Nitrate enhancement of CAM activity in two Kalanchoë species is associated with increased vacuolar proton transport capacity
Abstract
Among species that perform CAM photosynthesis, members of the genus Kalanchoë have been studied frequently to investigate the effect of environmental factors on the magnitude of CAM activity. In particular, different nitrogen sources have been shown to influence the rate of nocturnal CO2 fixation and organic-acid accumulation in several species of Kalanchoë. However, there has been little investigation of the interrelationship between nitrogen source (nitrate versus ammonium), concentration and the activity of the vacuolar proton pumps responsible for driving nocturnal organic-acid accumulation in these species. In the present study with Kalanchoë laxiflora and Kalanchoë delagoensis cultivated on different nitrogen sources, both species were found to show highest total nocturnal organic-acid accumulation and highest rates of ATP- and PPi-dependent vacuolar proton transport on 2.5 mM nitrate, whereas plants cultivated on 5.0 mM ammonium showed the lowest values. In both species malate was the principal organic-acid accumulated during the night, but the second-most accumulated organic-acid was fumarate for K. laxiflora and citrate for K. delagoensis. Higher ATP- and PPi-dependent vacuolar proton transport rates and greater nocturnal acid accumulation were observed in K. delagoensis compared with K. laxiflora. These results show that the effect of nitrogen source on CAM activity in Kalanchoë species is reflected in corresponding differences in activity of the tonoplast proton pumps responsible for driving sequestration of these acids in the vacuole of CAM-performing cells.
Abbreviations
-
- ATP
-
- adenosine triphosphate
-
- BTP
-
- bis-TRIS-propane
-
- CAM
-
- crassulacean acid metabolism
-
- DW
-
- dry weight
-
- MDH
-
- malate dehydrogenase
-
- NR
-
- nitrate reductase
-
- OAA
-
- oxaloacetate
-
- PEPC
-
- phosphoenolpyruvate carboxylase
-
- PPi
-
- inorganic pyrophosphate
Introduction
Crassulacean acid metabolism (CAM) is a photosynthetic pathway characterized by nocturnal CO2 fixation performed by the enzyme phosphoenolpyruvate carboxylase (PEPC), the accumulation of organic-acids during the night (mainly in the form of malic acid), and a high efficiency of water use (Osmond 1978, Cushman 2001, Borland et al. 2011, 2014). At least 343 genera from 35 plant families are capable of performing CAM photosynthesis (Smith and Winter 1996, Silvera et al. 2010). Among these genera, Kalanchoë species from the eponymous family Crassulaceae have been one of the largest groups studied (Kluge and Ting 1978, Osmond 1978, Widmann et al. 1990, 1993, Kluge et al. 1991, Santos and Salema 1991). It has been suggested that most species from the genus Kalanchoë are able to perform CAM, and also a correlation has been established between the capacity to perform CAM and the climate (especially aridity) of the habitats where the individual species grow naturally (Kluge et al. 1991).
The influence of nutrient availability on the expression of CAM photosynthesis has been relatively little studied, but nitrogen supply is one factor thought to affect the CAM activity. Kalanchoë blossfeldiana has been investigated in several studies to determine how nitrogen availability affects CAM photosynthesis (Ota 1988a, 1988b, Ota and Yamamoto 1991), and it has been observed that the expression of CAM photosynthesis was greater in plants supplied with 1.0 mM nitrate compared with 1.0 mM ammonium. However, this species exhibited the highest CAM expression when both nitrogen sources were removed from the nutrient solution (nitrogen starvation) and a water deficit was imposed (Ota 1988a), suggesting that N-depletion is an important factor in maximizing CAM performance in K. blossfeldiana. In another study with K. blossfeldiana, plants cultivated with 5.0 mM nitrate showed higher CAM activity compared with those cultivated with 5.0 mM ammonium in the absence of imposition of water deficit (Ota 1988b), suggesting that both nitrogen source and concentration are important factors influencing CAM expression in Kalanchoë species. In Kalanchoë pinnata, another much investigated CAM species, growth over a wider range of different nitrate concentrations (0.6–24.0 mM) showed that plants on higher nitrate concentrations possessed higher PEPC activity when compared with plants on lower nitrate concentrations (Winter et al. 1982). All these studies support the idea that both the chemical source of inorganic nitrogen sources and the concentration of available nitrogen influence the magnitude of CAM expression.
