Thiamin stimulates growth and secondary metabolites in turnip (Brassica rapa L.) leaf and root under drought stress
Edited by V. Hurry
Abstract
Thiamin, an important member of the vitamin B family, is believed to play a significant role in mitigating environmental stresses including drought stress. In turnip, drought stress causes a reduced growth, biomass yield, pigment content, total phenolics and ascorbic acid (AsA), particularly at 50% field capacity (F.C.) in the two cultivars (cv) studied. However, a significant enhancement was observed in the contents of leaf proline, glycinebetaine (GB), malondialdehyde (MDA), hydrogen peroxide (H2O2) and the activities of catalase (CAT) and superoxide dismutase (SOD) as well as root proline, GB, total phenolics, AsA, H2O2, MDA and the activities of peroxidase (POD) and SOD. However, foliar-applied thiamin significantly improved (particularly 100 mM) all the growth attributes, photosynthetic pigments, leaf and root osmoprotectants (GB and proline), AsA, total phenolics and the activities of enzymatic antioxidants such as SOD and POD as well as root CAT in both turnip cultivars under drought stress conditions. Foliar application of thiamin was effective in decreasing the leaf and root H2O2 and MDA content in both cultivars particularly at 50% F.C. Thiamin-induced growth of both turnip cultivars, particularly of cv. Purple Top, was found to be associated with increased photosynthetic pigments, proline and GB contents and antioxidant capacity, but reduced levels of reactive oxygen species (ROS) under water deficit conditions. So, it is suggested that exogenous application of thiamin can be effective in improving drought tolerance of plants.
Abbreviations
-
- AsA
-
- ascorbic acid
-
- CAT
-
- catalase
-
- cv
-
- cultivars
-
- F.C.
-
- field capacity
-
- GB
-
- glycinebetaine
-
- H2O2
-
- hydrogen peroxide
-
- MDA
-
- malondialdehyde
-
- POD
-
- peroxidase
-
- ROS
-
- reactive oxygen species.
-
- SOD
-
- superoxide dismutase
Introduction
Optimum water availability is one of the major ecological factors that affect the growth and development of plants (Ashraf et al. 2010, Jabeen et al. 2019). In contrast, if plants are subjected to water deficit conditions, they show stunted growth (Ahammed et al. 2020a, 2020b). In addition, water shortage causes considerable impairment in various plants physiological and biochemical processes such as hormonal metabolism, activities of key enzymes, synthesis/accumulation of ROS, stomatal regulation and other gas exchange characteristics (Kosar et al. 2015, Soltys-Kalina et al. 2016, Kamanga et al. 2018, Noori et al. 2018, Ahammed et al. 2020b).
It is now well established that drought stress induces overproduction of ROS that adversely affect the internal structure of plasma membrane, proteins, ribonucleic acid (RNA) and deoxyribonucleic acid (DNA), therefore, impeding the normal functioning of organelles (Gharibi et al. 2015, Kaya et al. 2015). To survive under stress-induced oxidative stress, plants possess various biochemical and molecular mechanisms for scavenging free radicals such as key antioxidant enzymes that is, catalase (CAT), superoxide dismutase (SOD), peroxidase (POD) and non-enzymatic antioxidants such as phenolics and ascorbic acid, so on. The upregulation of antioxidant defense system is associated with high drought tolerance (Ashraf 2009, Akram et al. 2018).
Thiamin (vitamin B1) belongs to the vitamin B group and thiamin pyrophosphate is a cofactor of energy production pathways including decarboxylation of alpha-keto acids (Goyer 2010). It also plays an essential role in the biosynthesis of fat and carbohydrates (Sajjad et al. 2017). Thiamin diphosphate (TDP) basically acts as a cofactor in many metabolic activities such as glycolysis, amino acid biosynthesis, Krebs cycle and the pentose phosphate pathway (Du et al. 2011). The assimilation of thiamin is a well-known phenomenon, taking place in leaves, seeds and roots. Thiamin imposes favorable effects on plant development and growth under environmental stresses (Kaya et al. 2015). An increase of vitamin B1 in tissue through exogenous application or through the enhancement of its biosynthesis could increase the antioxidant defense mechanism by involving the transketolase enzyme in the glycolytic cycle (El-Metwally and Sadak 2019). Thiamin plays a significant role in plant responses to abiotic stresses including drought (Dong et al. 2015, Toscano et al. 2019). Furthermore, exogenous application of thiamin increases its endogenous level conferring resistance to drought stress (Toscano et al. 2019).
