Microsphere stem blockage as a screen for nitrogen-fixation drought tolerance in soybean
Abstract
Symbiotic nitrogen-fixation of soybean (Glycine max [Merr.] L) commonly decreases in response to soil drying in advance of other plant processes. While a few soybean lines express nitrogen-fixation drought tolerance, breeding for genetic variation is hampered by laborious phenotyping procedures. The objective of this research was to explore the potential of an initial screen for nitrogen-fixation drought-tolerant genotypes based on a possible relationship with xylem-vessel diameter. The hypothesis was that nitrogen-fixation drought tolerance might result from fewer, large-diameter xylem vessels in the stem that are vulnerable to disrupted flow as water deficit develops. The disrupted flow could cause nitrogen products to accumulate in nodules resulting in negative feedback on nitrogen-fixation rate. The proposed screen involved exposing de-rooted shoots to a suspension containing microspheres (45–53 μm diameter) and recording the decrease in transpiration rate as a result of microsphere xylem-blockage. Two soybean populations were tested. One population was progeny derived from mating of two parents with high and low nitrogen-fixation drought sensitivity. A high correlation (R2 = 0.68; P < 0.001) was found in this population between decreasing transpiration rate resulting from the microsphere treatment and increasing sensitivity of nitrogen-fixation to soil drying. The second tested population consisted of 16 genotypes, most of which had been previously identified in germplasm screens as expressing nitrogen-fixation drought tolerance. Nearly half of the lines in this second population were identified in the screen as showing minimum blockage of transpiration when exposed to the microspheres. Overall, these results showed the potential of using the microsphere screen to identify candidate genotypes expressing nitrogen-fixation drought tolerance.
Abbreviations
-
- FTSW
-
- fraction of transpirable soil water
-
- HIF
-
- heterogeneous inbred families
-
- NTR
-
- normalized transpiration rate
1 INTRODUCTION
Soybean (Glycine max L.) is one of the most economically important crops worldwide because its seeds have high concentrations of protein and oil, supporting food, feed, and industrial needs. The capacity of soybean to biochemically fix much of its required nitrogen in symbiosis with Bradyrhizobium eliminates the need for nitrogen fertilizer application. However, symbiotic nitrogen-fixation in soybean is highly vulnerable to drought. Decreases in nitrogen-fixation rates occur with soil drying at relatively high soil water content, well before other plant physiological processes such as transpiration and photosynthesis are decreased (King et al., 2014). A simulation study of the impact on soybean yields of nitrogen-fixation sensitivity to soil drying across the United States showed increased nitrogen-fixation drought tolerance could result in high probabilities of yield increase (Sinclair et al., 2010). However, methods to select soybean genotypes with superior nitrogen-fixation tolerance to soil drying are very laborious and time-consuming. For example, Sinclair et al. (2000) undertook a three-stage screening processes involving multi-years of field and greenhouse study. To expedite the screening process, a protocol is needed to identify genotypes more rapidly that have a high probability of expressing nitrogen-fixation drought tolerance.
A recent study by Nogueira et al. (2020) showed that genotype PI 471938, which expresses nitrogen-fixation drought tolerance (Devi & Sinclair, 2013), had fewer large diameter xylem vessels than commercial cultivar Hutcheson, which expresses the normal, high sensitivity of nitrogen-fixation to soil drying (Riar et al., 2018). Nogueira et al. (2020) suggested that smaller-diameter xylem vessels may have made the xylem less vulnerable to cavitation with soil drying. Sustained mass flow of N products from nodules, which in soybean are ureides, in the smaller-diameter xylem vessels under developing water-deficit would result in increased nitrogen-fixation drought tolerance. This is hypothesized to result since sustained transport of N products out of the nodules would allow plants to avoid the negative feedback on nitrogen-fixation rates resulting from the accumulation of nitrogen products in the nodule (Sinclair & Nogueira, 2018). This hypothesis leads to the possibility of screening for nitrogen-fixation drought tolerance based on fewer, large-diameter xylem vessels in soybean stems such as observed in PI471938.