On compartmental grounds, and in order to maintain cytoplasmic homeostasis, almost all the malic acid synthesized in CAM plants as a result of dark CO2 fixation must be transported into the vacuole at night and remobilized in the following light period (Smith et al. 1996). Many studies investigating the transport properties of the vacuolar membrane have used another model CAM plant, Kalanchoë daigremontiana, for example to characterize the ATP- and PPi-dependent proton pumps at the tonoplast membrane and to investigate the pathway of malate transport (White and Smith 1989, White et al. 1990, Bettey and Smith 1993, Cheffings et al. 1997, Pantoja and Smith 2002, Hafke et al. 2003). White and Smith (1989) observed that the rate of proton pumping into the vacuole by the tonoplast H+-ATPase or H+-PPiase was constrained by transport of a charge-balancing anion, and that in the CAM plant K. daigremontiana the distinctive sequence of tonoplast anion permeability was fumarate2− > malate2− > chloride−. Another study with Nicotiana tabacum (Solanaceae), a C3 species, showed the influence of both nitrogen source and concentration on proton transport across the vacuole and on malate accumulation (Lüttge et al. 2000): the highest malate accumulation and the maximum ATP-dependent malate transport into the vacuole were observed when the species was cultivated in the presence of high nitrate concentrations (10 or 20 mM).
These prior studies raise some important questions about the degree to which the capacity for transport of organic-acids at the vacuolar membrane is coordinated with the magnitude of CAM expression. Would a higher vacuolar H+ transport capacity be accompanied by greater organic acid accumulation during the night in CAM species? Would nitrogen source and concentration affect the magnitude of CAM photosynthesis by changing proton transport rates into the vacuole and associated nocturnal organic-acid accumulation? A possible approach to address these questions would be to investigate H+ transport across the tonoplast in other Kalanchoë species that exhibit different levels of organic-acid accumulation to model species such as K. daigremontiana studied to date. Another approach would be to investigate the effects of alternative nitrogen sources at different concentrations and the extent to which changes in proton and organic-acid transport across the vacuole are correlated with higher or lower CAM expression in Kalanchoë. Thus, the present study is focused on a comparison of nitrate and ammonium as contrasting nitrogen sources, supplied at two different concentrations (2.5 or 5.0 mM), both of which are lower than the toxic concentration (6.5 mM) observed during preliminary experiments performed with Kalanchoë laxiflora and Kalanchoë delagoensis (data are not shown). The aim of these experiments is to establish how nitrogen nutrition influences the expression of CAM and the associated capacity for vacuolar organic-acid accumulation, as well as studying the effect of alternative nitrogen sources on CAM activity. This investigation specifically sought to establish the relationship between ATP- and PPi-dependent proton transport across the vacuolar membrane and the organic-acids accumulated at night under these different nutritional conditions.
Materials and methods
Plant material and growth conditions
Mature plants of K. laxiflora Baker and K. delagoensis Eckl. and Zeyh. (commonly referred to in the physiological and biochemical literature by its later synonym Kalanchoë tubiflora Raym.-Hamet) were obtained from the garden of the Department of Botany at the University of São Paulo, São Paulo, Brazil. Plants were transferred to controlled environment chambers and maintained under a photosynthetic flux density (PFD) of approximately 200 µmol m−2 s−1 of photosynthetically active radiation (measured at the surfaces of the uppermost leaves), 12 h photoperiod, day/night air temperature of 25/22 °C, and day/night relative humidity of 60/70%.
Plants were cultivated in pots (13 cm diameter and 7 cm height) containing fine sand, one plant per pot, and were watered daily with distilled water without supplementary nutrients for 2 weeks. After this acclimation period, experiments were performed for 60 days and each species was divided into six treatments based on nitrogen sources and their concentrations: (1) nitrogen-deficient (no supplementary nitrogen), (2) 2.5 mM ammonium + 2.5 mM nitrate, (3) 2.5 mM ammonium, (4) 2.5 mM nitrate, (5) 5.0 mM ammonium, and (6) 5.0 mM nitrate. The nitrogen concentrations selected were designed to be below any toxic level based on previous results obtained by Ota (1988a, 1988b) with Kalanchoë blossfeldiana, in which toxicity effects were observed under 10 mM but not under 5 mM ammonium (whereas toxicity effects were not observed for nitrate at either concentration). In addition, a preliminary test using 6.5 or 5.0 mM ammonium or nitrate was performed with K. delagoensis and K. laxiflora (data not shown). This test showed toxicity effects when K. delagoensis plants were kept under 6.5 mM ammonium, such as, necrosis, which decreased growth and caused the plants to lose most of their leaves. For this same species, 6.5 mM nitrate also showed a decrease in the growth. For K. laxiflora, the plants kept under 6.5 mM nitrate showed a decrease in their growth compared with the plants kept in 5.0 mM nitrate. For this same species, higher ammonium and nitrate concentrations (6.5 mM) caused early senescence (data are not shown). In the light of these preliminary results, we chose a lower concentration of nitrate and ammonium (5.0 mM).