Turnip (Brassica rapa L.) is a biennial herbaceous plant, belonging to the family Cruciferae (Paul et al. 2018). Turnip is native to Central Asia, Europe, Near East and Russia. It is widely cultivated as a potential vegetable and oil resource throughout the globe (Sun 2015). Turnip is also appreciated for its contents such as vitamin, carbohydrates, folate and minerals (calcium, iron, magnesium, manganese, copper, potassium and phosphorus). A significant reduction in turnip seed germination, yield formation and plant growth was observed under abiotic stress conditions (Mojarad et al. 2014, Tabatabaei and Larijani 2016). So, it was hypothesized that whether foliar-applied thiamin could minimize the drought-induced adverse effects on turnip plants. Keeping in view the efficiency of thiamin in enhancing stress tolerance, the present study was conducted to assess to what extent exogenously-applied thiamin could overcome the adverse effects of water deficiency on morphological, physiological and biochemical attributes in turnip plants.
Materials and methods
To evaluate the effect of exogenously-applied thiamin on plant growth, secondary metabolites and root quality of turnip (Brassica rapa L.) under different levels of drought stress, a pot experiment was conducted in a net house. The experiment was carried out from February through April 2019 in the Botanic Garden, Government College University Faisalabad, Pakistan under natural environmental conditions. There were a total of 54 plastic pots (diameter 30 cm) arranged in a completely randomized design with three replications of each treatment [drought stress (3 levels), foliar-applied thiamin (3 levels), cultivars (2)]. During this period, an average temperature of 18°C, relative humidity 59% and light period 10.9 h were recorded. The seeds of two turnip cultivars Purple Top and Golden Ball were obtained from the Vegetables Section of the Ayub Agricultural Research Institute, Faisalabad, Pakistan. Eight seeds were sown in each plastic pot filled with 8 kg sandy-loam soil. The soil had pH 8.7, EC 2.53 dS m−1, P 6.5 mg kg−1, K 4.40 mg kg−1, saturation percentage 34% and organic matter 0.83%. After seeds' germination, thinning was performed to maintain five seedlings per pot. Two weeks after seed germination (four-leaf stage), three water stress treatments [100% field capacity (F.C.) as control, 75% F.C. and 50% F.C.] were applied to the turnip plants. After 10 days, the required water stress levels were maintained. The different field capacities were optimized on the basis of soil saturation percentage. The drought stress treatments continued up to 4 weeks. After 30 days of water-deficit conditions, three levels [0 mM (control, distilled water), 50 and 100 mM)] of thiamin (MP Biomedical Inc.) prepared in distilled water along with Tween-20 (0.1%) were applied to all turnip plants (except the controls) as a foliar spray using a plastic hand pump. A total of 25 ml thiamine solution (of each thiamin level) were applied per pot and per treatment (Ghafar et al. 2019).
Growth attributes
Ten days after foliar application of thiamin, two plants were harvested from each pot. Root and shoot fresh and dry weights as well as root and shoot lengths were recorded. For dry weights, roots and shoots were initially air-dried for 4 days following oven-drying for 3 days at 70°C.
The remaining three plants were sampled and preserved in an ultra-low freezer at −20°C to determine the following primary and secondary metabolites of turnip leaves and roots.
Chlorophyll contents
The youngest leaf from the top (0.5 g) was ground in 10 ml of 80% acetone (Sigma-Aldrich) following the method of Arnon (1949). After centrifugation at 10 000g for 10 min, the absorbance was recorded at 645 and 663 nm using an Ultra Violet-visible spectrophotometer (Model Hitachi-U 2001).
Free proline contents
Following Bates et al. (1973), fresh leaf and root samples (each 0.5 g) were separately grinded in 10 ml of 3% (w/v) sulfosalicylic acid (MP, Biomedicals, Inc). Then, 2 ml of the leaf and root extract, 2 ml ninhydrin acid and 2 ml of glacial acetic acid were mixed in a test tube. All samples were placed in a water bath for 1 h at 100°C. After cooling, 3 ml of toluene were added to the solution. Then two layers were formed. The absorbance of the upper layer was recorded at 520 nm using a spectrophotometer. A standard curve was drawn using varying levels of free proline ranging from 1.0 to 10 mg l−1.
Glycinebetaine contents
Each of the fresh leaf and root samples (each 0.5 g) was extracted in 10 ml toluene (0.5%). The mixture was kept for overnight at 4°C. After centrifugation, 1 ml of the extract was taken in a test tube and 1 ml of 2 N sulfuric acid (H2SO4) was added. Then, 0.5 ml of this solution was shaken with a vortex mixer and 0.2 ml of potassium tri-iodide solution (KI3) was added. The contents were shaken again, cooled in an ice bath for 90 min and then 5 ml of 1, 2 dichloroethane were added. As a result of this, two layers were formed. The upper layer was discarded, and the absorbance of the lower layer was recorded at 365 nm using a spectrophotometer following Grieve and Grattan (1983). Different concentrations of glycine betaine (1.0–10 mg l−1) were used to draw a standard curve.
