Dew absorption by leaf trichomes in Caragana korshinskii: An alternative water acquisition strategy for withstanding drought in arid environments
Edited by: P. Ahmad
Funding information: National Natural Science Foundation of China 31670404 31971406 31422011; Second Tibetan Plateau Scientific Expedition and Research Program (STEP) 2019QZKK0301; Feitian Project, Grant/Award Number: 860059
Abstract
Investigating plant morphological traits can provide insights into plant drought tolerance. To date, many papers have focused on plant hydraulic responses to drought during dehydration, but atmospheric water absorption by trichomes to mitigate drought stress by influencing leaf hydraulics in plant species that inhabit arid environments has been largely ignored. The experiment in this study was designed to assess how dew absorbed by leaf trichomes helps Caragana korshinskii withstand drought. The results showed that under a drought stress and dew (DS & D) treatment, C. korshinskii displayed a strong capacity to absorb dew with trichomes; exhibited slow decreases in leaf water potential (Ψleaf), leaf hydraulic conductivity (Kleaf), and gas exchange; experienced 50% Kleaf and gas exchange losses at lower relative soil water content levels than plants treated with drought stress and no dew (DS & ND); and experienced 50% Kleaf loss (Kleaf P50) at similar Ψleaf levels as DS & ND plants. Its congener C. sinica, which does not have leaf trichomes, displayed little ability to absorb dew under drought stress and did not show any remarkable improvement in the above parameters under the DS & D treatment. Our results indicated that leaf trichomes are important epidermal dew-uptake structures that assist in partially sustaining the leaf hydraulic assimilation system, mitigate the adverse effects of drought stress and contribute to the distribution of C. korshinskii in arid environments.
1 INTRODUCTION
Plants usually face acute desiccation in arid environments that are characterized by few rainfall events and massive circadian fluctuations in temperature (Brodribb et al., 2020; Choat et al., 2012; Schreel & Steppe, 2019; Yao et al., 2020, 2021). Therefore, forms of occult precipitation, such as dewfall, which naturally condenses on leaf surfaces in the form of atmospheric moisture at dawn and occurs frequently during the growing season (Agam & Berliner, 2006; Hao et al., 2012; Schreel & Steppe, 2019), are viable water resources for plant survival in these harsh environments (Jacobs et al., 1999; Schreel & Steppe, 2019; Zhang et al., 2015). Accordingly, plants have evolved unique structures to retain and absorb dew (Berry et al., 2019; Malik et al., 2015), such as the water-collecting leaf hairs (awns) of Syntrichia caninervis (Pan et al., 2016), the spines of the cactus Opuntia microdasys (Ju et al., 2012) and the leaf trichomes of Combretum leprosum (Pina et al., 2016). These specific tissues are regarded as morphological structures adapted to absorb dew that might decrease the probability of the leaf water potential (Ψleaf) reaching the lower threshold value and contribute to plant survival in arid environments (Eller et al., 2016; Kim et al., 2017; Schreel et al., 2020). Therefore, illustrating the effects of foliar dew uptake by trichomes on leaf hydraulic conductivity and gas exchange would provide insight into plant drought resistance.
It has been shown that under drought conditions, leaf hydraulic conductance (Kleaf) is lost either from xylem tissue cavitation and embolus formation (embolism) (Brodribb et al., 2014, 2016) and/or from tissues outside of the xylem (Scoffoni et al., 2017; Yao et al., 2021). A decrease in Kleaf results in a decrease of photosynthetic rate and ultimately plant growth and survival, and the Ψleaf at 50% Kleaf loss (Kleaf P50) is regarded as an essential indicator of species resistance to drought (Blackman et al., 2014; Scoffoni & Sack, 2017). Indeed, the Kleaf P50 has been shown to be closely related to the mean annual precipitation (MAP), with species growing in the arid environments usually exhibiting stronger drought resistance (more negative values of Kleaf P50) than species growing in humid environments (Blackman et al., 2012, 2014; Nardini & Luglio, 2014; Yao et al., 2021). Moreover, atmospheric moisture absorption from aerial organs may alleviate drought stress. Indeed, dew absorption by leaf trichomes is widely observed in species growing in arid environments (Berry et al., 2019; Limm et al., 2009; Schreel & Steppe, 2019). Thus, foliar dew absorption may prevent a rapid decrease in Ψleaf, Kleaf, and gas exchange and contribute to plant drought resistance by inducing a lower relative soil water content (RSWC) at 50% loss of leaf hydraulic conductance (Kleaf RSWC50) during dehydration without changing the Kleaf P50.