Each plant received 30 ml of the appropriate nutrient solution with different nitrogen sources and their concentration every second day for a total of 60 days based on full-strength Hoagland solution (Hoagland and Arnon 1950). After 60 days, leaf samples were taken from six plants from each treatment and were used to determine proton transport rates in isolated tonoplast vesicles and nocturnal organic-acid accumulation.
Tonoplast vesicle isolation
Fully expanded leaves from K. laxiflora and K. delagoensis were harvested 1–1.5 h after commencement of the light period. Tonoplast fractions from the mesophyll tissue were isolated in accordance with White and Smith (1989) with minor modifications. The leaf margins were excised and the mesophyll tissue (approximately 80 g fresh mass) was sliced and suspended in 250 ml of ice-cold extraction buffer containing 450 mM mannitol, 3.0 mM MgSO4, 10.0 mM ethylenediaminetetraacetic acid, disodium salt (Na2EDTA), 10 mM dithiothreitol (DTT), 0.5% (w/v) polyvinylpyrrolidone (PVP-40) and 100 mM tris(hydroxymethyl)aminomethane (Trizma® base, Saint Louis, Missouri, USA) buffered to pH 8.0 with HCl. After homogenization in a commercial blender, the homogenate was filtered through two layers of cheesecloth and the filtrate centrifuged at 18 000 g for 20 min. The resulting supernatant was centrifuged at 80 000 g for 60 min, yielding a microsomal pellet that was resuspended in a glycerol-containing medium consisting of 1.1 M glycerol, 1.0 mM Na2EDTA, 10 mM Tricine buffered to pH 8.0 with bis-tris-propane (BTP) and 2.0 mM DTT. The membrane suspension was layered onto a 23% (w/v) sucrose cushion made up in glycerol medium and the sucrose step gradient centrifuged at 100 000 g for 70 min. Tonoplast vesicles were carefully removed from the interface by aspiration using a Pasteur pipette, resuspended 1:1 in glycerol medium buffer, and pelleted at 100 000 g for 50 min. The resulting pellet was finally gently re-suspended using a paintbrush in a small volume of glycerol medium. All steps were performed at 4°C. Preparations were snap frozen in liquid nitrogen, stored at −80 °C, and were used within one month. Vesicle yield did not show any significant differences among the N treatments used in this study.
Proton-transport assays
Measurement of proton transport into isolated tonoplast vesicles was conducted using a quinacrine-fluorescence-quenching method according to White and Smith (1989) with minor modifications. Initial rates of H+ transport into the vesicles at 25°C were determined from the initial rates of fluorescence quenching following addition of 3.0 mM Tris-ATP or 500 µM Na4PPi to the reaction medium in buffered solution. For assays of ATP-dependent H+ transport, the reaction medium contained 3.0 µM quinacrine, 6.0 mM MgSO4, 0.3 mM Na2EDTA, 150 mM mannitol and 25 mM BTP buffered to pH 8.0 with Mes. For measurements of PPi-dependent H+ transport, the reaction medium was identical except that the MgSO4 concentration was increased to 7.5 mM and the medium also contained 100 mM K-Mes. Charge-balancing anions (fumaric acid, malic acid or citric acid buffered to pH 8.0 with BTP), 50 mM potassium nitrate (inhibitor of vacuolar membrane), or 100 µM sodium orthovanadate plus 100 µM sodium azide (inhibitor of non-vacuolar membrane) were used in the ATP- and PPi-dependent proton transport assays. Approximately 3 µg of tonoplast protein per reaction was used in all of the ATP- and PPi-dependent proton transport assays. Fluorescence quenching was measured using a model Perkin-Elmer LS-55 luminescence spectrometer (Perkin-Elmer Ltd., Llantrisant, Wales, UK) with excitation at 422 nm and emission at 495 nm, both with a slit width of 5 nm. Relative quinacrine fluorescence intensity (%F) was calculated based on the maximum slopes obtained after addition of ATP or PPi to calculate the rate of proton pumping in each preparation.
