Unraveling the roles of brassinosteroids in alleviating drought stress in young Eucalyptus urophylla plants: Implications on redox homeostasis and photosynthetic apparatus
Funding information: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil); Fundação Amazônia de Amparo a Estudos e Pesquisas (FAPESPA/Brazil); Researches Supporting Project Number RSP 2020/236 from King Saud University; Universidade Federal Rural da Amazônia (UFRA/Brazil)
Abstract
Water deficit is the most limiting abiotic stress to plants because it affects several physiological and biochemical processes. Brassinosteroids, including 24-epibrassinolide (EBR), are steroids that regulate growth and positively act on gas exchange. This research aims to determine whether EBR can attenuate the negative effects of water deficit, revealing possible contributions of this steroid on photosynthetic machinery of young Eucalyptus urophylla plants under water deficit. The experiment had a completely randomized factorial design with two water conditions (control and water deficit) and three levels of EBR (0, 50, and 100 nM EBR). Water deficit caused a decrease in the levels of total chlorophyll and carotenoids, but these photosynthetic pigments increased by 135 and 226%, respectively, in plants sprayed with EBR when compared to the water deficit + 0 nM EBR treatment. Regarding the antioxidant system, 100 nM EBR induced significant increments in superoxide dismutase (42%), catalase (52%), ascorbate peroxidase (147%), and peroxidase (204%). Steroid application in E. urophylla plants exposed to water deficit increased the effective quantum yield of the photosystem II (PSII) photochemistry and electron transport rate. However, interestingly, it decreased the nonphotochemical quenching and relative energy excess at the PSII level, indicating improvements related to PSII efficiency. This research revealed that application of 100 nM EBR attenuated the negative effects caused by water deficit, being explained by the positive repercussions on antioxidant enzyme activities, chloroplastic pigments, PSII efficiency, electron flux, and net photosynthetic rate.
Abbreviations
-
- ΦPSII
-
- effective quantum yield of PSII photochemistry
-
- APX
-
- ascorbate peroxidase
-
- BR
-
- brassinosteroid
-
- Car
-
- carotenoid
-
- CAT
-
- catalase
-
- Chl a
-
- chlorophyll a
-
- Chl b
-
- chlorophyll b
-
- Ci
-
- intercellular CO2 concentration
-
- E
-
- transpiration rate
-
- EBR
-
- 24-epibrassinolide
-
- EL
-
- electrolyte leakage
-
- ETR
-
- electron transport rate
-
- EXC
-
- relative energy excess at the PSII level
-
- gs
-
- stomatal conductance
-
- HBL
-
- 28-homobrassinolide
-
- LDM
-
- leaf dry matter
-
- MDA
-
- malondialdehyde
-
- NPQ
-
- nonphotochemical quenching
-
- PN
-
- net photosynthetic rate
-
- POX
-
- peroxidase
-
- PQ
-
- plastoquinone
-
- PSI
-
- photosystem I
-
- PSII
-
- photosystem II
-
- qP
-
- photochemical quenching
-
- RDM
-
- root dry matter
-
- ROS
-
- reactive oxygen species
-
- SDM
-
- stem dry matter
-
- SOD
-
- superoxide dismutase
-
- TDM
-
- total dry matter
-
- WUE
-
- water-use efficiency
1 INTRODUCTION
The genus Eucalyptus has more than 700 species, distributed across more than 20 million hectares of the world's temperate and tropical regions (Mora et al., 2017; Mora & Serra, 2014). Of this total, approximately 5.7 million hectares are cultivated in commercial forests in Brazil (IBA, 2017). The interest in species of this genus is justified by their rapid growth and broad environmental adaptability in addition to the wide variety of products generated by their cultivation, such as wood, cellulose pulp, and essential oils (Mora et al., 2009).
Chloroplasts are fundamental organelles in photosynthetic organisms. They are composed of thylakoids and stroma, in which reside the components intrinsically linked to the photosynthetic machinery (Staehelin, 2003; Zoschke & Bock, 2018). In the granum lamellae, the solar energy that reaches the antenna of the light-harvesting systems (light-harvesting complex II) is captured by the photosynthetic pigments, including chlorophylls a (Chl a) and b (Chl b) and carotenoids (Car), which transfer the electrons to the plastoquinone (PQ), primary acceptor of the reaction center (P680) in the photosystem II (PSII) (Baker, 2008; Kume et al., 2018). The PQ then transfers the excited energy to the reaction center (P700) of photosystem I (PSI) through the electron transport chain in the thylakoid membrane. This energy is used for the synthesis of ATP and nicotinamide adenine dinucleotide phosphate during reactions connected to carbon dioxide (CO2) fixation (Belyaeva et al., 2016; Cherepanov et al., 2017).
Water deficit is the abiotic stress most limiting to plants (Liu et al., 2019; Lobato et al., 2020; Wyka et al., 2019), causing a reduction in leaf water potential (Rivas et al., 2016), a reduction in stomatal conductance and transpiration (Pereira et al., 2016), and thus a decrease in the photosynthetic rate (Silva et al., 2015). This stress also reduces the synthesis of photosynthetic pigments (Santos et al., 2015) and photochemical efficiency (Singh & Reddy, 2011) and induces the accumulation of reactive oxygen species (ROS), leading to oxidative stress (Sheikh-Mohamadi et al., 2017; Silva et al., 2012), which reduces growth rates (Caser et al., 2017).