The genus Caragana comprises deciduous perennial shrubs or small trees that are widely distributed across Eastern Europe and temperate Asia (Fang et al., 2014; Yao et al., 2021). They have important ecological and economic value, including playing a key role in vegetation succession from shifting dunes to sandy grasslands, helping to restore degraded land by fixing atmospheric nitrogen, forming shelterbelts for crops and pastures, serving as supplemental livestock forage (leaves and flowers), providing fuel (re-sprouted shoots) for local farmers and aiding in preventing desertification and soil erosion (Fang et al., 2008, 2013). C. korshinskii, one of the most important drought-resistant species in this genus, is widely distributed in desert areas where the MAP is less than 200 mm and has long, dense trichomes on both leaf sides. Its congener, C. sinica, which has no leaf trichomes, is distributed in humid environments, where the MAP is more than 1400 mm (Fang et al., 2011, 2017). In this study, both species were studied to gain insight into the role of leaf trichomes in absorbing dew and ameliorating water relations, Kleaf and gas exchange. We hypothesized that the species that grow in arid environments (C. korshinskii) would use leaf trichomes to absorb dew efficiently to prevent a rapid decrease in Ψleaf, Kleaf, and gas exchange, which would lower the soil water content level at which 50% Kleaf (Kleaf RSWC50), photosynthesis (A RSWC50), and stomatal conductance (gs RSWC50) loss occurred in response to dehydration. We also hypothesized that a closely related species that grows in humid environments (C. sinica) would have higher values of Kleaf RSWC50, A RSWC50, and gs RSWC50.
2 MATERIALS AND METHODS
2.1 Experimental site and plant materials
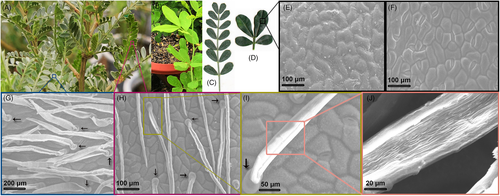
The experiment was conducted at Lanzhou University, Lanzhou, Gansu Province, China (Yuzhong campus, 35°51′N, 104°07′S, altitude 1620 m) in naturally lit glasshouses with average environmental conditions (temperature, 23 ± 2°C; humidity, 40–60%). We selected two Caragana species that occur in regions with dramatically different MAP. C. korshinskii grows widely in arid environments. It has pinnate compound leaves with 12–16 leaflets (Figure 1), and its leaves are covered with dense trichomes on both the adaxial (upper) and abaxial (lower) surfaces. C. sinica grows in humid environments and has four leaflets and no trichomes (Figure 1).
In June 2017, seeds of C. korshinskii were collected from wild populations at physiological maturity when the pods changed color from green to brown. After collection, ripe pods were spread out in the laboratory at 20°C until they opened, and the seeds were removed. The seeds were stored at 4°C until the start of the experiment. In February 2018, seeds were placed in Petri dishes lined with wet filter paper for germination at 20°C. After germination, when the seedling roots were approximately 0.3 mm long, three seedlings were transferred into each of 100 plastic pots for each species, that were 270 mm high and 170 mm in diameter and contained 3.6 kg of a 1:1 (v:v) mixture of sieved peat soil and Perlite. C. sinica does not form seeds, so C. sinica tissue was cultured from more than 50 individuals as described by Yao et al. (2021). Then, the tissue-cultured seedlings of C. sinica were moved to pots (one individual per pot, 100 pots). All pots received ample amount of water and nutrient supply. Eighty to ninety percent of RSWC was maintained by weighing the pots and replacing the lost water every 2 days.