Hydrogen peroxide
Following Velikova et al. (2000), fresh leaf and root samples (0.25 g) were separately extracted with 5 ml of trichloroacetic acid [TCA (w/v) 0.1%] in a pre-chilled mortar and pestle, and centrifuged at 12 000 g for 15 min. To 0.5 ml of the supernatant in a test tube, 1 ml of potassium iodide (KI) and 0.5 ml of potassium phosphate buffer (pH 7.8) were added and the mixture was shaken vigorously. Thereafter, the absorbance of the colored mixture was read at 390 nm using a spectrophotometer and H2O2 contents were determined using different concentrations of H2O2 to obtain a standard curve.
Malondialdehyde
The method proposed by Cakmak and Horst (1991) was used to determine the MDA content of turnip leaves and roots. The leaf and root samples (0.25 g) were homogenized in 3 ml of TCA (1%; w/v). The mixture was centrifuged at 12 000 g for 15 min and then 4 ml of 0.5% (w/v) of thiobarbituric acid (MW 144.5, Sigma-Aldrich GmbH) were mixed in 1.0 ml of the supernatant. The mixture was incubated for 50 min in a water bath at 95°C, cooled in chilled water for 15 min and absorbance recorded at 532 and 600 nm using a spectrophotometer.
Total phenolics
According to the method of Julkunen-Tiitto (1985), total phenolics in the leaf and root tissues were determined using the Folin-Ciocalteau phenol reagent (FCPR; MP Biomedicals). Fresh material (each 0.1 g) was homogenized in 3 ml of 80% acetone. After centrifugation at 10 000g, 1 ml of the FCPR, 2 ml of distilled water and 1 ml of supernatant were mixed in a test tube. After mixing the solution, 5 ml of sodium carbonate (20%) were added and the volume was complemented to 10 ml using distilled water. The absorbance was recorded at 750 nm using a spectrophotometer.
Ascorbic acid
According to the protocol of Mukherjee and Choudhuri (1983), leaf and root tissues (0.25 g) were ground in 10 ml trichloroacetic acid (6%) solution. Then, 4 ml of the supernatant, 2 ml (2%) of dinitrophenyl hydrazine and one drop of thiourea were mixed in a test tube. This solution was kept in a water bath at 100°C for 15 min, and then the mixture was cooled at room temperature. After cooling, 5 ml of H2SO4 were added to it and recorded the absorbance at 530 nm on a spectrophotometer.
Antioxidant enzymes
In a pre-chilled pestle and mortar, fresh leaf and root samples (each 0.25 g) were separately homogenized in 10 ml of 50 mM potassium phosphate buffer (pH 7.8). After centrifugation at 15 000 g, the supernatant was used for the determination of the activities of the following antioxidant enzymes on the basis of total soluble proteins determined following the Bradford (1976) assay.
Activity of CAT enzyme
According to the protocol of Chance and Maehly (1955), the reaction mixture contained 0.1 ml of the extract, 1.9 ml potassium phosphate buffer (5.9 mM; pH 7.0) and 1.0 ml hydrogen peroxide (100 mM). Then, the absorbance of the reaction mixture was recorded at 240 nm using a spectrophotometer.
Activity of POD enzyme
The method proposed by Chance and Maehly (1955) was used to determine the activity of POD enzyme. The leaf and root extracts (0.1 ml) were mixed with (1 ml) hydrogen peroxide, 20 mM guiacol (100 μl) and 5.9 mM potassium phosphate buffer (1.9 ml; pH 5.0). Then the absorbance was measured at 470 nm for 180 fsec using a spectrophotometer.
Activity of SOD enzyme
According to the protocol of Giannopolitis and Ries (1977), each reaction mixture contained 75 μM EDTA, 13 mM L-methionine (100 μl), enzyme extract (0.1 ml), 20 mM potassium phosphate buffer (pH 7.8), riboflavin (1.3 μM) and nitroblue tetrazolium (50 μM). Then, the absorbance of the mixture was read at 560 nm using a spectrophotometer.
Statistical analysis
Analysis of variance (ANOVA) of data for each of all the above-mentioned attributes was carried out to test the effects of thiamin and drought stress levels on the turnip plants using the COSTAT statistical software (Costat v6.303). The least significant differences were calculated at 5% probability between the mean values of each treatments and replicates.