The exogenous application of brassinosteroids (BRs) may represent a mitigation option for plants' development under water deficit conditions. BRs are natural polyhydroxy steroid compounds classified according to the number of carbons. They are present in various plant organs, such as leaves, roots, seeds, and fruits, where they are rapidly absorbed and metabolized (Bajguz & Hayat, 2009; Bajguz & Tretyn, 2003). There are approximately 70 types of BRs, but 24-epibrassinolide (EBR), 28-homobrassinolide (HBL), and brassinolide are the most frequently used (Divi & Krishna, 2009; Oklestkova et al., 2015).
EBR is recognized as a steroid with intrinsic effects on growth. They participate in several physiological, biochemical, and molecular processes (Fonseca et al., 2020; Nazir et al., 2021; Planas-Riverola et al., 2019), including osmotic regulation (Shahid et al., 2014), gas exchange (Ma & Guo, 2014), chlorophyll biosynthesis (Yuan et al., 2012), and photochemical efficiency (Wang, Zheng, et al., 2015). This molecule can mitigate oxidative stress (Talaat et al., 2015) by stimulating the activity of antioxidant enzymes (Ali et al., 2008) and increasing growth (Shahbaz et al., 2008). In addition, EBR regulates the tolerance mechanisms to salinity stress (Reyes Guerrero et al., 2015), low and high temperatures (Cui et al., 2016; Wu et al., 2014), heavy metal toxicity (Ramakrishna & Rao, 2015), nutrient uptake (Karlidag et al., 2011), and nutrient deficiency (Wang, Li, & Zhang, 2015).
Researchers have demonstrated the beneficial effects of the application of EBR on plants exposed to water deficit, such as increases in the growth, relative water content, and minor production of H2O2 in Lycopersicon esculentum plants (Jangid & Dwivedi, 2017). In Hordeum vulgare plants sprayed with EBR, biomass production and gas exchange were maximized (Gill et al., 2017). In Vigna unguiculata, plants pretreated with EBR had an increase in leaf water potential and carbon fixation (Lima & Lobato, 2017).
The hypothesis of this research considered the negative effects induced by water deficit on absorption and utilization of light (Ju et al., 2018; Lang et al., 2018). Important results on the protective roles exercised by the EBR are available in literature, including benefits on carbon fixation (Talaat, 2020). Therefore, the objective of this research is to determine whether EBR can attenuate the negative effects resulting from water deficit, revealing possible contributions of this steroid on antioxidant system and photosynthetic machinery in Eucalyptus urophylla under water deficit.
2 MATERIALS AND METHODS
2.1 Location and growth conditions
The experiment was performed at the Campus of Paragominas of the Universidade Federal Rural da Amazônia, Paragominas, Brazil (2°55′S, 47°34′W). The study was conducted in a greenhouse with the temperature and humidity semi-controlled. The minimum, maximum, and median temperatures were 20, 31, and 24.5°C, respectively. The relative humidity during the experimental period varied between 60 and 80%. Day length of 12 h. During the measurement period (12:00 p.m.), the photosynthetically active radiation varied between 585 and 1854 μmol photons m−2 s−1.
2.2 Plants, containers, and acclimation
Thirty-eight-day-old seedlings of E. urophylla, similar in aspects and sizes, were selected and placed in 1.2 L containers filled with a mixed substrate of sand and vermiculite in a 3:1 ratio supplied with 500 ml of nutrient solution (semi-hydroponic conditions) in agreement with Oliveira et al. (2019), with minor modifications. Ionic strength started at 50% (38th day) and was modified to 100% after 5 days (40th day). After this period, the nutritive solution remained at total ionic strength.
2.3 Experimental design
The experiment was a factorial design with the completely randomized factors of two water conditions (control and water deficit) and three levels of EBR (0, 50, and 100 nM EBR). A total of 30 experimental units (five replicates for each of six treatments) was used, with one plant in each unit. The steroid concentrations were chosen according to Amzallag and Vaisman (2006).
2.4 EBR preparation and application
Forty-day-old seedlings were sprayed with EBR or Milli-Q water (containing a proportion of ethanol equal to that used to prepare the EBR solution) at 6-day intervals until Day 76. The 0, 50, and 100 nM EBR (Sigma-Aldrich) solutions were prepared by dissolving the solute in ethanol (100 μl) followed by dilution with Milli-Q water (ethanol: water [v/v] = 1:10,000) (Ahammed et al., 2013). On Day 74, half of the plants were subjected to water restriction, while the others were properly watered.
2.5 Water deficit treatment
Plants received the following macro- and micronutrients in the nutrient solution in agreement with Oliveira et al. (2019), with minor modifications. To simulate water deficit, the nutrient solution was removed completely, and the root system was placed in similar pots without water/solution. The water deficit was applied for 4 days (between Day 74 and Day 78). During the study, the nutrient solutions were changed at 07:00 h at 3-day intervals with the pH adjusted to 6.0 using HCl or NaOH. On Day 78 of the experiment, physiological and morphological parameters were measured for all plants, and leaf tissues were harvested for nutritional and biochemical analyses.