Protein determination
Protein concentration was measured using the method described by Bradford (1976), with bovine serum albumin as the standard.
Organic-acid quantification
To determine the nocturnal accumulation of organic-acids (Δcitrate, Δfumarate and Δmalate), samples (100 mg fresh mass) from fully developed leaves from six individual plants were collected 1 h after the start of the light period (dawn), and 1 h before the end of the light period (dusk). Samples were ground in liquid nitrogen and subsequently homogenized with 500 µl of methanol, chloroform and water (MCW) solution (12:5:1) containing benzoic acid as a standard (1 mg ml−1 of methanol). Samples were then incubated at 60°C for 30 min. All samples were centrifuged at 16 000 g at 4°C for 10 min. The supernatant was collected (50 µl) and dried for 1 h at 60°C in a CentriVap Vacuum Concentrator® (Labonco, Kansas City, MO). The dried sample was then re-suspended in 25 µl of pyridine and 25 µl of N-tert-butyldimethylsilyl-N-methyltrifluoroacetamide (MTBSTFA) and incubated in a dry bath for 1 h at 92°C. An aliquot of 1 µl of the incubated sample was used to quantify organic-acids by gas chromatography coupled with a chromatographic system (Shimadzu-QP2010SE, Kyoto, Japan), an Agilent DB5MS column (30 m, D.I. 0.25 mm, I.T. 0.5 µM), with helium as a carrier gas in a 1 ml min−1 flux and an auto sampler (Shimadzu AOC-20i). The column remained at 100°C for 6 min, with a temperature ramp from 100 to 300 °C at a rate of 6 °C min−1. The injector temperature was 290°C, total flux 24 ml min−1 and linear velocity 37.2 cm s−1. Standard curves for citric, fumaric and malic acids were used to determine the concentrations of individual organic-acids in the samples. Results are expressed as millimoles per gram of dry weight (mmol g−1 DW).
Data analysis
All data are presented as mean values ± standard deviation (SD). One-way anova was used to analyse the results. Significant differences among the different anions or treatments in the same species were tested using the Tukey–Kramer test at P < 0.05 using JMP 5.01 software (SAS Institute 2002). The difference between the inhibitors in the same treatment and species was tested by Student's t-test at 5% significance (P < 0.05).
Results
Proton transport rates in the presence of inhibitor of vacuolar or non-vacuolar membranes
To determine the relative contribution of vacuolar and non-vacuolar membranes in these two species kept in different nitrogen treatments, we used a specific inhibitor of vacuolar membranes (V-ATPases) (KNO3) or non-vacuolar membranes (non-V-ATPases) (NaN3 plus Na3VO4) (Table 1). In all the nitrogen treatments, KNO3 caused a substantial (up to 99%) inhibition of ATP-dependent proton transport. When the isolated tonoplast membranes were tested in the presence of NaN3 plus Na3VO4, there was a small (up to 32%) decrease in proton transport, but this degree of inhibition was much less than that caused by KNO3 (Table 1). These results indicate that most of the extracted vesicles obtained are indeed derived from the tonoplast. All of these nitrogen treatments, for both Kalanchoë species, showed an inhibition percentage of ATP-dependent proton transport, in the presence of KNO3, higher than 54% (Table 1). On the other hand, in the presence of NaN3 plus Na3VO4, all of the other treatments showed an inhibition percentage between 2% and 32% when a specific inhibitor of non-V-ATPases was added (Table 1).