2.2 Experimental design
In the growing season of June 2019, 16-month-old healthy plants of both species with a height about 1.0 m were randomly chosen for measurements of physiological parameters. Three treatments were imposed: well-watered (WW), drought stress with dew (DS & D), and drought stress with no dew (DS & ND) (10 replicates of each treatment for both species). WW plants were irrigated every 2 days to maintain approximately 80–90% RSWC by weighing the pots every day. In the DS & D and DS & ND treatments, water was withheld throughout the experiment until the RSWC reached approximately 5%. As a previous dew assessment showed that considerable dew formation takes place between 03:00 and 06:00 am, each pot of the DS & D treatment was sprayed with 10 ml distilled water (artificial dew) at 03:00 h on a daily basis following the protocols described by Breshears et al. (2008). During the experiment, Ψleaf, Kleaf, gas exchange, and RSWC were measured every day for approximately 25 days.
2.3 Leaf water potential and relative soil water content
The predawn, morning, and midday Ψleaf (Ψpd, Ψmor, Ψmd) were measured from four different individual of each species at 05:00, 09:00, and 12:00 am Beijing Standard Time (BST) at each sampling time, respectively. One upper fully expanded mature leaf was excised, immediately placed into a plastic bag to prevent transpiration, and inserted promptly into a pressure chamber (Plant Moisture Stress model 1000, PMS Instrument Co.) to measure the leaf water potential following the method of Fang et al. (2010). After Ψmor measurement, pots were weighed with a balance, and the RSWC was determined by the following formula: RSWC = (Wday−Wfinal)/(Whigh−Wfinal) × 100%, where Wday was the pot weight on each day, Whigh was the maximum weight of a pot at 100% soil field capacity and Wfinal was the pot weight when the pot weight reached a minimum due to dehydration.
2.4 Gas exchange
After Ψmor measurement, the leaves close to the leaves used to measure Ψmor were chosen from each individual to monitor gas exchange. Photosynthesis (A) and stomatal conductance (gs) were measured by a portable open-gas exchange system (LI-6400, LiCor) with 1200 μmol m−2 s−1 photosynthetic photon flux density, a leaf temperature of 22°C, a CO2 level of 400 ppm, a vapor pressure difference (VPD) between 1.1 and 1.4 kPa, and a flow rate of 400 mL per minute. After recording the values, the leaflets inserted into the cuvette were incised and scanned to calculate the rate of A and the gs per unit leaf area.
2.5 Leaf hydraulic conductance
Kleaf was measured between 9:00–10:00 h BST using the evaporative flux method described by Sack and Scoffoni (2012). Three leaves close to the leaf used to measure Ψmor were chosen from each individual after measurement of gas exchange. The upper and lower leaves were used to determine the initial leaf water potential (Ψinitial) as described above (if the Ψinitial of the upper and bottom leaves was more than 0.1 MPa, the samples were discarded), and the middle leaf was excised immediately under double-distilled water and connected to silicone tubing to determine the transpiration rate under a light source (1000 μmol m-2 s−1 photosynthetically active radiation). The leaf was allowed to transpire for more than 30 min until the flow rate stabilized. When the flow rate had stabilized, the leaf was disconnected from the silicon tube, immediately put inside a sealable plastic bag with 100% humidity, and equilibrated in the dark for at least 20 min. After equilibration, the final leaf water potential (Ψfinal) was measured with a pressure chamber, and the leaf was scanned. Kleaf was calculated as the ratio of transpiration to ΔΨleaf (where ΔΨleaf = Ψfinal − 0 MPa), which was further normalized to the leaf area and expressed in mmol m−2 s−1 MPa−1. The vulnerability curve was obtained by plotting Kleaf against whichever is lowest, Ψinitial or Ψfinal (Sack & Scoffoni, 2012).
2.6 Morphological traits
The leaves of C. korshinskii and C. sinica were photographed with a digital camera (EOS 5D Mark III, Canon). The role of C. korshinskii leaf trichomes in dew collection was observed and recorded using a digital microscope (DVM6, Leica Microsystems Ltd.,) after the artificial dew had been spread through humidifier. The trichomes of leaves close to leaves used for measuring physiological parameters were observed in five different C. korshinskii plants under well-watered and severe drought stress condition, and images of them were captured under the multi-magnification power of a scanning electron microscope (SEM) (S-3400N, Hitachi Ltd.) following the method as described by Ning et al. (2016). Briefly, leaf samples from the WW and DS replicates were collected, fixed in 4% glutaraldehyde (4°C, 6 h), cleansed using 0.1 M phosphate buffer solution (three times), fixed in 1% osmic acid (4°C, 2 h), and cleansed again using 0.1 M phosphate buffer solution (three times). Then, the leaves were dehydrated with a series of ethanol solutions and rinsed with tert-butyl alcohol twice to remove ethanol. The samples were kept overnight in a refrigerator (−40°C). A layer of gold was sprayed onto the samples, and the samples were observed under the SEM.