2.6 Measurement of chlorophyll fluorescence and gas exchange
The chlorophyll fluorescence was measured in fully expanded leaves under light using a modulated chlorophyll fluorometer (model OS5p; Opti-Sciences). Preliminary tests determined the location of the leaf, the part of the leaf, and the time required to obtain the highest Fv/Fm ratio; therefore, the acropetal third of leaves located at the middle third of the plant and adapted to the dark for 30 min, was used in the evaluation. The intensity and duration of the saturation light pulse were 7500 μmol m−2 s−1 and 0.7 s, respectively. The gas exchange was evaluated in all plants under constant CO2 concentration using an infrared gas analyzer (model LCPro+; ADC BioScientific). The photosynthetically active radiation, air-flow rate, and temperature were 360 μmol mol−1 CO2, 800 μmol photons m−2 s−1, 300 μmol s−1, and 28°C, respectively, when the measurements occurred (between 10:00 a.m. and 12:00 p.m.).
2.7 Determination of antioxidant enzymes, superoxide, and soluble proteins
Antioxidant enzymes (superoxide dismutase [SOD], catalase [CAT], ascorbate peroxidase [APX], and peroxidase [POX]), superoxide (O2−), and soluble proteins were extracted from totally expanded leaf (0.5 g) according to the method of Badawi et al. (2004), in which this extraction methodology presents as advantages to be rapid, low cost, and efficient. Total soluble proteins were quantified using the methodology described by Bradford (1976). The SOD activity was measured at 560 nm by Unit m−1 proteins (Giannopolitis & Ries, 1977). The CAT activity was detected at 240 nm (Havir & McHale, 1987) by μmol H2O2 mg−1 protein min−1. The APX activity was measured at 290 nm (Nakano & Asada, 1981) in μmol AsA mg−1 protein min−1. POX activity was detected at 470 nm (Cakmak & Marschner, 1992), with the activity expressed in μmol tetraguaiacol mg−1 protein min−1. The determination of O2− was measured at 530 nm (Elstner & Heupel, 1976).
2.8 Quantification of hydrogen peroxide, malondialdehyde, and electrolyte leakage
Stress indicators (hydrogen peroxide [H2O2] and malondialdehyde [MDA]) were extracted from leaf tissue using the methodology described by Wu et al. (2006). It has the advantage of applying a single extraction procedure to both compounds. H2O2 was measured with the procedures defined by Velikova et al. (2000). MDA was determined by the method of Cakmak and Horst (1991), using the extinction coefficient of 155 mM−1 cm−1. Electrolyte leakage (EL) was measured according to Gong et al. (1998), being calculated by the formula EL (%) = (EC1/EC2) × 100.
2.9 Quantification of photosynthetic pigments and biomass
The chlorophyll and Car determinations were performed using a spectrophotometer (model UV-M51; Bel Photonics), according to the methodology of Lichtenthaler and Buschmann (2001). The biomass of roots, stems, and leaves was measured based on constant dry weights (g) after drying in a forced-air ventilation oven at 65°C.
2.10 Data analysis
The normality of data was verified with Shapiro–Wilk test. Data were submitted to two-way ANOVA (water condition and EBR concentration) and applied Scott–Knott test at a probability level of 5% (Steel et al., 2006). All statistical procedures used the Assistat software.
3 RESULTS
3.1 EBR contributions to water status and light capture
Plants exposed to water deficit had a significant reduction in leaf water potential (Ψw) values, but the application of 100 nM of EBR caused an increase of 18% when compared to plants under water deficit without EBR (Figure 1). Water restriction led to significant decreases in maximal quantum yield of PSII photochemistry (Fv/Fm) and maximal fluorescence yield of the dark-adapted state (Fm) values, while plants sprayed with EBR (100 nM) increased those parameters by 40 and 54%, respectively, compared to water deficit + 0 nM EBR. Water deficit significantly increased the minimal fluorescence yield of the dark-adapted state (F0) values, while the application of 100 nM EBR to plants under water deficit induced a significant reduction of 15% in F0 values when compared to the equal treatment without EBR.
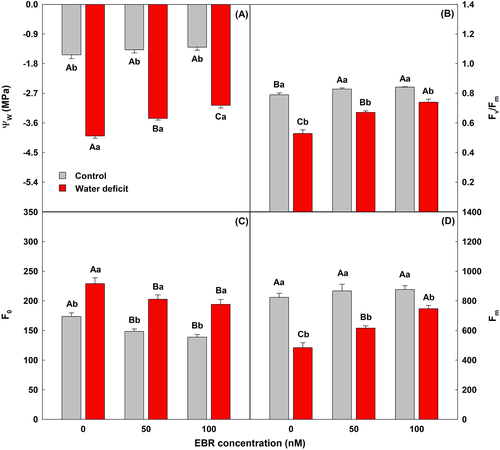
3.2 Steroid increased photosynthetic efficiency and electron transport
The effective quantum yield of PSII photochemistry (ΦPSII), photochemical quenching (qP), and electron transport rate (ETR) were significantly reduced under water deficit. In contrast, plants under water deficit + 100 nM of EBR increased those parameters by 40, 124, and 62%, respectively, compared to the water deficit + 0 nM EBR treatment. Significant increases in nonphotochemical quenching (NPQ), relative energy excess at the PSII level (EXC), and the ratio between the ETR and net photosynthetic rate (PN) were detected after water deficit. The application of 100 nM EBR reduced NPQ, EXC, and ETR/PN by 30, 6, and 33%, respectively, compared to the equal treatment without EBR (Table 1).