| Specific activity (% min−1 mg−1 protein) (inhibition relative to control) | |||
|---|---|---|---|
| Control | +KNO3 | NaN3 + Na4VO3 | |
| Kalanchoë laxiflora | |||
| Nitrogen-deficient | 3279 ± 30.8 A | 161 ± 3.5 C (95%)* | 2857 ± 21.7 B (13%)* |
| 2.5 mM ammonium + 2.5 mM nitrate | 4501 ± 76.2 A | 171 ± 4.8 C (96%)* | 3487 ± 43.3 B (23%)* |
| 2.5 mM ammonium | 2781 ± 19.1 A | 92.0 ± 18.9 C (97%)* | 2254 ± 78.9 B (19%)* |
| 2.5 mM nitrate | 4498 ± 33.4 A | 58.6 ± 6.5 C (99%)* | 4396 ± 31.9 B (2.3%) |
| 5.0 mM ammonium | 697 ± 27.5 A | 71.8 ± 3.2 C (90%)* | 612 ± 26.4 B (13%)* |
| 5.0 mM nitrate | 1119 ± 47.2 A | 89.8 ± 6.2 C (92%)* | 838 ± 94.4 B (25%)* |
| Kalanchoë delagoensis | |||
| Nitrogen-deficient | 3735 ± 54.6 A | 459 ± 50.9 C (88%)* | 3010 ± 30.8 B (19%)* |
| 2.5 mM ammonium + 2.5 mM nitrate | 3936 ± 136.1 A | 203 ± 47.5 C (95%)* | 2659 ± 86.2 B (32%)* |
| 2.5 mM ammonium | 3673 ± 97.0 A | 232 ± 31.2 B (94%)* | 3580 ± 108.7 A (2.4%) |
| 2.5 mM nitrate | 4757 ± 146.7 A | 269 ± 23.5 B (94%)* | 4622 ± 168.7 A (2.8%) |
| 5.0 mM ammonium | 311 ± 28.2 A | 141 ± 13.4 C (54%)* | 237.8 ± 13.1 B (23%)* |
| 5.0 mM nitrate | 1964 ± 132.4 A | 264 ± 57.0 C (86%)* | 1628 ± 55.6 B (17%)* |
ATP-dependent proton transport rates
ATP-dependent proton transport rates (%F) in tonoplast vesicles prepared from K. laxiflora and K. delagoensis leaves were assayed in the presence of fumarate, malate and citrate anions and were calculated from the initial slope of the quinacrine fluorescence quenching reaction as shown in Figs S1 and S2, Supporting Information.
Fumarate supported the highest rates of vesicle acidification in all treatments in both species, followed by malate (not considering the control). In the presence of fumarate, K. laxiflora plants cultivated in 2.5 mM nitrate presented the highest ATP-dependent proton transport rates (about 4061% min−1 mg−1 protein: Table 2), followed by plants cultivated in the presence of 2.5 mM ammonium + 2.5 mM nitrate, nitrogen-deficient, 2.5 mM ammonium, 5.0 mM nitrate and 5.0 mM ammonium (Table 2). For comparison, ATP-dependent proton transport in the presence of 2.5 mM nitrate (highest rates) was about 9 times higher than in the presence of 5.0 mM ammonium (lowest rates). For K. delagoensis, in the presence of fumarate, the highest proton transport rates were also observed in plants cultivated in 2.5 mM nitrate, 4172% min−1 mg−1 protein, followed by 2.5 mM ammonium + 2.5 mM nitrate, 2.5 mM ammonium, nitrogen-deficient, 5.0 mM nitrate and 5.0 mM ammonium. When this Kalanchoë species was cultivated in 2.5 mM nitrate, ATP-dependent H+ transport was about 3.5 times higher than when the species was in the presence of 5.0 mM ammonium.
| ATPase Specific Activity (% quench min−1 mg−1 protein) | ||||
|---|---|---|---|---|
| Fumarate | Malate | Citrate | Control | |
| Kalanchoë laxiflora | ||||
| Nitrogen-deficient | 3040 ± 9.6 C a | 1053 ± 15.3 C b | 38.8 ± 7.6 D d | 274 ± 6.8 A c |
| 2.5 mM ammonium +2.5 mM nitrate | 3113 ± 14.9 B a | 1516 ± 15.3 B b | 53.08 ± 9.0 CD b | 208 ± 7.0 C c |
| 2.5 mM ammonium | 2131 ± 12.6 D a | 522 ± 6.9 D b | 126 ± 7.7 AB c | 21.7 ± 2.3 F d |
| 2.5 mM nitrate | 4061 ± 24.2 A a | 1623 ± 12.0 A b | 142 ± 10.7 A d | 239 ± 5.1 B c |
| 5.0 mM ammonium | 445 ± 7.7 F a | 149 ± 8.4 F c | 73.0 ± 6.2 C d | 190 ± 10.5 D b |
| 5.0 mM nitrate | 878 ± 16.4 E a | 210 ± 6.0 E b | 111 ± 8.1 B c | 104 ± 3.5 E c |
| Kalanchoë delagoensis | ||||
| Nitrogen-deficient | 2809 ± 8.2 D a | 1424 ± 6.8 C b | 73.3 ± 5.8 C d | 145 ± 4.02 C c |