2.7 Statistical analysis
To examine the significance of differences between groups, analysis of variance (independent samples t-test), and a generalized linear model (univariate GLM) were performed with spss 25.0 (SPSS Inc.). Differences were considered statistically significant at P < 0.05. The graphs were plotted using SigmaPlot 10.0 (Systat Software Inc.), and nonlinear regressions were fitted to determine the correlations among the curves. Kleaf RSWC50, A RSWC50, gs RSWC50, and Kleaf P50 were calculated from the best-fitting equation. The trichome length and density were measured by using Image J software (https://imagej.nih.gov/ij/).
3 RESULTS
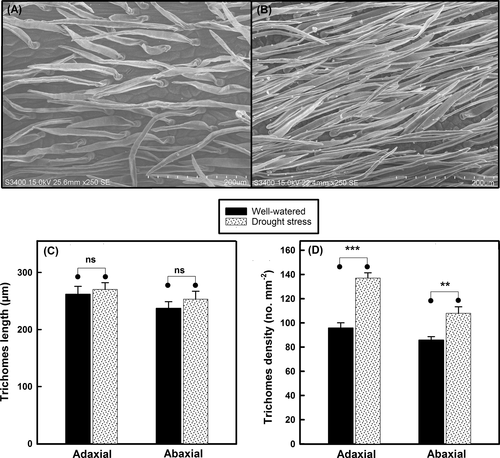
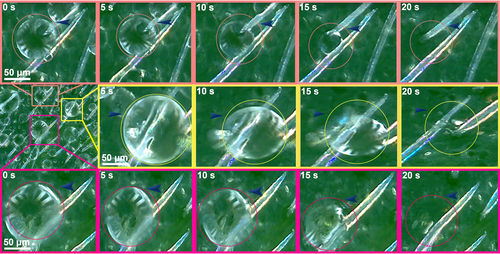
There was no significant difference in trichome length between the well-watered and drought-stressed plants of C. korshinskii, but trichome density on both the adaxial and abaxial leaf surfaces in drought-stressed plants increased by approximately 50 and 25% compared with that in well-watered plants, respectively (Figure 2). To test whether the trichomes absorbed dew, we spread dew on C. korshinskii leaf surfaces and observed dew absorption using digital microscopy. The observations revealed that the dew drops were absorbed rapidly by the C. korshinskii leaf trichomes; the large water droplets became smaller quickly and absorbed, within approximately 20 s (Figure 3).
In well-watered C. korshinskii plants, Ψpd, Ψmor, and Ψmd were approximately −0.5, −1.1, and − 1.8 MPa, respectively. Similar values were also observed in C. sinica (Figure 4). With the decrease in RSWC, the Ψleaf dropped gradually, but the Ψpd decreased more slowly in plants with dew than in plants without dew in C. korshinskii. Thus, the Ψpd in plants with dew was about −2.6 MPa higher than that in plants without dew when the RSWC decreased below 5% (Figure 4, Table 1). A similar effect was also observed in the Ψmor (−3.5 MPa in the plants with dew vs. −5.9 MPa in the plants without dew) and in the Ψmd (−5.3 MPa in the plants with dew vs. −6.2 MPa in the plants without dew) (Figure 4, Table 1). The generalized linear model demonstrated that the difference between the regression lines of plants with dew (DS & D) and those without dew (DS & ND) was significant (Figure 4). In C. sinica, dew improved the Ψpd in plants, but the degree of increase was far less than that observed in C. korshinskii, and the Ψmor and Ψmd were similar in C. sinica plants with dew and without dew with decreasing RSWC (Figure 4, Table. 1).