| Water condition | EBR (nM) | ΦPSII | qP | NPQ | ETR (μmol m−2 s−1) | EXC (μmol m−2 s−1) | ETR/PN |
|---|---|---|---|---|---|---|---|
| Control | 0 | 0.79 ± 0.01Ba | 0.67 ± 0.01Ca | 0.36 ± 0.03Ab | 80.9 ± 1.8Ba | 0.30 ± 0.01Ab | 5.6 ± 0.5Ab |
| Control | 50 | 0.83 ± 0.01Aa | 0.76 ± 0.03Ba | 0.35 ± 0.03Ab | 89.9 ± 2.7Aa | 0.26 ± 0.02Bb | 5.7 ± 0.3Ab |
| Control | 100 | 0.84 ± 0.01Aa | 0.88 ± 0.04Aa | 0.34 ± 0.02Ab | 94.8 ± 2.8Aa | 0.23 ± 0.01Bb | 5.3 ± 0.1Ab |
| Water deficit | 0 | 0.53 ± 0.02Cb | 0.25 ± 0.02Cb | 0.61 ± 0.01Aa | 23.2 ± 2.9Bb | 0.70 ± 0.02Aa | 46.4 ± 1.7Aa |
| Water deficit | 50 | 0.67 ± 0.02Bb | 0.40 ± 0.03Bb | 0.55 ± 0.03Ba | 27.3 ± 1.9Bb | 0.72 ± 0.01Aa | 39.0 ± 2.1Ba |
| Water deficit | 100 | 0.74 ± 0.02Ab | 0.56 ± 0.03Ab | 0.43 ± 0.01Ca | 37.5 ± 3.7Ab | 0.66 ± 0.02Ba | 31.2 ± 2.4Ca |
| Interaction effects | |||||||
| W × E (P-value) | 0.025 | <0.001 | 0.027 | 0.018 | 0.022 | 0.019 | |
- Notes: Columns with different uppercase letters between EBR levels (0, 50, and 100 nM EBR under equal water condition) and lowercase letters between water conditions (control and water deficit under equal EBR concentration) indicate significant differences from the Scott–Knott test (P < 0.05). W = water condition, E = EBR, means ± sd, n = 5.
- Abbreviations: ΦPSII, effective quantum yield of PSII photochemistry; ETR, electron transport rate; EXC, relative energy excess at the PSII level; ETR/PN, ratio between the electron transport rate and net photosynthetic rate; NPQ, nonphotochemical quenching; qP, photochemical quenching coefficient.
3.3 Gas exchange was maximized by EBR
Plants under water deficit suffered significant reductions in PN, transpiration rate (E), and stomatal conductance (gs), but the application of 100 nM EBR increased them by 140, 33, and 133%, respectively, compared to the water deficit + 0 nM EBR treatment. Water-use efficiency (WUE) and carboxylation instantaneous efficiency (PN/intercellular CO2 concentration [Ci]) were significantly reduced during water deficit; however, they increased by 88% and 300%, respectively, upon 100 nM EBR application compared to cultivated plants without EBR (Table 2).
| Water condition | EBR (nM) | PN (μmol m−2 s−1) | E (mmol m−2 s−1) | gs (mol m−2 s−1) | Ci (μmol mol−1) | WUE (μmol mmol−1) | PN/Ci (μmol m−2 s−1 Pa−1) |
|---|---|---|---|---|---|---|---|
| Control | 0 | 14.3 ± 0.3Ca | 2.5 ± 0.1Ba | 0.26 ± 0.01Ca | 258 ± 8Ab | 5.7 ± 0.3Ba | 0.055 ± 0.002Ca |
| Control | 50 | 15.7 ± 0.4Ba | 2.7 ± 0.1Aa | 0.29 ± 0.01Ba | 254 ± 7Ab | 5.8 ± 0.1Ba | 0.062 ± 0.003Ba |
| Control | 100 | 17.7 ± 0.5Aa | 2.8 ± 0.1Aa | 0.34 ± 0.02Aa | 244 ± 9Ab | 6.3 ± 0.1Aa | 0.072 ± 0.003Aa |
| Water deficit | 0 | 0.5 ± 0.1Bb | 0.6 ± 0.0Bb | 0.03 ± 0.01Ab | 358 ± 21Aa | 0.8 ± 0.1Bb | 0.001 ± 0.001Ab |
| Water deficit | 50 | 0.7 ± 0.2Bb | 0.8 ± 0.1Ab | 0.04 ± 0.01Ab | 344 ± 21Aa | 0.9 ± 0.1Bb | 0.002 ± 0.002Ab |
| Water deficit | 100 | 1.2 ± 0.1Ab | 0.8 ± 0.1Ab | 0.07 ± 0.01Ab | 334 ± 22Aa | 1.5 ± 0.1Ab | 0.004 ± 0.001Ab |
| Interaction effects | |||||||
| W × E (P-value) | 0.034 | 0.041 | 0.029 | 0.047 | 0.038 | 0.026 | |
- Notes: Columns with different uppercase letters between EBR levels (0, 50, and 100 nM EBR under equal water condition) and lowercase letters between water conditions (control and water deficit under equal EBR concentration) indicate significant differences from the Scott–Knott test (P < 0.05). W = water condition, E = EBR, means ± sd, n = 5.