| 2.5 mM ammonium +2.5 mM nitrate | 3776 ± 5.3 B a | 1810 ± 6.7 B b | 107 ± 9.1 B d | 243 ± 3.5 A c |
| 2.5 mM ammonium | 3715 ± 5.0 C a | 1310 ± 10.0 D b | 142 ± 7.4 A d | 220 ± 12.2 B c |
| 2.5 mM nitrate | 4172 ± 15.5 A a | 2088 ± 6.9 A b | 108 ± 10.4 B c | 108 ± 7.0 D c |
| 5.0 mM ammonium | 1207 ± 8.9 F a | 531 ± 7.6 F b | 21.4 ± 1.0 E d | 67.2 ± 5.4 E c |
| 5.0 mM nitrate | 2009 ± 5.2 E a | 774 ± 9.9 E b | 45.2 ± 8.3 D d | 26.7 ± 1.2 F c |
In the presence of malate, the H+ transport rates showed a similar pattern for both Kalanchoë species. Kalanchoë plants cultivated with 2.5 mM nitrate presented the highest proton transport rates, while the plants kept under 5.0 mM ammonium showed the lowest rates (Table 2). In the presence of citrate, the proton transport rates in K. laxiflora and K. delagoensis did not show any discernible pattern. Generally, ATP-dependent proton transport in all of the treatments in K. delagoensis showed significantly higher rates while in the presence of fumarate or malate compared with when in the presence of all of the treatments for K. laxiflora (Table 2).
PPi-dependent proton transport rates
PPi-dependent proton transport rates (%F) were calculated in a similar way from the quinacrine fluorescence quenching reactions as shown in Figs S3 and S4.
The pattern of PPi-dependent H+ transport rates in the presence of all of the anions was different when compared with ATP-dependent proton transport in K. laxiflora and K. delagoensis. For K. laxiflora, in the presence of fumarate, the highest H+ transport rates were observed in 2.5 mM nitrate, 2124% min−1 mg−1 protein, while the lowest rate was observed in the nitrogen-deficient treatment (Table 3). For K. delagoensis, in the presence of the same anion, the highest proton transport rates were observed in 2.5 mM nitrate, and the lowest rates in 5.0 mM ammonium (Table 3).
| PPiase Specific Activity (% quench min−1 mg−1 protein) | ||||
|---|---|---|---|---|
| Fumarate | Malate | Citrate | Control | |
| Kalanchoë laxiflora | ||||
| Nitrogen-deficient | 315 ± 7.1 E a | 203 ± 8.5 C b | 33.2 ± 1.7 C d | 51.8 ± 4.5 D c |
| 2.5 mM ammonium + 2.5 mM nitrate | 688 ± 3.7 B a | 536 ± 6.8 B b | 219 ± 8.4 A c | 87.3 ± 5.3 C d |
| 2.5 mM ammonium | 511 ± 10.2 C a | 220 ± 12.1 C b | 25.3 ± 4.6 C d | 51.0 ± 3.8 D c |
| 2.5 mM nitrate | 2125 ± 25.0 A a | 753 ± 15.3 A b | 75.4 ± 3.9 B d | 126 ± 5.2 A c |
| 5.0 mM ammonium | 404 ± 13.9 D a | 119 ± 5.1 D b | 20.8 ± 0.7 C c | 106 ± 5.2 B b |
| 5.0 mM nitrate | 523 ± 8.9 C a | 142 ± 8.1 D b | 87.7 ± 7.8 B c | 73.3 ± 6.7 C c |
| Kalanchoë delagoensis | ||||
| Nitrogen-deficient | 829 ± 16.5 D a | 906 ± 54.9 C a | 34.4 ± 0.7 CD b | 70.2 ± 3.4 DE b |
| 2.5 mM ammonium + 2.5 mM nitrate | 1125 ± 21.8 B a | 1057 ± 10.0 B a | 23.4 ± 2.43 D b | 49.8 ± 13.3 E b |
| 2.5 mM ammonium | 1025 ± 5.0 C a | 854 ± 10.0 C b | 42.0 ± 7.1 CD d | 90.6 ± 9.2 CD c |
| 2.5 mM nitrate | 1280 ± 6.7 A a | 1303 ± 11.9 A a | 66.3 ± 1.53 AB c | 108 ± 13.1 BC b |
| 5.0 mM ammonium | 468 ± 10.1 E a | 405 ± 8.3 E b | 82.9 ± 11.9 A d | 141 ± 7.9 A c |
| 5.0 mM nitrate | 846 ± 10.8 D a | 674 ± 1.5 D b | 49.7 ± 10.0 BC d | 120 ± 8.5 AB c |
In the presence of malate, the highest rates were observed in both Kalanchoë species cultivated with 2.5 mM nitrate (Table 3). In the presence of citrate, K. laxiflora plants cultivated in 2.5 mM ammonium + 2.5 mM nitrate showed the highest proton transport rates (Table 3). On the other hand, for K. delagoensis, the highest PPi-dependent proton transport rates were observed in the presence of 5.0 mM ammonium and 2.5 mM nitrate (Table 3).