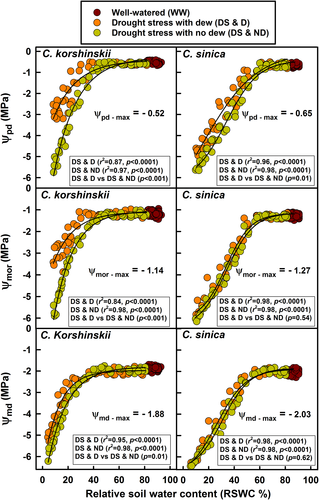
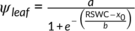 ) are listed. The differences between the two curves are also shown as P-values, with P < 0.05 indicating significance. The maximum values of Ψpd, Ψmor, and Ψmd for each species are listed in each panel
) are listed. The differences between the two curves are also shown as P-values, with P < 0.05 indicating significance. The maximum values of Ψpd, Ψmor, and Ψmd for each species are listed in each panel| Species | Habitat | Trichomes | Ψpd (-MPa) | Ψmor (-MPa) | Ψmd (-MPa) | Kleaf (mmol m−2 s−1 MPa−1) | A (μmol m−2 s−1) | gs (mol m−2 s−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DS & D | DS & ND | DS & D | DS & ND | DS & D | DS & ND | DS & D | DS &ND | DS & D | DS & ND | DS & D | DS & ND | |||
Caragana korshinskii |
Arid |
Long and dense trichomes present |
3.1 aA |
5.7 aB |
3.5 aA |
5.9 aB |
5.3 aA |
6.2 aB |
8.4 aA |
0.0 aB |
7.4 aA |
0.9 aB |
0.14 aA |
0.01 aB |
C. sinica |
Humid |
Trichomes absent |
4.9 bA |
5.6 aB |
5.9 bA |
6.1 aA |
6.2 bA |
6.4 aA |
0.91 bA |
0.0 aA |
0.88 bA |
0.03 aA |
0.03 bA |
0.01 aA |
- Note: Differences between values of species (C. korshinskii vs. C. sinica) and treatments (DS & D vs. DS & ND) were evaluated with analysis of variance (t-test). Values with the same lowercase letter within a column are not significantly different between two speceis (p > 0.05), and with the same capital letter with each parameter are not significantly different between two treatments (P > 0.05) (n = 4).
- Abbreviations: A, photosynthesis; DS & D, drought-stressed plants with dew; DS & ND, drought-stressed plants without dew; gs, stomatal conductance; Ψpd, predawn leaf water potential; Ψmor, morning leaf water potential; Ψmd, mid-day leaf water potential; Kleaf, leaf hydraulic conductivity.
In C. korshinskii, the Kleaf declined more slowly in plants with dew than in those without dew, and a significant difference was observed between the regression lines. When the RSWC was less than 5%, the Kleaf was about 8 mmol m−2 s−1 MPa−1 in plants with dew and approximately zero in plants without dew (Figure 5, Table 1). However, the effect of dew on the Kleaf in C. sinica was not observed, and the Kleaf was almost the same between C. sinica plants with dew and without dew (Figure 5, Table. 1). The dew also markedly ameliorated the gas exchange parameters of C. korshinskii, with A of 7.4 μmol m−2 s−1 and gs of 0.15 mol m−2 s−1 at 5% RSWC, while those in plants with no dew decreased to approximately zero. In C. sinica, no significant difference in A and gs was observed between the DS & D and DS & ND treatments, and both A and gs decreased to zero. Thus, the Kleaf RSWC50, A RSWC50, and gs RSWC50 were 7.6, 13.5, and 19.4% in DS & D and 20.5, 26.5, and 35.5% in DS & ND in C. korshinskii, respectively. In C. sinica, both A and gs were more sensitive to decreasing RSWC than in C. korshinskii, and the Kleaf RSWC50, A RSWC50 and gs RSWC50 were almost the same between DS & D and DS & ND (Figure 5, Table 2). The Kleaf P50 was about −2.7 and − 1.7 MPa in C. korshinskii and C. sinica, respectively, in DS & ND treatment, and dew absorption had no effect on the Kleaf P50 in either species (Figure 6; Table 2).