- Abbreviations: Ci, intercellular CO2 concentration; E, transpiration rate; gs, stomatal conductance; PN, net photosynthetic rate; PN/Ci, carboxylation instantaneous efficiency; WUE, water-use efficiency.
3.4 Antioxidant metabolism improved in plants pretreated with EBR
Water deficit caused an increase in all enzyme activities evaluated (Figure 2). The application of 100 nM EBR intensified this effect, and SOD, CAT, APX, and POX activities significantly increased by 42, 52, 147, and 204%, respectively, when compared to plants cultivated under the water deficit + 0 nM EBR.
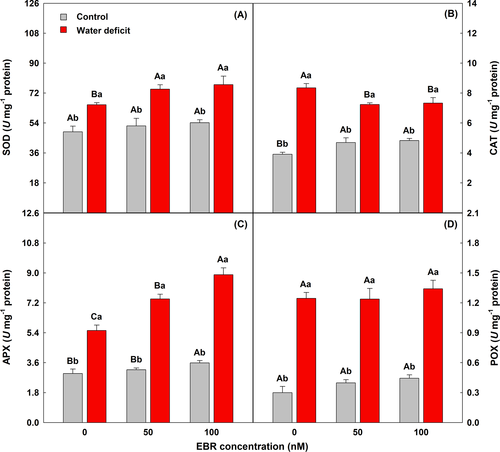
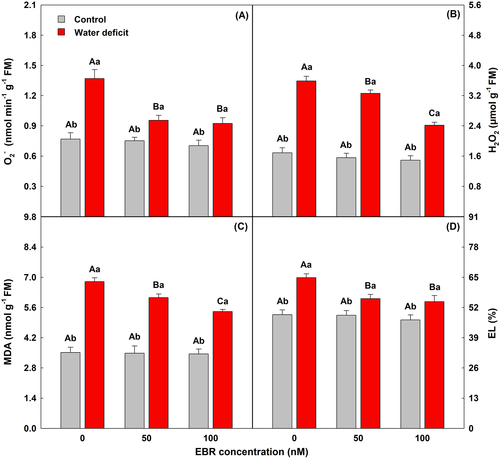
3.5 The oxidative stress was mitigated in plants exposed to water deficit
Plants exposed to water deficit had significant increases in O2−, H2O2, MDA, and EL levels; however, the application of 100 nM EBR significantly attenuated these effects with a reduction of 33, 33, 20, and 16%, respectively, when compared to plants grown under water deficit without EBR (Figure 3).
3.6 Attenuation of the effects of water deficit on photosynthetic pigments
The water deficit promoted significant reductions in Chl a, Chl b, total chlorophyll (total Chl), and Car (Table 3). However, treatment with 100 nM EBR caused a significant increase in those pigments by 109, 212, 135, and 226%, respectively, when compared to the water deficit + 0 nM EBR treatment. In addition, the Chl a/Chl b and total Chl/Car ratios significantly increased in plants under water deficit compared with control plants under the same concentration of EBR (Table 3).
| Water condition | EBR (nM) | Chl a (mg g−1 FM) | Chl b (mg g−1 FM) | Total Chl (mg g−1 FM) | Car (mg g−1 FM) | Ratio Chl a/Chl b | Ratio total Chl/Car |
|---|---|---|---|---|---|---|---|
| Control | 0 | 8.24 ± 0.27Ba | 6.27 ± 0.19Ca | 14.51 ± 0.79Ba | 1.17 ± 0.04Ca | 1.31 ± 0.11Ab | 12.40 ± 1.22Ab |
| Control | 50 | 8.43 ± 0.29Ba | 7.08 ± 0.19Ba | 15.51 ± 0.61Ba | 1.31 ± 0.06Ba | 1.19 ± 0.09Ab | 11.84 ± 0.83Ab |
| Control | 100 | 9.23 ± 0.37Aa | 8.08 ± 0.28Aa | 17.31 ± 1.03Aa | 1.47 ± 0.05Aa | 1.14 ± 0.08Ab | 11.78 ± 0.85Ab |
| Water deficit | 0 | 2.75 ± 0.25Bb | 0.98 ± 0.09Cb | 3.74 ± 0.32Cb | 0.19 ± 0.01Cb | 2.80 ± 0.18Aa | 19.68 ± 1.02Aa |
| Water deficit | 50 | 5.58 ± 0.24Ab | 2.01 ± 0.20Bb | 7.60 ± 0.48Bb | 0.46 ± 0.04Bb | 2.78 ± 0.23Aa | 16.52 ± 1.31Ba |
| Water deficit | 100 | 5.74 ± 0.31Ab | 3.06 ± 0.17Ab | 8.80 ± 0.37Ab | 0.62 ± 0.03Ab | 1.88 ± 0.14Ba | 14.20 ± 0.41Ca |
| Interaction effects | |||||||
| W × E (P-value) | 0.024 | <0.001 | 0.010 | <0.001 | 0.041 | 0.027 | |
- Notes: Columns with different uppercase letters between EBR levels (0, 50, and 100 nM EBR under equal water condition) and lowercase letters between water conditions (control and water deficit under equal EBR concentration) indicate significant differences from the Scott–Knott test (P < 0.05). W = water condition, E = EBR, means ± sd, n = 5.