The variations in the PPi-dependent proton transport rates in these species were also dependent upon the different nitrogen sources and their relative concentrations. In K. laxiflora and K. delagoensis, ATP- or PPi-dependent proton transport rates presented a control (no anion used) higher than zero, and in most of the treatments the rates in this control were higher than the rates measured in the presence of (Table 2). Generally, ATP- or PPi-dependent proton transport rates were higher in the presence of fumarate than in the presence of malate or citrate in both Kalanchoë species, and in all of the treatments. Also, this transport seems to exhibit a preference for ATPase rather than PPiase, because the H+ transport rates in the presence of ATP were always higher than in the presence of PPi for both species.
Nocturnal organic-acid accumulation
Nocturnal citrate, fumarate and malate accumulation was measured in the leaves of K. laxiflora and K. delagoensis (Fig. 1). In all of the nitrogen treatments for both Kalanchoë species, the highest nocturnal organic acid accumulation was observed for malate. For example, in K. laxiflora plants cultivated with 2.5 mM nitrate, the amount of malate accumulated during the dark period was about 8 times higher than fumarate and about 72 times higher than citrate in this same treatment. Plants from this same species treated with 5.0 mM nitrate or 5.0 mM ammonium exhibited the lowest amount of malate accumulated during the night (Fig. 1A). K delagoensis plants cultivated in 2.5 mM nitrate, the treatment that showed the highest amount of organic acids accumulated during the night, exhibited nocturnal malate accumulation of about 20 times higher than fumarate, and about 4 times higher than citrate. On the other hand, the lowest nocturnal malate accumulation was observed in 5.0 mM ammonium (Fig. 1B). In K. laxiflora, fumarate showed the second highest nocturnal accumulation, while in K. delagoensis citrate was the second most accumulated organic acid in all of the nitrogen treatments (Fig. 1).
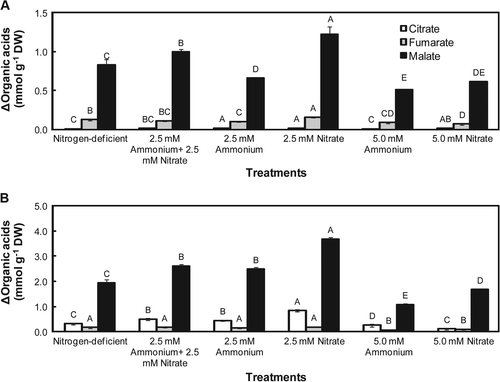
The rank order of total organic acids (malate + fumarate + citrate) accumulated during the night in K. laxiflora under different nitrogen treatments was as follows: 2.5 mM nitrate > 2.5 mM ammonium + 2.5 mM nitrate = nitrogen-deficient > 2.5 mM ammonium ≥ 5.0 mM nitrate = 5.0 mM ammonium (Fig. 1A). For K. delagoensis, this order showed a slight difference compared with K. laxiflora: 2.5 mM nitrate > 2.5 mM ammonium + 2.5 mM nitrate > 2.5 mM ammonium > nitrogen-deficient > 5.0 mM nitrate > 5.0 mM ammonium (Fig. 1B). As shown in the previous results, nocturnal organic-acid accumulation varied according to the species, nitrogen sources and their concentrations. K. delagoensis exhibited higher nocturnal total organic acids accumulation, about 3.5 times, compared with K. laxiflora (Fig. 1).
Discussion
Although several studies have demonstrated the influence of nutrients on CAM expression, our study is the first to show, to our knowledge, that the rates of vacuolar proton and anion transport in CAM plants, and the associated nocturnal accumulation of malate, fumarate and citrate, are affected by growth of plants at differing concentrations and sources of inorganic nitrogen in two Kalanchoë species, K. laxiflora and K. delagoensis.