Unfortunately, the microscopic approach used by Nogueira et al. (2020) to characterize vessel diameter is extremely time consuming such that it is not useful for genotypic screening. In this paper, a more expeditious method for screening for possible differences in stem xylem-vessel diameter was developed and applied to two soybean populations. The method explored in the current study was to expose de-rooted shoots to microspheres of a selected diameter that could enter large-diameter xylem vessels, but not smaller diameter vessels, and thereby cause at least partial blockage of xylem flow. Those genotypes with small-diameter xylem elements would express smaller decreases in transpiration rate than those with large-diameter, blocked xylem elements. The hypothesis, therefore, is that small decreases in transpiration rate following exposure to microspheres would indicate candidate genotypes for expressing nitrogen-fixation drought tolerance. The objective of this study was to determine if a microsphere screen fed to de-rooted soybean stems resulted in differences among genotypes in altered transpiration rate, and if so, do those differences correspond to differences among genotypes in the expression of nitrogen-fixation drought tolerance. Two population of soybean were studied that had been previously documented for their nitrogen-fixation drought sensitivity.
2 MATERIALS AND METHODS
2.1 Plant material
The initial study was done with a population consisting of 13 heterogeneous inbred families (HIF) that were developed from the cross of PI 471938 (nitrogen-fixation drought tolerant) × Hutcheson (nitrogen-fixation drought sensitive). At the F4 stage, individual progeny plants were selected based on heterogeneous molecular markers associated with wilting and yield in the field. Riar et al. (2018) characterized for all these HIF families the fraction of transpirable soil water (FTSW) breakpoint at which the decline in nitrogen-fixation activity was initiated with soil drying. The measurements were made in situ by flowing a 1:9 acetylene: air mixture through sealed pots for 15 min each day during 2-week, dry-down cycles (Riar et al., 2018). The FTSW breakpoint varied widely among genotypes from one that expressed substantial drought tolerance (FTSW breakpoint = 0.08) to one that expressed extreme drought sensitivity (FTSW breakpoint = 0.70).
A second population of genotypes was tested that was created from previous studies reporting genotypes expressing nitrogen-fixation drought tolerance. The purpose of the current research was to determine if some of these tolerant genotypes showed only small decreases in transpiration rates when exposed to the microspheres. The studied genotypes had been previously identified in three studies. Sinclair et al. (2000) undertook a sequence of screens in the field and greenhouse beginning with 3500 soybean lines that ultimately led to the identification of seven lines that exhibited nitrogen-fixation drought tolerance (Table 1). In a separate study, King et al. (2014) found two additional lines that showed especially high nitrogen-fixation tolerance to soil drying. In a comparison of advanced breeding lines, Devi et al. (2014) identified two lines with high tolerance of nitrogen-fixation to soil drying. Also, two lines were included in the current experiment that either did not express nitrogen-fixation drought tolerance (NC-Roy; Bellaloui et al., 2013) or its sensitivity was unknown (Holladay). A total of 16 soybean lines were compared.
| Genotype | Slope | N2 fixation measurement |
|---|---|---|
| PI 423890c | −0.00158 | King et al. (2014) |
| PI 227557 | −0.00161 | Sinclair et al. (2000) |
| PI 507039 | −0.00161 | Sinclair et al. (2000) |
| PI 471938 | −0.00163 | Riar et al. (2018) |
| PI 429328 | −0.00164 | Sinclair et al. (2000) |
| PI 548657 (Jackson) | −0.00168 | Sall and Sinclair (1991) |
| PI 423886 | −0.00172 | Sinclair et al. (2000) |
| PI 374163 | −0.00195 | Sinclair et al. (2000) |
| N04 9646 | −0.00206 | Bellaloui et al. (2013); Devi et al. (2014) |
| PI 222547 | −0.00212 | Sinclair et al. (2000) |
| NC Roya | −0.00213 | Bellaloui et al. (2013) |
| Holladaya | −0.00216 | None |
| PI 518664 (Hutcheson)a | −0.00246 | Riar et al. (2018) |
| PI 597477 | −0.00256 | King et al. (2014) |
| N 8002 | −0.00261 | Devi et al. (2014) |
| PI 578315B | −0.00282 | Sinclair et al. (2000) |
- a Either nitrogen-fixation drought sensitive or unknown sensitivity.