- Abbreviations: Car, carotenoids; Chl a, chlorophyll a; Chl b, chlorophyll b; Total chl, total chlorophyll.
3.7 EBR maximized the growth rate in control and water deficit conditions
Water deficit reduced leaf dry matter (LDM), root dry matter (RDM), stem dry matter (SDM), and total dry matter (TDM) of E. urophylla plants, while plants sprayed with both concentrations of EBR (50 and 100 nM) suffered less of the water deficit. The best effect was observed with 50 nM EBR, where LDM, RDM, SDM, and TDM increased by 15, 18, 18, and 16%, respectively, compared to plants under water deficit without EBR treatment (Figure 4).
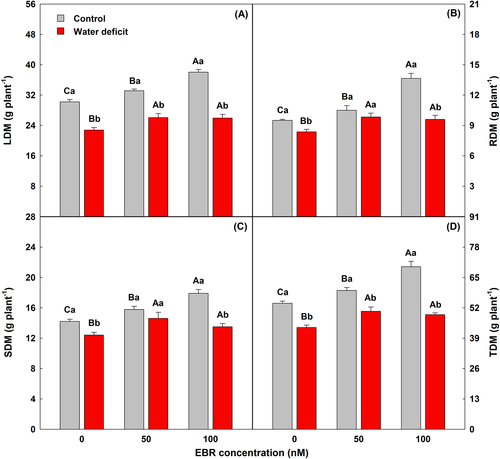
4 DISCUSSION
Our study unraveled the roles of BRs in alleviating drought stress in young E. urophylla plants with clear benefits on redox homeostasis and photosynthetic apparatus (Figure 5). This research confirms by physiological and biochemical approaches the insights obtained mainly at molecular level by other research groups. In this context, Xia et al. (2011) evaluated the EBR properties inducing stress tolerance in plants, and they confirmed that the overexpression of CPD and DWF4 genes is connected to the biosynthetic pathway of this steroid. Li et al. (2016) found that tomato dwarf mutants modified in EBR biosynthesis presented an increased gas exchange, also ratified by our results.
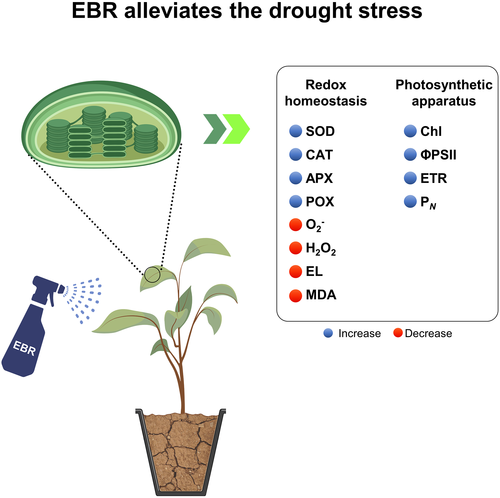
The pretreatment with EBR promoted the increase in Ψw values in plants exposed to water deficit, suggesting that this steroid improved the osmotic adjustment of E. urophylla plants through the accumulation of organic solutes, such as proline and soluble sugars (Behnamnia, Kalantari, & Ziaie, 2009; Liu et al., 2014). Farooq et al. (2009) reported an increase in Ψw values after foliar treatment of two forms of BRs (HBL and EBR) in Oryza sativa plants grown under water deficit. These results are due to the improvements in leaf water economy and CO2 assimilation promoted by the steroid. The beneficial contributions of EBR on Ψw and growth were associated with enhanced levels of soluble proteins and free proline in three cultivars of Sorghum vulgare exposed to osmotic stress with application of 2 and 3 μM of this steroid (Vardhini & Rao, 2003).
Higher values of Fv/Fm revealed that EBR increased the photochemical efficiency of the reaction center linked to PSII, attenuating the photoinhibition due to water deficit. The reductions in F0 and increase in Fm indicated that EBR application reduced the photo-oxidative damage caused by water deficit on the structure and function of the primary PQ acceptor in the reaction center of PSII, increasing the cyclic electron flow in the photosystems (Huang et al., 2012; Lima Neto et al., 2017). Wang, Zheng, et al. (2015) evaluated chlorophyll fluorescence, leaf morphology, and cell ultrastructure and also found increases in the Fv/Fm of Vitis vinifera plants under 12 days of water deficit. Li et al. (2012) reported similar results for F0 and Fm in Chorispora bungeana plants sprayed with 0.1 μM EBR and exposed to water deficit for 3 days: F0 reduced by 6% and Fm increased by 7% after application of EBR.
Increases in ΦPSII and qP in the plants treated with EBR were associated with higher values of Fv/Fm, indicating that this steroid increased the ratio linked to the reaction center of PSII and maximized the light capture efficiency by the light-harvesting system (Figure 5). The increase in ETR is related to the reduction of PSII overexcitation, represented by the lower EXC values. It revealed that EBR increased the photosynthetic electron transport efficiency of the primary PQ acceptor preventing damage to the electron transport chain in the thylakoid membrane (Fariduddin et al., 2014; Zhang et al., 2013). Jiang et al. (2012) evaluated the CO2 assimilation and photosynthetic electron transport in Cucumis sativus plants and reported increases in ΦPSII and qP after application of 0.1 μM EBR. An increase in ETR was also verified by Thussagunpanit et al. (2015) investigating the photosynthetic efficiency of O. sativa plants sprayed with 1 nM EBR.