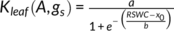 ) are listed. The differences between the two curves are also shown as P-values, with P < 0.05 indicating significance. The solid vertical and dotted vertical lines indicate the RSWC at which 50% loss of Kleaf, A, and gs occurred in drought stress with dew (DS & D) and drought stress without dew (DS & ND). The maximum values for Kleaf, A, and gs are listed in each panel
) are listed. The differences between the two curves are also shown as P-values, with P < 0.05 indicating significance. The solid vertical and dotted vertical lines indicate the RSWC at which 50% loss of Kleaf, A, and gs occurred in drought stress with dew (DS & D) and drought stress without dew (DS & ND). The maximum values for Kleaf, A, and gs are listed in each panel| Species | Kleaf P50 (MPa) | Kleaf RSWC50 (%) | A RSWC50 (%) | gs RSWC50 (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DS& D | DS & ND | DS & D | DS & ND | ΔRSWC | DS & D | DS & ND | ΔRSWC | DS & D | DS & ND | ΔRSWC | |
Caragana korshinskii |
−2.7 |
−2.8 |
7.6 |
20.5 |
12.9 |
13.5 |
26.5 |
13 |
19.4 |
35.5 |
16.1 |
C. sinica |
−1.7 |
−1.6 |
47.1 |
49.4 |
2.3 |
35.3 |
36.8 |
1.5 |
45.3 |
46.4 |
1.1 |
- Note: Kleaf P50 is the leaf water potential value at 50% Kleaf loss. Kleaf RSWC50, A RSWC50, and gs RSWC50 are the relative soil water content (RSWC) at 50% of the Kleaf, A, and gs loss. ΔRSWC is the difference of RSWC between drought stress with dew (DS & D) and drought stress without dew (DS & ND).

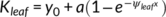 , in C. korshinskii and a sigmoidal function,
, in C. korshinskii and a sigmoidal function,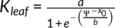 ,in C. sinica) are listed. The solid and dotted vertical lines indicate the Ψleaf at which 50% Kleaf loss occurs (KleafP50) in drought stress with dew (DS & D) and drought stress without dew (DS & ND)
,in C. sinica) are listed. The solid and dotted vertical lines indicate the Ψleaf at which 50% Kleaf loss occurs (KleafP50) in drought stress with dew (DS & D) and drought stress without dew (DS & ND)4 DISCUSSION
In arid environments, plants consistently face environmental challenges, such as water scarcity (Brodribb et al., 2020; Choat et al., 2012; Yao et al., 2021) Therefore, plants have evolved diverse coping strategies to adapt to these conditions, including developing deeper root architecture to maximize water absorption from soil (Gibbens & Lenz, 2001), having smaller leaf surface area to minimize water loss through transpiration and exhibiting rapid stomatal closure (Ahammed, Li, Mao, et al., 2020; Ahammed, Li, Wan, et al., 2020; Ahammed, Li, Yang, et al., 2020; Li et al., 2019; Li et al., 2020; Yao et al., 2020). Other structural modifications in the form of epidermal appendages designed to collect atmospheric water, such as the unique conical spines on cactus stems, the awns of desert mosses (Ju et al., 2012; Pan et al., 2016), and the leaf trichomes in numerous xeromorphic plant species (Huttunen et al., 2010; Ning et al., 2016; Schreel et al., 2020). The species inhabiting arid environments usually have dense foliar trichomes, as evident by the leaf trichomes of Phlomis fruticosa, Plectranthus australis, Acacia verniciflua, Solanum pennellii, Combretum leprosum, Arabidopsis lyrata, Arabidopsis kamchatica, Caragana korshinskii, and Conocarpus lancifolius (Huttunen et al., 2010; Ning et al., 2016; Pina et al., 2016; Redha et al., 2011; Sletvold & Agren, 2012). The same results were also observed in the Caragana genus in this study; C. korshinskii, which inhabits regions where precipitation is less than 200 mm/year, exhibited dense trichomes on both its adaxial and abaxial leaf surfaces.