Results
Growth attributes
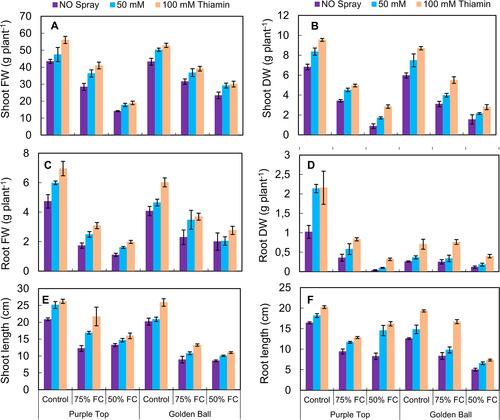
Both turnip cultivars (Golden Ball and Purple Top) were subjected to control [100% field capacity (F.C.)] and water deficit regimes (75 and 50% F.C.). Under drought stress conditions, particularly at 50% F.C., a significant (P ≤ 0.001) decrease (67.01%) was observed in shoot and root fresh and dry weights of both turnip cultivars. However, foliar application of thiamin significantly (P ≤ 0.001) improved all growth attributes and a maximum increase (46%) was observed in both turnip cultivars at 100 mM of thiamin under control and water-deficit (75 and 50% F.C.) conditions (Fig. 1). Of both turnip cultivars, cv. Purple Top performed better in terms of shoot fresh and root dry weights at 100% F.C. While at 50% water deficit conditions, cv. Golden Top was better in shoot fresh and root dry weights. However, both turnip cultivars were similar in terms of shoot dry weight and root fresh weight under varying (75 and 50% F.C.) water stress and thiamin regimes (Fig. 1, Table 1).
| Source of variation | df | Shoot fresh weight | Shoot dry weight | Root fresh weight | Root dry weight | Shoot length |
|---|---|---|---|---|---|---|
| Cultivars (Cvs) | 1 | 259.6*** | 0.297 ns | 0.444 ns | 3.406*** | 316.5*** |
| Drought (D) | 2 | 439.7*** | 218.9*** | 81.79*** | 5.536*** | 843.8*** |
| Thiamin (T) | 2 | 557.2*** | 28.98*** | 13.71*** | 1.704*** | 164.8*** |
| Cvs × D | 2 | 209.2*** | 1.207** | 4.393*** | 3.235*** | 38.18*** |
| Cvs × T | 2 | 12.16 ns | 0.006 ns | 0.069 ns | 0.216* | 7.581 ns |
| D × T | 4 | 25.69 ns | 1.046** | 0.956 ns | 0.237** | 12.78** |
| Cvs × D × T | 4 | 10.76 ns | 0.413 ns | 0.202 ns | 0.155 ns | 9.831* |
| Error | 54 | 14.01 | 0.225 | 0.481 | 0.063 | 2.911 |
| Root length | Chl a | Chl b | Chl a/b ratio | Total chlorophyll | ||
|---|---|---|---|---|---|---|
| Cultivars (Cvs) | 1 | 164.3*** | 0.327* | 0.006 ns | 1.854 ns | 0.239 ns |
| Drought (D) | 2 | 368.5*** | 0.755*** | 1.165*** | 0.901 ns | 3.661*** |
| Thiamin (T) | 2 | 200.4*** | 1.435*** | 0.171 ns | 0.506 ns | 2.597*** |
| Cvs × D | 2 | 65.33*** | 0.032 ns | 0.006 ns | 0.471 ns | 0.059 ns |
| Cvs × T | 2 | 3.665 ns | 0.0316 ns | 0.006 ns | 0.062 ns | 0.066 ns |
| D × T | 4 | 6.922** | 0.149* | 0.081 ns | 0.131 ns | 0.394* |
| Cvs × D × T | 4 | 14.49* | 0.058 ns | 0.007 ns | 0.081 ns | 0.055 ns |
| Error | 54 | 1.959 | 0.058 | 0.077 | 0.374 | 0.117 |
| Proline | GB | Ascorbic acid | MDA | H2O2 | ||
|---|---|---|---|---|---|---|
| Cultivars (Cvs) | 1 | 13.02*** | 9092*** | 27.91*** | 42.53* | 21.39*** |
| Drought (D) | 2 | 5.171*** | 482.3*** | 11.31* | 321.6*** | 16.24*** |
| Thiamin (T) | 2 | 6.655*** | 928.9*** | 21.11 *** | 69.53*** | 49.31*** |
| Cvs × D | 2 | 0.505 ns | 440.9*** | 22.93*** | 166.4*** | 40.18*** |
| Cvs × T | 2 | 0.685 ns | 14.72 ns | 5.713 ns | 9.629 ns | 19.07*** |
| D × T | 4 | 0.962 ns | 23.81 ns | 1.597 ns | 6.456 ns | 2.807 ns |