2.2 Microsphere test
The microsphere test was done on plants that had developed two fully expanded trifoliolate leaves (15 to 18 days after sowing). Individual plants were collected by cutting the stem below the cotyledonary node and quickly transferring the shoot into deionized water. The stem was cut a second time with a sharp blade above the cotyledonary node while holding the stem underwater. Each shoot was immediately placed in a 50-ml Erlenmeyer flask that was filled with deionized water. The flask was sealed with paraffin ‘M’ film (Pechiney Plastic Packaging) around the stem to avoid direct water evaporation. The sealed flasks were placed in an oscillating shaker bath with the bath temperature set at 30°C and 40 oscillations per minute, which provided a stable temperature during measurement and stirred the suspension of microspheres at the cut surface of the stem. The shaker water bath was positioned under a LED light source (SPYDER 600) providing 590 μmol m−2 s−1 of photosynthetically active radiation at plant height.
Due to the limited capacity of the shaker bath, a maximum of only 32 shoots could be tested at one time so the measurements had to be done in batches. Measurements were done on five replicates of each genotype in all tests. For the HIF population of 13 genotypes derived from the mating of PI 471938 × Hutcheson, each batch included up to five progeny genotypes plus three replicates of each of the parents. For the general population of 16 genotypes, each batch included up to six genotypes.
To confirm that microspheres actually entered xylem vessels when the de-rooted shoots were supplied with a microsphere suspension, the shoots were exposed to red microspheres that facilitated visual observation of the microspheres in the plant stem. After approximately a 200 min exposure to the microsphere suspension, a 2-cm piece of the stem was cut from the end that had been submerged in the suspension of microspheres. The tissue was fixed and blocked as described (Livingston III et al., 2009) without dehydration. The block was sectioned at 50 μm intervals and only the surface of the tissue remaining within the block (i.e., not the 50 μm section) was photographed. This non-conventional approach was required because the microspheres were likely to have been dislodged from the thin cross-sections. As a result, the surface images do not provide the same resolution expected of conventional thin section histology.
To document the impact of the microsphere treatment on transpiration rate, a reference transpiration rate was first measured for each shoot by supplying the de-rooted shoots with de-ionized water only. After placing the de-rooted shoots in water, the weight of the flask plus de-rooted shoot was measured every 30 min for about 180 min to determine reference transpiration rate for each shoot. Immediately following the determination of the reference transpiration rate, the de-rooted shoots were removed from the flasks containing the de-ionized water and transferred to 50-ml flasks containing a suspension of polyethylene microspheres (Cospheric LLC) in a mixture of water and Tween 80. Water loss rate from the de-rooted shoots was measured after transference to the microsphere suspension every 30 min for 210 to 240 min.
Preliminary studies with PI 471938 and Hutcheson showed that placing the stem of de-rooted shoots in a Tween solution of 0.2 ml L−1 sustained the stable transpiration rate for up to 4 h. Polyethylene microspheres (0.2 g L−1) of 45–53 μm diameter (Cospheric LLC) were suspended in the Tween 80 solution. The microsphere diameter was selected for possible difference in their uptake between PI 471938 and Hutcheson based on previous measurements of differences in the distribution of xylem-vessel diameter in the stems of these two genotypes (Nogueira et al., 2020). A difference in microsphere uptake between the two parent genotypes was expected to be reflected in differences in the decrease in transpiration rate following exposure to the microsphere.
The transpiration rates of each de-rooted shoot following exposure to the microspheres were normalized using its initial reference transpiration rate in water. Normalized transpiration rates (NTRs) for all replicate shoots of each genotype were combined to calculate the rate of decrease in NTR with time. Linear regression analysis was performed for each line beginning about 80 to 90 min after transferring the shoots to the microsphere mixture to determine the rate of decrease in transpiration rate (Figure 1). A decrease in transpiration rate was interpreted as indicative of xylem vessel blockage by the microspheres.
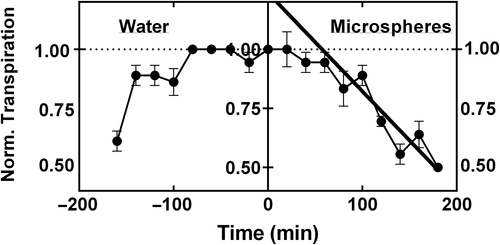
For the test of the HIF genotypes, the results of Riar et al. (2018) for the FTSW remaining in the soil at the initiation of decrease in nitrogen-fixation were plotted against the slope of decrease in transpiration rate following exposure to the microspheres. A linear regression was applied to this plot to determine if there was a relationship between the FTSW breakpoint in nitrogen-fixation rate and the normalized decrease in transpiration rate following exposure to microspheres.