Trichomes can be regarded as one of the superficial evolutionary structures with which plants contend with hostile environments because of their numerous ecophysiological functions, such as protecting the plant from ultraviolet (UV) radiations (Karabourniotis & Bornman, 1999), reducing heat loading (Skelton et al., 2012), discharging salts in saline environments, acting as water storehouses and producing hydrophilic substances (Paiva & Martins, 2011). Apart from these functions, there is evidence of foliar water uptake in an increasing number of species, for example, conifers (Breshears et al., 2008; Simonin et al., 2009), herbaceous vegetation (Zhuang & Zhao, 2010), broadleaf trees (Yan et al., 2015), and diverse species in multiple biomes, from tropical montane cloud forests to mangrove forests (Steppe et al., 2018) and desert ecosystems (Yan et al., 2015). In this study, we found that the leaf trichomes of C. korshinskii facilitate foliar water uptake as an adaptive strategy for acquiring water from alternative sources in arid environments. Similar results have also been documented in Tillandsia ionantha, Syntrichia caninervis, and Combretum leprosum, which generally dominate arid and semiarid biomes (Ohrui et al., 2007; Pan et al., 2016; Pina et al., 2016).
Foliar water absorption not only contributes to maintaining the leaf water status but also allows substantial water to pass through the branches and stems towards the belowground components through water-conducting xylem (Burgess & Dawson, 2004; Eller et al., 2013), forming an upside-down water transport system. The results of this study show a close relationship between absorption of dew and a slow decrease in Ψleaf similar to that reported in other plant species (Breshears et al., 2008; Pina et al., 2016; Zhuang & Zhao, 2010). Our results, in combination with previously published results, emphasize that absorption dew is a viable water resource for plant survival in harsh environments. In beech (Fagus sylvatica), foliar trichomes, which exhibit strong hygroscopic properties as a result of their structural and chemical design, constitute a major foliar water uptake pathway, while foliar water uptake is less driven by cuticular uptake and does not occur through the stomata (Schreel et al., 2020). In this study, we suggest that dew is mainly absorbed by trichomes rather than by cuticles or stomata due to (1) the rapid absorption of dew drops by C. korshinskii leaf trichomes observed under the digital microscope (Figure 2); (2) the amelioration of Kleaf and gaseous exchange with decreasing RSWC under drought and dew treatments in C. korshinskii but not in C. sinica, which does not have leaf trichomes (Figures 4 and 5); these results suggest that water was mainly absorbed via the trichomes, not via the stomata or cuticles; and (3) xerophytes being more likely than species in humid environments to have thick cuticles, which suggests that the chance of dew absorption by cuticles is relatively low in C. korshinskii growing in arid environments. Indeed, some experimental studies have explored how water is absorbed into the trichomes by capillary action, diffuses into leaf tissues with the aid of several aquaporin (water channel) proteins (Schreel et al., 2020), and moves intracellularly through apoplastic or symplastic pathways (Buckley, 2015; Chrispeels & Agre, 1994). In drought-stressed C. korshinskii plants, trichome density on both the adaxial and abaxial leaf surfaces increased greatly compared with that in well-watered plants, which might arise from drought-induced leaf shrinkage (Scoffoni et al., 2014), would facilitate foliar water uptake greatly and efficiency.
Intensifying soil water deficits induce a more negative Ψleaf, which causes a deterioration in xylem tension that leads to hydraulic dysfunction and consequent decreases in Kleaf (Brodribb et al., 2014; Scoffoni & Sack, 2017; Yao et al., 2021). Plant hydraulic failure acts as a primary physiological driver of drought-driven plant mortality by completely disconnecting the pathway between soil water and leaves (Brodribb et al., 2020; Choat et al., 2012). Recently, some studies showed that rehydration through water uptake from the shoot surface induced hydraulic recovery after drought-induced embolism repair (Earles et al., 2016; Fuenzalida et al., 2019; Mayr et al., 2014); these findings suggest that the role of foliar water absorption in contributing to the maintenance of hydraulic conductivity, which, in turn, mediates the functionality of gas exchange. Here, the outcomes of our investigation revealed that dew absorption through trichomes changed the Ψleaf in C. korshinskii and that slowly decreasing Ψleaf had a positive influence on the plant hydraulic system by slowing Kleaf loss as well as by inducing a lower Kleaf RSWC50 during soil water deficiency (Figure 7). According to the vulnerability segmentation hypothesis, the distal plant parts (leaves) are more vulnerable to hydraulic dysfunction than the basal parts (stem and root) (Creek et al., 2018; Scholz et al., 2014; Tyree et al., 1993). Here, we also exposed another function of leaves, that is, reducing the risk of stem hydraulic dysfunction under severe drought stress by absorbing dew through trichomes to improve the leaf water status. In addition, foliar water uptake may also mitigate the greater evaporative loads induced by the high vapor pressure deficits that occur in a daily cycle in arid environments (Scoffoni et al., 2011).
