 40 DEGs, while carotenoid biosynthesis (zma00906), diterpenoid biosynthesis (zma00904) and benzoxazinoid biosynthesis (zma00402) had 13, 8 and 6 DEGs, respectively. For the other three pathways, protein processing in the endoplasmic reticulum, glycolysis/gluconeogenesis and carbon fixation in photosynthetic organisms had 40, 29 and 24 enriched DEGs, respectively.
3.9 Transcriptomic responses to drought in maize, rice and Arabidopsis
To further analyze the drought regulatory mechanism in different plants, we compared transcriptome data in maize with those in Arabidopsis (Clauw et al., 2015) and rice (Tarun et al., 2020) leaves.
The upregulated DEGs in drought-treated Arabidopsis, rice and maize compared to control were used for GO analysis. 133, 73 and 138 BP terms were enriched in Arabidopsis, rice and maize, respectively (Figure 7A). Venn diagram analysis revealed that there were 11 identical BP terms enriched in all three species, while 76, 45 and 83 unique BP terms were specific in Arabidopsis, rice and maize (Figure 7A). The 11 shared BP terms could be classified into two parts. The first refers to the responses to the stress signal process, like the “response to water” and “response to osmotic stress.” The other one is related to carbohydrate metabolism process, like the “cellular carbohydrate metabolic process” and “polysaccharide catabolic process” (Figure 7B). In addition, more similar BP terms were enriched in maize and Arabidopsis than in rice. There were 48 BP terms shared by the maize and Arabidopsis, which referred to stimulus responses, cell wall modification and metabolism process (Table S9).
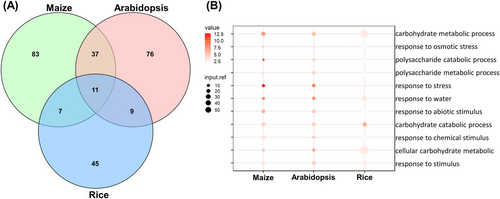
Moreover, most BP terms were unique in one species. The secondary metabolite-related processes were specifically enriched in maize, like the “wax biosynthesis process,” “chitin catabolic process” and “brassinosteroid biosynthesis process” (Table S9). The signal transduction-related processes were uniquely enriched in Arabidopsis, like the “regulation of signal transduction” and “cation transmembrane transport,” while the transcription regulation-related process was enriched in rice, like the “regulation of transcription” and “regulation of RNA metabolic process” (Table S9).
Therefore, all results indicated that the stress response and carbohydrate metabolism-related processes could be a universal response mechanism to drought in Arabidopsis, rice and maize. In addition, different species also had specific regulatory mechanisms to adapt to drought stress.
3.10 Proposed molecular model of maize seedlings for drought stress tolerance
Based on our main findings of the significant differential expression of drought-responsive genes and their associated pathways/networks, in combination with the related relevant published literature in this study, we proposed a molecular model for drought stress tolerance in maize seedlings (Figure 8). Drought stress causes injury to maize, leading to curly and dried leaves. Moreover, the physiological and biochemical indices showed significant differences after drought stress in the drought-tolerant line compared with the drought-susceptible line. Next, the drought stress signal was transmitted through the phytohormone and ROS components to the cellular level. The transcription factors accept these signals and induce the expression of downstream drought-responsive genes. These genes participate in several key processes, such as secondary metabolism and carbohydrate metabolism, which play key roles in keeping the moisture content in plants, maintaining cellular homeostasis and the reaction environment. As a feedback, these increased processes would enhance drought resistance under drought stress.
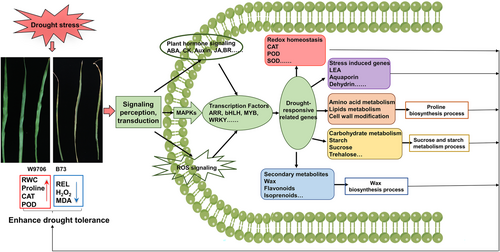
4 DISCUSSION
As we know, drought stress is an environmental factor limiting plant growth and development, and drought stress tolerance is a complex regulatory process. Plants have evolved various mechanisms at the physiological, biochemical, cellular and molecular levels for adaptation and response to drought conditions. However, several gaps in knowledge remain on the molecular mechanism for drought tolerance. Therefore, in our current report, we performed RNA-seq analysis on the drought-tolerant line W9706 and drought-susceptible B73 to identify the essential regulatory-associated genes and investigate the elusive and complex molecular mechanisms underpinning drought tolerance in maize. Overall, our findings support new insights into drought stress response mechanisms, which could provide a molecular basis for designing new drought-tolerant maize lines.
4.1 Differences in physiological and biochemical responses between inbred lines W9706 and B73 under drought stress
Maize is a commercial crop whose production is limited by water deficit. To adapt to water deficit conditions, maize undergoes a series of physiological and biochemical changes under drought stress. Furthermore, the drought-tolerant line and drought-susceptible line show different performances under drought stress probably due to the different physiological responses. Here, in our present study, significant differences in the physiological and biochemical indices were found between the maize inbred lines W9706 and B73. Compared with B73, W9706 exhibited a higher drought tolerance under drought stress.