The reduction in NPQ and EXC in E. urophylla plants by EBR indicates that this steroid improved the efficiency of light conversion, maximizing the energy proportion available to the photosynthetic electron transport after the light was absorbed by the antenna of PSII, attenuating the photoinhibitory damages imposed to PSII. EXC is intrinsically related to the energy that is neither used for electron transport or dissipated as heat (Kato et al., 2003). Additionally, the reduction in ETR/PN was related to the increase in PN and reduction of O2−. The application of EBR induced the plants to use more photochemical energy in the fixation of CO2 instead of other metabolic processes, such as the photoreduction of O2 to O2− (Lima Neto et al., 2017; Silva et al., 2012). Lima and Lobato (2017) evaluated the PSII efficiency, gas exchange, antioxidant enzymes, and growth in V. unguiculata plants exposed to water deficit for 2 days and reported reductions of 30, 19, and 12% in NPQ, EXC, and ETR/PN, respectively.
EBR spray increased PN, which was explained by the higher electron flow between the photosystems (ETR) detected in this study, resulting in greater photosynthetic activity (Figure 5). Increases in E and gs are corroborated by higher water content in leaf tissue (Ψw) induced by EBR, which led to the increased opening of the stomata (Xia et al., 2014; Zhang et al., 2008). Zhao et al. (2017) evaluated the effects of the application of 0.1 mg L−1 of EBR on photosynthesis and ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) of Triticum aestivum plants exposed to water deficit for 8 h and verified an increase in PN. Increases in PN (29%), E (43%), and gs (70%) were found by Hayat et al. (2011) studying the comparative effects of two EBR homologs on the growth, carbonic anhydrase activity, and photosynthetic efficiency in L. esculentum plants.
Steroid application attenuated the effect of water deficit on WUE, and this response was related to the combined increases in PN and E, verified in this study, because WUE is the physiological variable that expresses the amount of carbon fixed (PN) per plant per unit of lost water (E) (Barros Junior et al., 2017). The increase in PN/Ci in plants treated with EBR indicated that the steroid increased the assimilation of CO2 (PN) with concomitant reduction of the concentrations of Ci due to the increase in the activity of the enzyme RuBisCO (Lima & Lobato, 2017; Yusuf et al., 2011). Fariduddin et al. (2009) evaluated the effects of EBR in Brassica juncea plants exposed to water deficit for 7 days and verified an increase in WUE after the application of 0.01 μM EBR. Anjum et al. (2011) studied the modulation of enzymatic antioxidants and leaf gas exchange and verified an increase in PN/Ci induced by the application of EBR in Zea mays plants after 6 days of water restriction.
The increase in enzyme activity (SOD, CAT, APX, and POX) in plants sprayed with 100 nM EBR revealed the intrinsic action of this molecule on the antioxidant metabolism. These increases in activity contributed to the reduction of the photoinhibitory damage caused to PSII (detected by lower NPQ), resulting in increases in Fv/Fm and ETR, ratified by the lower oxidative stress (MDA and EL). NPQ is a variable that measures the amount of absorbed light energy dissipated in heat (Ruban, 2016); the excess light often causes deleterious effects on the photosynthetic efficiency due to inefficient electron flux and ROS accumulation into chloroplasts that damages membranes. In other words, ROS removal by the antioxidant enzymes is essential to the adequate performance of the photosynthetic apparatus. Li et al. (2008) evaluated the effects of different concentrations of EBR on Robinia pseudoacacia seedlings exposed to three water regimes (75, 55, and 35% of the field capacity) for 10 days and detected peaks in SOD, CAT, and APX activities upon treatment with 0.2 mg L−1 EBR. Zhang et al. (2008) studied the adverse effects of water deficits on photosynthesis and the antioxidant metabolism of Glycine max after application of 0.1 mg L−1 of EBR in plants exposed to 7 days of water deficit and found increases of 29 and 30% for POX and SOD, respectively.
Decreases in O2− and H2O2 levels in plants sprayed with 100 nM EBR are related to the increases in the activities of SOD, CAT, APX, and POX enzymes promoted by the steroid (Figures 2 and 5). The lower level of O2− is associated with the increased activity of the SOD enzyme, which is responsible for converting O2− into H2O2 and O2 (Gill & Tuteja, 2010; Reyes Guerrero et al., 2015), while the reduction in H2O2 concentrations is linked to APX activity. This enzyme is responsible for dismutation of H2O2 into H2O and O2, contributing to cell detoxification and reducing oxidative stress (El-Beltagi & Mohamed, 2013; Gratão et al., 2005). Reduction in O2− level induced by the application of EBR was observed by Lima and Lobato (2017) with V. unguiculata plants exposed to water deficit for 2 days. Behnamnia, Kalantari, and Rezanejad (2009) evaluated the mitigation effects of exogenous EBR application on drought-induced oxidative stress in L. esculentum plants and reported significant reductions in H2O2 levels after the application of two concentrations of EBR (0.01 and 1 μM) in plants under water restriction for 3 and 5 days.