| Cvs × D × T | 4 | 0.044 ns | 140.2* | 4.101 ns | 6.245 ns | 2.237 ns |
| Error | 54 | 0.456 | 45.01 | 2.302 | 7.994 | 1.346 |
| Phenolics | SOD | Catalase | POD | |||
|---|---|---|---|---|---|---|
| Cultivars (Cvs) | 1 | 1702*** | 19.79* | 0.011 ns | 311.4* | |
| Drought (D) | 2 | 1736*** | 185.6*** | 27.95*** | 167.2 ns | |
| Thiamin (T) | 2 | 894.7*** | 185.6*** | 2.353 ns | 229.2* | |
| Cvs × D | 2 | 263.9*** | 109.1*** | 4.901** | 229.2 ns | |
| Cvs × T | 2 | 412.1*** | 35.71*** | 0.766 ns | 51.62 ns | |
| D × T | 4 | 34.45 ns | 31.84*** | 0.602 ns | 58.13 ns | |
| Cvs × D × T | 4 | 33.54 ns | 54.191*** | 0.243 ns | 80.53 ns | |
| Error | 54 | 30.11 | 2.899 | 0.945 | 63.34 |
- ns, non-significant; df, degree of freedom. *, ** and *** = significant at 0.05, 0.01 and 0.001 levels, respectively.
Shoot and root lengths were adversely (P ≤ 0.001) affected at 75 and 50% F.C. (Fig. 1). However, thiamin application as a foliar spray significantly improved (45.05%) the shoot and root lengths of both turnip cultivars under varying water regimes. Of both turnip cultivars, cv. Purple Top was better than the other cultivar in shoot length and root length under water-deficit conditions.
Leaf characteristics
Chlorophyll a, b and total chlorophyll contents were drastically (P ≤ 0.001) affected by 75 and 50% F.C. However, thiamin application had a significant (P ≤ 0.001) positive effect on chlorophyll a and total chlorophyll contents of both turnip cultivars (Fig. 2). Chlorophyll a content was slightly (P ≤ 0.05) better in cv. Purple Top than Golden Top, while the response of both turnip cultivars was almost similar for all other photosynthetic pigments measured in the present study.
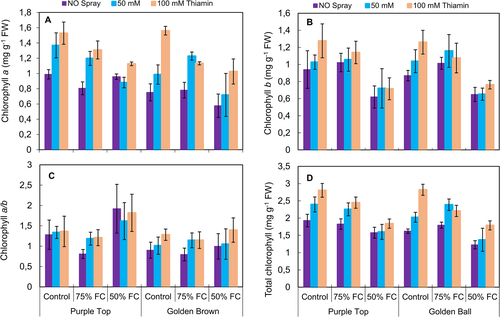
A significant (P ≤ 0.001) increase was observed in leaf free proline and glycinebetaine (GB) contents under varying (75 and 50% F.C.) water deficit levels. Exogenously-applied thiamin, particularly at the concentration of 100 mM, also significantly (P ≤ 0.001) improved the proline and GB contents in both turnip cultivars. Of both turnip cultivars, cv. Purple Top had a relatively higher content in osmoprotectants under stress and non-stress conditions (Fig. 3).
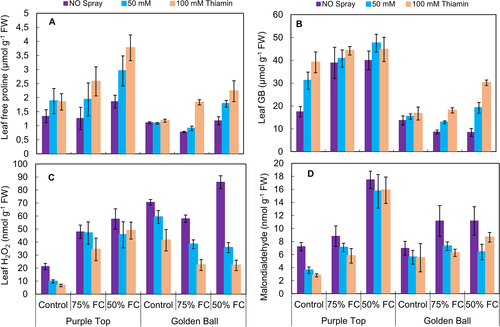
Leaf H2O2 and MDA contents increased significantly in both turnip cultivars at 75 and 50% F.C. The application of thiamin was effective in decreasing the H2O2 and MDA contents, particularly at 50% F.C. Overall, cv. Golden Ball was lower than the other cultivar in accumulation of H2O2 and MDA contents at 75 and 50% F.C. (Fig. 3).
The total phenolics decreased significantly (P ≤ 0.001) at 75 and 50% F.C. Foliar-applied thiamin had a significant increasing effect on total phenolics and a maximum improvement in total phenolics was observed at 100 mM thiamin under control and stress conditions (Fig. 4A). Of both turnip cultivars, cv. Purple Top accumulated more total phenolics under stress and non-stress regimes.