3 RESULTS
To visually confirm that microspheres were taken into the xylem, de-rooted shoots were exposed to a suspension of red microspheres and subsequently observed for the presence of the microspheres in the xylem. In fact, the microspheres could be readily seen in the cut surface of the de-rooted stem. Figure 2 is an example showing the microspheres in a cross-section taken 0.135 mm from the original exposed end of the stem. These observations indicated the ability of the microspheres to enter and migrate at least in some of the xylem vessels. As the distance progressed farther from the original cut surface, there were fewer and eventually no observable microspheres in the cross sections. This is illustrated in Figure 2 by the surface cut at 0.80 mm from the original cut surface. The lack of microspheres at a greater distance from the original cut surface was taken as evidence that the microspheres did not pass through the vessel end plates.

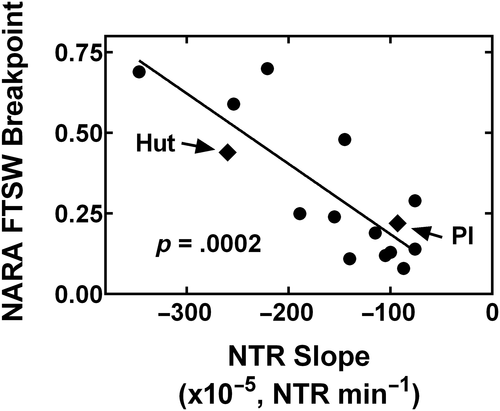
The measurements of water loss rate from the de-rooted shoots exposed to microspheres were done to indicate differences in the extent of xylem blockage by the microspheres. The initial test was on the population of the 13 HIF genotypes derived from the PI 471938 × Hutcheson cross that expressed wide diversity in nitrogen-fixation drought tolerance. This population also showed a wide range in the decrease in transpiration rate following the microsphere treatment from −0.00076 NTR min−1 to −0.00347 NTR min−1. Since the nitrogen-fixation responses to soil drying of these lines were all obtained under common experimental conditions (Riar et al., 2018), it was possible to plot the nitrogen-fixation breakpoint with soil drying against the decrease in transpiration following treatment with the microspheres (Figure 3). A strong, negative correlation (P = 0.0002) between the two variables indicated that those lines with the smaller decline in transpiration rate in response to feeding with microspheres had the greater nitrogen-fixation tolerance to drought. The regression, which included the two parents, was well described by a linear relationship (r2 = 0.678).
The second test was done on a population of genotypes and cultivars that were selected from previous studies for their nitrogen-fixation tolerance to soil drying. Among these lines, there was nearly a twofold range in the rate of decrease in transpiration (Table 1). Interestingly, there were roughly three groups expressing different decreases in transpiration rates following the microsphere treatment. One group, which included PI 471938, had the lowest range in decrease in transpiration from −0.00158 to −0.00172 NTR min−1. Seven of the 16 lines were in this grouping, indicating that these lines likely had a low number of large-diameter xylem vessels. All members of this group of seven had nitrogen-fixation tolerance to drought indicating a correspondence between nitrogen-fixation drought tolerance and relative insensitivity to the microsphere treatment.
At the other extreme of transpiration decrease following exposure to the microspheres, there were four lines with transpiration rate decreases of −0.00246 to −0.00282 NTR min−1 indicating substantial xylem blockage (Table 1). This group included Hutcheson, the nitrogen-fixation drought-sensitive line. There was an intermediate group, which included six genotypes between the two extreme groups, with decreases in transpiration rate of −0.00195 to −0.00216 NTR min−1.