The reductions in MDA and EL levels in E. urophylla plants after treatment with EBR were directly connected to the lower accumulation of O2− and H2O2, influenced by the action of the antioxidant enzymes. O2− and H2O2 are highly reactive and toxic biomolecules that, in high concentrations, cause the structural and functional deterioration of membranes leading to lipid peroxidation in response to water deficit (Siddiqui et al., 2015; Yuan et al., 2010). In addition, EBR improves membrane structure and stability, decreasing lipid peroxidation (Rady, 2011). Li and Feng (2011) verified the effects of four concentrations of EBR (0.1, 0.2, 0.3, and 0.4 mg L−1) on Xanthoceras sorbifolia plants exposed to mild (12–13% soil moisture) and severe (7–8% soil moisture) water deficit and reported reductions in MDA and EL levels promoted by EBR. Zhu et al. (2014) confirmed the protective role triggered by BRs in Salvia miltiorrhiza plants under drought stress due to the synergistic effects of a more performant antioxidant system and membrane stability.
Increases in the Chl a, Chl b, total Chl, and Car levels of E. urophylla plants sprayed with EBR revealed a lower accumulation of ROS (O2− and H2O2), reducing the oxidative damages, benefiting the chloroplast membranes (Figure 5). In parallel, Ali et al. (2020) described that BRs protect plant cells against environmental stresses, including drought. These results were confirmed by the reductions in the MDA and EL levels previously described in this study, combined with increases in the biosynthesis of these pigments (Hayat et al., 2010). The lowest Chl a/Chl b and total Chl/Car ratios found after the application of EBR were related to the beneficial effects on Chl b and Car. Our results are similar to what observed in H. vulgare plants sprayed with three concentrations of EBR (0.01, 0.1, and 1 μM) and exposed to water deficit (PEG 6000 at 8%) for 15 days where Chl a, Chl b, total Chl, and Car increased 30, 59, 18, and 54%, respectively (Gill et al., 2017).
Increments in LDM, RDM, SDM, and TDM after the exogenous application of EBR suggested that this steroid probably accelerated cell division and expansion in leaves, stems, and roots, increasing the biomass. In agreement with Ahmad et al. (2018), this molecule plays a crucial role connected to growth and development in higher plants. In addition, EBR increased the growth based on the higher PN and enzymatic activities (SOD, CAT, APX, and GPX) previously verified in this study (Khalid & Aftab, 2016; Shahbaz et al., 2008). Talaat et al. (2015) studied the effects of dual application of 0.1 mg L−1 of EBR and 25 mg L−1 of spermine in two Z. mays hybrids under water deficit (50 and 75% of field capacity) for 30 days and found increases in RDM in both treatments (only EBR and EBR + spermine) related to the elevation of antioxidant enzyme activities and the improvement in the redox state of ascorbate and glutathione. Gomes et al. (2013) evaluated the effects of the analogue of BRs (Biobras 16) in two genotypes of Carica papaya plants exposed to 15 days of water restriction. They reported an increase in shoot biomass (leaf + stem) of the UC 01 genotype and suggested that BR improves the flux of photoassimilates and other compounds to the youngest leaves.
Based on our results and the ones from the literature, we propose an action mechanism triggered by the EBR in E. urophylla plants under drought stress (Figure 5). Pretreatment with this molecule clearly induced direct and indirect responses on chloroplasts, modulating positively antioxidant enzymes (SOD, CAT, APX, and POX), and consequently protecting the membranes against the oxidative stress generated by ROS as measured by the reductions in EL, MDA, O2−, and H2O2. Indirectly, this protective role alleviated the damages on chloroplastic pigments, improving the performance of the photosynthetic apparatus, more specifically the PSII efficiency (ΦPSII), electron flux (ETR), and CO2 fixation (PN).
5 CONCLUSIONS
This research confirmed the negative effects induced by water deficit on photosynthetic machinery, more specifically on absorption and utilization of light. Our results confirmed that the water deficit tolerance triggered by EBR in E. urophylla plants was associated with the enhancement on photosynthetic pigments, PSII efficiency, electron flux, and CO2 fixation, being explained by the increments detected in all antioxidant enzymes evaluated, contributing to minor accumulation of oxidative compounds. Our results revealed that 100 nM EBR gives the best results.
ACKNOWLEDGMENTS
This research had financial support from Fundação Amazônia de Amparo a Estudos e Pesquisas (FAPESPA/Brazil), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil), and Universidade Federal Rural da Amazônia (UFRA/Brazil) to Allan K. da Silva Lobato. Udson de Oliveira Barros Junior and Michael D. R. Lima were supported by scholarships from Universidade Federal Rural da Amazônia (UFRA/Brazil). The authors would like to extend their sincere appreciation to the Researches Supporting Project Number (RSP-2020/236) from King Saud University, Riyadi, Saudi Arabia.
AUTHOR CONTRIBUTIONS
Allan K. da Silva Lobato was the advisor of this project, planning all phases of the research, and critically revised the manuscript. Udson de Oliveira Barros Junior and Michael D. R. Lima conducted the experiment and performed the physiological, biochemical and morphological determinations, as well as wrote and edited the manuscript. Abdulaziz A. Alsahli critically revised and edited the manuscript. All authors read and approved the final version of the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
Data are available upon request from the corresponding author.