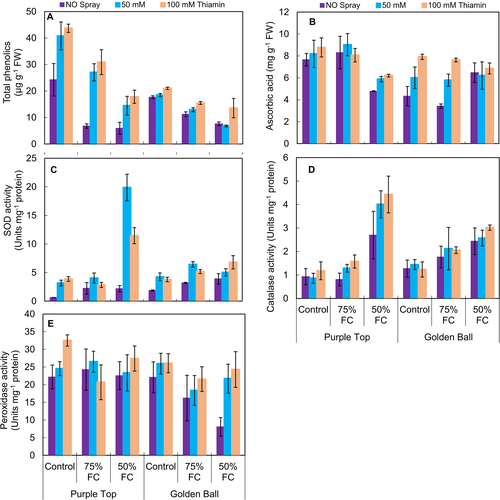
No change was observed in ascorbic acid (AsA) contents of cv. Golden Ball, while a significant decrease was observed in turnip cv. Purple Top under drought stress conditions (75 and 50% F.C.; Fig. 4B, Table 1). However, exogenous application of thiamin significantly increased (P ≤ 0.001) the ascorbic contents in both turnip cultivars. Of both turnip cultivars, cv. Golden Ball accumulated more AsA contents than that by the other cultivar at 75 and 50% F.C. (Fig. 4B).
The activities of superoxide dismutase and catalase enzymes enhanced (P ≤ 0.001) considerably, while no change was observed in peroxidase under water deficit conditions (both 75 and 50% F.C.). However, foliar-applied thiamin significantly improved only the activities of SOD and POD, while no change was observed in the activity of CAT under varying water regimes. Of both turnip cultivars, Purple Top was superior to the other cultivar in SOD and POD activities (Fig. 4D,E).
Root characteristics
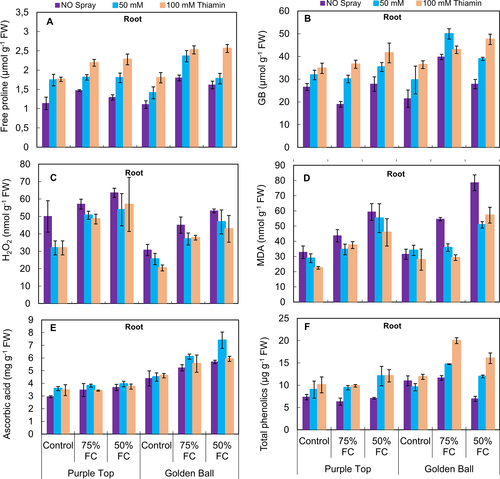
It was observed that under water-limited conditions, root proline and GB contents increased significantly (P ≤ 0.001). Foliar-applied thiamin considerably improved the GB and proline contents in both turnip cultivars under control and water deficit conditions (Fig. 5A,B, Table 2). Of both cultivars, cv. Golden Ball was higher than the other line in these root attributes under different water and thiamin regimes.
| Source of variation | df | Proline | GB | Total phenolics | Ascorbic acid |
|---|---|---|---|---|---|
| Cultivars (Cvs) | 1 | 0.681*** | 663.6*** | 221.2*** | 68.25 *** |
| Drought (D) | 2 | 1.499*** | 243.5*** | 24.27** | 7.377*** |
| Thiamin (T) | 2 | 4.101*** | 1175*** | 148.6*** | 2.926** |
| Cvs × D | 2 | 0.226** | 364.1*** | 54.71*** | 2.548** |
| Cvs × T | 2 | 0.043 ns | 27.11 ns | 15.86* | 0.512 ns |
| D × T | 4 | 0.087 ns | 24.89 ns | 17.27** | 0.325 ns |
| Cvs × D × T | 4 | 0.088 ns | 105.9** | 6.874 ns | 0.468 ns |
| Error | 54 | 0.043 | 24.28 | 4.351 | 0.464 |
| H2O2 | DA | SOD | |||
| Cultivars (Cvs) | 1 | 2641*** | 276.5 ns | 0.004 ns | |
| Drought (D) | 2 | 2968*** | 5132*** | 5.668*** | |
| Thiamin (T) | 2 | 781.2** | 1200*** | 14.83*** | |
| Cvs × D | 2 | 15.78 ns | 104.3 ns | 2.274* | |
| Cvs × T | 2 | 20.67 ns | 161.5 ns | 1.057 ns | |
| D × T | 4 | 32.11 ns | 114.3 ns | 0.485 ns | |
| Cvs × D × T | 4 | 28.28 ns | 170.1 ns | 0.554 ns | |
| Error | 54 | 139.8 | 91.86 | 0.593 |
| CAT | POD | ||||
|---|---|---|---|---|---|
| Cultivars (Cvs) | 1 | 0.187 ns | 159.4*** | ||
| Drought (D) | 2 | 0.462 ns | 48.59** | ||
| Thiamin (T) | 2 | 7.261*** | 799.2*** | ||
| Cvs × D | 2 | 1.343 ns | 53.53*** | ||
| Cvs × T | 2 | 1.741 ns | 18.05 ns | ||
| D × T | 4 | 1.146 ns | 51.28*** | ||
| Cvs × D x T | 4 | 1.163 ns | 18.26* | ||
| Error | 54 | 0.551 | 6.511 |
- ns = non-significant; *, ** and *** = significant at 0.05, 0.01 and 0.001 levels, respectively.