RWC, which represents the water retention capacity in leaves, is regarded as one key factor for distinguishing the degree of drought tolerance in plants (Martinez-Vilalta et al., 2019; Zhang et al., 2020). Plants with higher RWC could maintain relatively steady cellular water content, provide an intact reaction environment and decrease the cell oxidative damage under drought stress.
REL, a measurement of the ionic stream in cells, is used to evaluate the membrane integrality and capacity for drought tolerance. In addition, MDA, the product from the plasma membrane attacked by oxidants, is used to evaluate the degree of lipid peroxidation. Reactive oxygen species (ROS) are a normal product in plant cells, but these compounds, such as superoxide radicals (˙O2), hydroxyl radicals (˙OH), and hydrogen peroxide (H2O2), which can cause oxidative damage to the cell membrane, accumulate rapidly under drought conditions (Anjum et al., 2017; Pan et al., 2020). These three indices all represent the degree of cell structure integrity under drought stress as well as the capacity of normal reactions in cells. In the present study, the drought-tolerant line W9706 had lower REL, MDA and H2O2 content compared to the drought-susceptible line B73, suggesting that W9706 had a more stable cellular environment than B73.
Proline, as a basic amino acid, is a common substance that can adjust the osmotic equilibrium under drought stress. It can accumulate under drought stress to increase drought tolerance (Kumar et al., 2021; Ozturk et al., 2021). Catalase (CAT), which is involved in H2O2 elimination, are key enzyme for protecting maize from oxidative damage (Blokhina et al., 2003). Taken together, higher RWC, a more stable cellular environment, higher antioxidant activities and a lower MDA content contributed to the higher drought tolerance of W9706 compared to B73.
4.2 TF-related genes are an essential component for maize drought responses
Maize has a complex regulatory network in response to drought stress. TFs play a critical role in regulating various abiotic stresses, such as temperature, drought and high-light, which could be involved in promoting and inhibiting the expression of downstream functional genes. In the present study, a large number of TF-related genes were identified, including AP2/EREBP, ARR, bHLH, bZIP, ERF, HSF, MYB, NAC and WRKY, and they could be involved in various physiological processes, such as stomatal movement and hormone signal transduction. The functions of TFs in tolerance toward various abiotic stresses in crops have been well analyzed. Previously, several transcriptome studies on maize drought stress responses all identified various TF gene families, including AP2/EREBP. Overexpression of OsERF115/AP2EREBP110 could enhance rice drought stress and provide a molecular basis for the development of new lines (Park et al., 2021). Arabidopsis response regulator (ARR) transcription factors were identified as contributing to the drought response, and the B-type ARR1/10/12 could negatively regulate Arabidopsis responses to drought stress (Nguyen et al., 2016). In the present study, the number of bHLH transcription factor families was greater than that of other TF families. ZmPTG1, encoding a basic helix-loop-helix (bHLH) transcription factor, was found to play a positive role in root development and drought tolerance in maize (Li, Liu, et al., 2019). Moreover, ZmbZIP4 (basic leucine zipper) could also enhance drought tolerance by regulating ABA synthesis and root morphogenesis in maize (Ma et al., 2018). Additionally, the NAC, WRKY and MYB TF families also play an essential role in the response to drought stress, and overexpression of ZmNAC111, ZmWRKY106 and ZmMYB-CC10 conferred drought tolerance in maize (Mao et al., 2015; Wang et al., 2018; Zhang et al., 2022).
4.3 Enhanced wax biosynthesis is essential for plants to tolerate drought stress
Cuticular wax, a compound comprising long-chain fatty acids and other aldehydes, alkanes and ketones, was the first protective part of the leaves of the plants. As wax can contribute to decreasing water loss and controlling gas exchange, it could play a key role in protecting plants from biotic and abiotic stresses such as drought. In a previous study, several genes involved in wax biosynthesis were identified from various species, such as Arabidopsis, maize, rice, wheat and tomato, and they are key genes in drought tolerance (Lee & Suh, 2015; Rahman et al., 2021; Seo & Park, 2011). Here, the “wax biosynthesis process” was enriched by the upregulated DEGs. Additionally, we evaluated the expression pattern of the other related genes in the same gene families of these DEGs in these two lines. Most genes in CER and FAR gene families identified in maize were specifically enriched in the W9706. FAR5 was found to contribute to wax biosynthesis and be induced by drought treatment in wheat (Wang et al., 2015). Additionally, the other two wax biosynthesis-related gene families, glossy and KCS, had higher expression in W9706 than in B73. Among them, Zmglossy6 could be involved in wax deposition and contribute to enhancing drought tolerance in maize (Li, Du, et al., 2019). Moreover, the ectopic expression of CsKCS6 could enhance drought tolerance in Arabidopsis (Guo, Wu, et al., 2020). In addition, the wax biosynthesis-related processes were uniquely enriched in W9706 but not in B73, suggesting that W9706 could accelerate wax biosynthesis and accumulate more epidermal wax than B73, which contributed to increased drought resistance. Together, wax biosynthesis-related genes could be induced by drought stress as more genes related to this process were enriched in W9706.