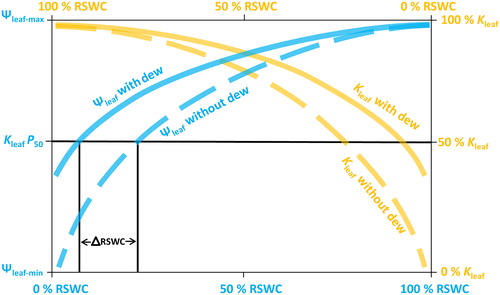
It has been shown that the ability of plants to withstand leaf hydraulic dysfunction shapes species distributions across precipitation gradients (Blackman et al., 2012, 2014; Nardini & Luglio, 2014; Yao et al., 2021). Our current study showed that Caragana species growing in arid environments achieved more negative Kleaf P50 values than Caragana species growing in humid regions (Yao et al., 2021). The results were consistent with findings that Kleaf P50 values are more negative in species growing in lower-rainfall environments (Blackman et al., 2009, 2012, 2014). However, none of the studies considered leaf dew absorption, which occurs widely in arid environments. Indeed, with regard to the role of dew in leaf hydraulics, as leaf wetting events occur >100 days/year in various ecosystems (Dawson & Goldsmith, 2018), the importance of foliar water uptake in the rehydration of leaves cannot be ignored (Breshears et al., 2008; Schreel & Steppe, 2019). Here, we clearly observed that dew absorption amends leaf hydraulic functions during dehydration. It has been shown that stems and roots can be rehydrated by the redistribution of water absorbed by the leaves and that this redistributed water can relieve tension on the water column (Steppe et al., 2018) and may coincide with whole-plant xylem cavitation recovery. The dew absorption maintains leaf hydraulic function during dehydration and does not induce a more negative Kleaf P50. Indeed, it induces lower Kleaf RSWC50 as well as A RSWC50 and gs RSWC50 by decoupling the leaf water status from soil water availability. The results suggest that leaf water uptake in C. korshinskii can alleviate drought stress, which broadens the distribution of this species and allows it to grow in more arid regions that experience non-rainfall events such as fog and dew rather than relying on soil water availability alone. However, C. sinica, which grows in humid areas and has no trichomes, showed poor foliar water uptake. The collection of occult precipitation during water shortages is an influential mechanism enabling plants to escape drastic hydraulic stress during the dry season; therefore, we should consider foliar water uptake when testing plant drought tolerance in arid environments.
5 CONCLUSIONS
The ability of C. korshinskii trichomes to absorb dew slowed the decrease in Ψleaf, Kleaf, and gas exchange with decreasing soil water content and resulted in lower Kleaf RSWC50, A RSWC50, and gs RSWC50 than were observed in the absence of dew. Its congener C. sinica, which does not have trichomes, displayed little ability to absorb dew under drought stress and did not show improvement in the above parameters. The results here provide new insights into the adaptability of species to arid environments. Further work is needed to elucidate the water pathway from the epidermal trichomes of C. korshinskii to the entire plant, and dew absorption should be tested in additional species growing along precipitation gradients.
ACKNOWLEDGMENTS
We thank four anonymous referees for their valuable comments on the manuscript. We thank core faculty of School of life Sciences, Lanzhou University. This work was supported by National Natural Science Foundation of China (Nos. 31670404, 31971406, 31422011), the Second Tibetan Plateau Scientific Expedition and Research Program (STEP) (2019QZKK0301), and Feitian Project (860059).
AUTHOR CONTRIBUTIONS
XWF designed experiments, XWF and YX revised manuscript. GQY and MMH performed the data analysis. ZFN partially involved in field experiments. MW had primary responsibility for experiment conduction and writing the manuscript. All authors have read and approved the final manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