Hydrogen peroxide and malondialdehyde contents in turnip roots increased significantly (P ≤ 0.001) in both cultivars at 75 and 50% F.C. Foliar application of thiamin effectively decreased the H2O2 and MDA contents particularly at 50% F.C. conditions. Overall, cv. Purple Top was higher than the other line in H2O2 and MDA contents at 75 and 50% F.C. (Fig. 5C,D).
A significant increase was observed in root AsA and total phenolics contents under both drought stress conditions (75 and 50% F.C.) and exogenously applied thiamin (Fig. 5E, F). Of both turnip cultivars, cv. Golden Ball was superior to the other cultivar in terms of total phenolics and AsA accumulation under stress and non-stress conditions.
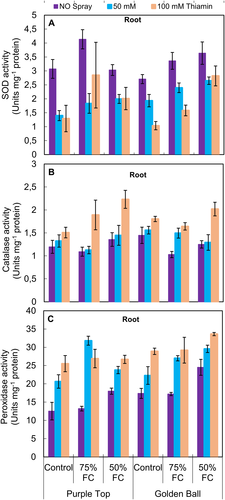
A significant (P ≤ 0.001) increase was observed in the activities of SOD and POD, while no change was observed in the activity of CAT at 75 and 50% F.C. However, foliar-applied thiamin significantly (P ≤ 0.001) improved the activities of CAT and POD, while a significant decrease was observed in SOD at 75 and 50% F.C. (Fig. 6A,C)). Of both turnip cultivars, cv. Golden Ball was superior in POD activity, while both cultivars had similar activities of SOD and CAT under varying water regimes.
Discussion
To attain optimum growth and yield production, plants require both water and nutrients in the proper amount to fulfill their basic needs. Under water-limited conditions, a significant reduction in plant growth and yield production has been observed in different crops, for example, canola (Shafiq et al. 2014), cauliflower (Latif et al. 2016), maize (Shafiq et al. 2018), sunflower (Kosar et al. 2018) and mungbean (Sadiq et al. 2019). In all these studies, it has been reported that water stress-induced reduction in plant growth can be related to oxidative stress, nutritional imbalance, hormonal alterations, suppression/deterioration of proteins, deactivation of enzymes and disturbance in secondary metabolism. In the present study, shoot and root fresh and dry weights of both turnip cultivars decreased significantly, particularly at 50% F.C. But foliar-applied thiamin at the concentration of 50 and 100 mM significantly improved plant growth under stress as well as non-stress conditions. Vitamins (thiamin) particularly of group B are the precursors of metabolic cofactors for enzymes and co-enzymes, but they are commonly destroyed in plants upon exposure to abiotic stresses for short or long periods (Hanson et al. 2016, Sanjari et al. 2019). So, stress-induced deficiency in the accumulation of vitamins is one of the primary symptoms for metabolic knock-on under water deficiency (Hanson et al. 2016, Sanjari et al. 2019). In a previous study with white clover (Trifolium repens L.), Ghafar et al. (2019) reported that foliage spray of 50 mM thiamin improved plant growth under drought stress conditions. They attributed this growth improvement to thiamin-induced increase in the photosynthetic pigments and total phenolics of white clover plants under water deficit conditions.