4.4 Upregulated DEGs related to the “response to desiccation” in W9706 under drought stress
Maize is susceptible to environmental water change. The “response to desiccation” process enriched in W9706 suggested that this genotype could better sense the stress signal than B73.
The genes involved in “response to desiccation” are five late embryogenesis abundant (LEA) genes, two Delta-1-pyrroline-5-carboxylate synthases, one BURP gene, one aquaporin gene and one dehydrin gene; all showing higher expression in W9706. These groups of genes were induced by drought stress, and they play essential roles in maintaining water retention.
From previous studies, we know that overexpression of the drought-induced LEA genes could enhance drought tolerance under water deficit conditions (Liang et al., 2019; Olvera-Carrillo et al., 2010). Moreover, DEHYDRINS, which belong to the group II LEA gene family, are induced by various environmental stresses, such as temperature and drought (Sun et al., 2021). This group of proteins has been reported to play an important role in enhancing drought stress. Overexpression of CaDHN3 could increase the tolerance to drought in pepper (Meng et al., 2021). Concerning aquaporine, the overexpression of aquaporin gene ZmPIP1;1 can confer drought and salt tolerance in Arabidopsis (Zhou et al., 2018).
In addition, the phytohormone abscisic acid (ABA) is an essential regulator for plants in response to various abiotic and biotic stresses, such as drought, and could also play a key role in plant growth and development. Stomata are involved in controlling water and gas exchange with the environment. Under drought stress, the stomatal aperture can be regulated rapidly by sensing ABA to decrease water loss and enhance drought tolerance (Agurla et al., 2018; Li, Li, et al., 2020; Lim et al., 2015; Raghavendra et al., 2010). In the present study, the term “response to abscisic acid” was enriched among the DEGs, indicating that the drought-tolerant line W9706 responded faster or more intensively to drought stress than the drought-susceptible line.
4.5 Carbohydrate metabolism is important for drought resistance
Carbohydrate metabolism is a key metabolic process that can provide essential saccharides and energy for maintaining cellular activity. The change in the expression pattern in this metabolic pathway could be induced by drought stress and contribute to the adaptation to water deficit conditions. Moreover, the carbohydrate metabolism-related processes were all enriched in the Arabidopsis, rice and maize, suggesting that it could be a universal regulatory mechanism for enhancing drought tolerance. In the present study, we identified carbohydrate metabolism-related terms such as “oligosaccharide metabolic process” enriched by upregulated DEGs. We also analyzed the expression of several saccharide key enzyme-related genes using MapMan software. Among these genes, several genes involved in starch synthesis and trehalose synthesis were upregulated under drought stress, such as sucrose synthase, trehalose-6-phosphate synthase and phosphatase, which could enhance drought tolerance. Drought stress could promote starch synthesis-related gene expression, which is involved in sugar accumulation. In turn, sugars can maintain osmotic homeostasis and provide the energy required for cellular activity under water-deficit conditions. Additionally, trehalose-6-phosphate synthase and phosphatase involved in trehalose synthesis play essential roles in drought response.
5 CONCLUSION
In the present study, we compared the drought-tolerant line W9706 and drought-sensitive line B73 after 10 days of drought treatment at physiological, biochemical and transcriptomic levels. Only analyzing the three levels can explain the drought-tolerant mechanisms. W9706 had better biochemichal and physiological indexes compared to B73: lower REL, higher RWC and higher CAT; this suggests that W9706 has an enhanced drought tolerance. By using transcriptome analysis, we focus on the different changes under drought treatment in two different lines, and the results revealed that the drought tolerance in maize is associated with the regulated expression of several essential genes, such as the ones related to wax biosynthesis, brassinosteroid biosynthetic process, chitin catabolic process, phytohormone signaling transduction and transcription factors. Our findings elucidate potential maize drought-tolerant mechanisms at seedling stage as well as provide a molecular basis for drought-tolerant maize breeding.
AUTHOR CONTRIBUTIONS
Yaping Yuan, Yuan Jiang, and SS planned and designed the research. Yuan Jiang, Haixiao Dong, Hao Chen, Shipeng Li, Xiaohui Shan, He Li and Hongkui Liu performed the experiments, analyzed the data and performed the field work. Yuan Jiang, Haixiao Dong, SS, and Yaping Yuan wrote the manuscript. All authors contributed to the article and approved the submitted version.
ACKNOWLEDGMENTS
We would like to thank the American Journal Experts for performing the English language editing of the manuscript.
FUNDING INFORMATION
This work was funded by the Major Science and Technology Project of Jilin Province, China (20210302003NC), the National Key Research and Development Program of China (2016YFD0101203-4), and the Scientific and Technologic Development Program of Jilin Province (20200402028NC).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
All data and supplementary materials for supporting the findings of this analysis are available from the corresponding author upon the reasonable request. RNA-seq data are available at Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/) under the accession numbers GSE223667.