4 DISCUSSION
Symbiotic nitrogen-fixation is a major advantage in soybean production worldwide by avoiding the necessity of applying nitrogen fertilizer to the crop. However, nitrogen-fixation in soybean is very sensitive to soil drying, with decreases in nitrogen-fixation rates occurring well before other physiological responses in the plant (King et al., 2014). This study was undertaken to explore the possibility that fewer, large-diameter vessels may be associated with nitrogen-fixation drought tolerance, and thereby offer a possible screening approach in genotype selection for nitrogen-fixation drought tolerance. The hypothesis for this study arose from the microscopic observations of Nogueira et al. (2020) that soybean PI 471938, a nitrogen-fixation drought-tolerant line, had smaller-diameter xylem vessels than Hutcheson, a nitrogen-fixation drought-sensitive line.
Since the microscopic evaluation of xylem anatomy is very laborious, an approach to evaluate xylem-vessel diameter by tracking transpiration rate following exposure of de-rooted shoots to microspheres was employed. In the HIF population, there was a linear, negative correlation between the nitrogen-fixation tolerance to drought and the decrease in transpiration rate following exposure to the microspheres (Figure 3). These results offered strong evidence of the negative impact of a larger number of large-diameter xylem vessels on soybean nitrogen-fixation tolerance to soil drying. Those genotypes with the smallest decreases in transpiration rate following the microsphere treatment consistently expressed the lowest FTSW breakpoint for nitrogen-fixation. Genotypes with slopes in transpiration decrease of −0.00120 NTR min−1 and higher had the lowest FTSW breakpoints (Figure 3).
The second population of soybean genotypes was used to test the ability of the microsphere blockage of transpiration to determine the possible utility of this protocol for screening the existence of nitrogen-fixation drought tolerance in a diverse population. Almost half (7 out of 16) of the genotypes in this population had low decreases in transpiration rate with exposure to microspheres (Table 1), all of which expressed nitrogen-fixation drought-tolerance. The genotypes in this nitrogen-fixation drought-tolerant group had slopes of −0.00172 NTR min−1 and higher, which is fully consistent with the observations for the tolerant genotypes in the HIF population (Figure 3).
At the other extreme, four lines in this second test population had substantial decreases in transpiration rates of −0.00246 NTR min−1 and lower. Based on the results of the HIF population (Figure 3), these lower slopes reflect vulnerability of nitrogen-fixation to drought stress. However, three (PI 597477, N8002, and PI 578315B) out of the four lines in this extreme group were originally identified as having nitrogen-fixation drought tolerance. This apparently conflicting result raises the possibility that nitrogen-fixation drought tolerance in these three lines might result from a mechanism apart from the hypothesized negative impact of large-diameter xylem vessels. Therefore, although the microsphere test showed a lack of transpiration blockage was consistently associated with nitrogen-fixation drought tolerance, the converse cannot be concluded. That is, transpiration blockage by microspheres does not necessarily reflect nitrogen-fixation drought sensitivity. In a screen, those genotypes that exhibited large decreases in response to the microsphere treatment might still be nitrogen-fixation drought tolerant, that is, false negatives in the screen.
Overall, microsphere results from the two studied populations were consistent with the hypothesis that fewer, large-diameter xylem vessels are advantageous in maintaining nitrogen-fixation rate at lower soil water contents. The regulation of nitrogen-fixation in nodules presented by Walsh et al. (1992) emphasized the importance of sustained transport from the nodules into the stem xylem to facilitate removal of N products from the nodule and avoidance of negative feedback of accumulation of nitrogen products in the nodule. The advantage of smaller-diameter vessels may be linked to the avoidance of cavitation in the xylem as hydraulic potential decreases with soil drying, and as a result, sustain higher xylem hydraulic conductance with soil drying. Hence, a population of xylem vessels that does not include larger-diameter vessels could hypothetically allow for sustained transport of nitrogen products from nodules as drought develops. Although the microsphere protocol was found to result in false negative identifications, in a large-scale plant screening effort the existence of false negatives, and hence a failure to select these genotypes, is generally expected in a breeding program.
ACKNOWLEDGMENT
This research was supported in part by a grant from the North Carolina Soybean Producers Association.
AUTHOR CONTRIBUTIONS
M.N. and T.S. conceived the experiment. D.P. developed the microsphere protocol. D.B. and D.P performed the transpiration measurements. D.L. did the micro cross-sections. T.C. provided the seeds and supported the micro cross-section experiment. T.S. drafted the manuscript with input from all other authors. All authors approved the final version of the paper.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