Photosynthetic pigments (chlorophyll a and b) play a significant role in plant growth and development (Ashraf and Harris 2013). Abiotic stresses adversely influence the synthesis of chlorophyll pigments and their utilization in photosynthesis, a vital physiological process. Accumulation of chlorophyll pigments, their fluorescence and efficiency of photosystems can be used as indicators of stress tolerance in a number of plant species (Ashraf et al. 2007, Ashraf and Harris 2013). Drought-induced reduction in chlorophyll concentration has been considered as a general indicator of oxidative stress and could be the result of photosynthetic pigment degradation or pigment photooxidation (Anjum et al. 2011). In the present study, chlorophyll a and b contents decreased in both cultivars under both water-deficit regimes (75 and 50% F.C.). In addition, exogenous application of thiamin improved the chlorophyll pigments in both turnip cultivars under stress and non-stress conditions. Likewise, in another study, foliar-applied thiamin enhanced the chlorophyll content at 100 mM in two cultivars of white clover under water-deficit conditions (Ghafar et al. 2019). An earlier study by Alipoor and Mohsenzadeh (2012) showed that thiamin improved chlorophyll pigments in Aloe vera under nickel stress and in sunflower under saline conditions (Sayed and Gadallah 2002).
Under stress conditions, production of ROS as well as accumulation/synthesis of osmoprotectants are very common phenomena (Ashraf and Foolad 2007). Long or short exposure to drought is believed to initiate oxidative stress in plant species. In reaction to it, plants have the ability to trigger their defense system including osmoprotection (Ashraf and Foolad 2007). So, the better growth of cv. Purple Top compared with the other cultivar can be ascribed to high accumulation of proline and GB contents. Moreover, osmoprotection safeguards the plants from overaccumulation of ROS as high concentration of ROS results in reduced plant growth and yield production (Ashraf 2009). In the present study, production of H2O2 and MDA increased significantly in both leaves and roots of both turnip cultivars upon drought stress. However, exogenous application of thiamin was effective in lowering the oxidative stress both in leaves and roots by enhancing the antioxidants content (AsA and total phenolics) and the activities of antioxidant enzymes (SOD, POD and CAT) in both turnip cultivars under drought stress conditions. It is somewhat similar to what has earlier been observed by Ghafar et al. (2019) in white clover, where ascorbic acid concentration increased in both white clover cultivars under water deficit conditions. However, in contradiction to the present study, thiamin spray did not improve these contents in the white clover plants. Nevertheless, analogous to our results, foliar spray of vitamin B increased the endogenous levels of AsA in flax plants (Emam et al. 2011). In addition, Kaya et al. (2015) reported that thiamin application reduced the MDA and H2O2 contents and improved growth of maize plants under drought stress conditions. Thiamin acts as an antioxidant as it has O2▪−/▪OH scavenging potential (Ahn et al. 2007). The antioxidant properties of thiamin were reported in a study on Arabidopsis where paraquat-treated plants showed reduction in protein carbonyls and dichlorofluorescein diacetate (indicator of oxidative stress) on exposure to thiamin (Tunc-Ozdemir et al. 2009). Moreover, thiamin monophosphate triggered the defense responses in plants (Zhou et al. 2013). Previously, exogenous application of thiamin to wild-type Arabidopsis plants showed a significant tolerance to oxidative stress induced by paraquat (Tunc-Ozdemir et al. 2009). Thiamin-induced tolerance to oxidative stress was accompanied by a decreased production of ROS in plants, such as reduced protein carbonylation and H2O2 accumulation (Tunc-Ozdemir et al. 2009).
Overall, a significant reduction was noted in shoot and root fresh and dry weights, root and shoot lengths, chlorophyll a, b and total chlorophyll, total phenolics and AsA contents in both turnip cultivars under water stress (50% F.C.). A significant increase was observed in leaf proline, glycinebetaine, H2O2, MDA contents and the activities of SOD and CAT enzymes as well as root proline, GB, total phenolics, AsA, H2O2, MDA and the activities of SOD and POD enzymes. However, foliar-applied thiamin, particularly 100 mM, significantly improved the growth, photosynthetic pigments, leaf and root of osmoprotectants (GB, proline), AsA, total phenolics, activities of antioxidants and root CAT in both turnip cultivars under drought stress conditions. The higher growth of both turnip cultivars, specifically of cv. Purple Top, was found to be associated with increased photosynthetic pigments, GB and proline contents, improved antioxidant capacity and reduced amount of ROS under water deficit regimes. Overall, exogenous application of thiamin was found to be effective in the present investigation conducted under semi-controlled conditions. However, some large-scale field trials need to be conducted to work out the efficiency and cost–benefit ratio of thiamin application.
Author Contributions
N.A.A., M.A. designed the experimental structure and M.J. and N.A.A. performed the experiments. M.N.A. and P.A. analyzed the data. N.A.A., M.A. and P.A. critically revised the manuscript to the current form. All authors have read and agreed to the published version of the manuscript.
Acknowledgements
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP-2020/180), King Saud University, Riyadh, Saudi Arabia.
Open Research
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