Winter and Holtum (2011) demonstrated an influence of KNO3 on CAM photosynthesis in the inducible species Calandrinia polyandra. It was observed that after 77 days without nutrients this species showed a slight dark CO2 fixation. However, after 20 mM KNO3 was provided to the soil, a loss of nocturnal CO2 fixation was noted within 48 hours. In other words, the authors observed that soil nutrient supply affects the balance between C3 and CAM photosynthesis. Ota and Yamamoto (1991) previously observed in K. blossfeldiana a higher degree of CAM expression when this plant was grown in the presence of nitrate. Our results corroborate this study, as we observed in K. laxiflora and K. delagoensis that the highest concentrations of organic acids, mainly malate, were accumulated during the night in the plants kept under 2.5 mM nitrate (low concentration). Maftoun et al. (1980) reported that Kalanchoë verticillata and K. laxiflora grew equally well on NO3− and NH4+ as N source, while Sedum telephoides showed reduced leaf growth in the presence of NH4+. Therefore, CAM species seem to show different preferences for inorganic nitrogen sources in regard to growth. In our investigation, we verified in K. laxiflora and K. delagoensis that the highest ammonium concentration (5 mM) decreased the overall accumulation of organic-acids (malate, fumarate and citrate) during the night. It is probable that even 5.0 mM ammonium would show slight toxicity effects to K. laxiflora and K. delagoensis compared with lower ammonium concentration (2.5 mM), and this toxicity might be responsible for the lowest rates of nocturnal organic-acids accumulation in these species. In K blossfeldiana, a higher ammonium concentration (10 mM) presented toxicity effects such as necrosis in the basal portion of the stem of this species (Ota 1988a). Britto et al. (2001) explained NH4+ toxicity as a result of the high energetic cost associated with pumping this ion back out of the cells, after entering at high rates in ammonium-sensitive species.
Generally, when the results from this study are combined with the observations made in earlier publications with Kalanchoë species, the data support the theory that species from the genus Kalanchoë show a higher degree of CAM expression when cultivated on NO3− compared with NH4+ (Ota 1988a, 1988b, Ota and Yamamoto 1991). One hypothesis for the preference for nitrate over ammonium could be a higher production of nitric oxide (NO) in the presence of NO3− compared with NH4+ in these Kalanchoë species. NO could be a signal to increase CAM expression in these species, as it is known that nitrate reductase (NR), an enzyme that uses NO3− as its substrate, is one of the main enzymes responsible for increasing NO production by plants tissues (Hao et al. 2008, Zhao et al. 2009). The presence of NO3− could thus increase NR activity and this enzyme could increase NO production in the leaves of K. laxiflora and K. delagoensis. Freschi et al (2010a), studying Ananas comosus, reported that the upregulation of CAM photosynthesis was accompanied by increase in NO production and endogenous levels and that decreased CAM expression coincided with a decrease in NO emission. On the other hand, the metabolic consequences of NO3− nutrition seem to differ among Kalanchoë species. For K. pinnata, a high nitrate concentration (24 mM) increased PEPC activity and, consequently CAM expression, by about threefold when compared with a low concentration (0.6 mM) (Winter et al. 1982). For K. blossfeldiana, a slight increase in nocturnal malate accumulation was observed in the presence of a high nitrate concentration (10 mM), but the CO2 absorbed during the night tended to decrease at this high concentration (10 mM) (Ota and Yamamoto 1991). In the present study, K. laxiflora and K. delagoensis appeared to show a preference for lower nitrogen concentrations (2.5 mM) regardless of the source of nitrogen. Another revealing result is that these species prefer either the combination of nitrogen sources (2.5 mM ammonium + 2.5 mM nitrate) or the nitrogen-deficiency, rather than the high concentration of each nitrogen source individually.
In addition to showing the influence of nitrogen sources and their concentrations on CAM photosynthetic expression, this study is also significant in that it differentiates between which species of organic acids are accumulated in the vacuole during the night in these Kalanchoë species. K. laxiflora exhibited the highest accumulation of malate, followed by fumarate and citrate during the night. Other studies have shown high (daytime) fumarate accumulation in Arabidopsis thaliana (Chia et al. 2000). However, the reason that some plants preferentially accumulate fumarate over other organic acids is still unclear. This organic acid is an important component of the tricarboxylic acid cycle and it can be metabolized and used to provide energy and carbon skeletons via anaplerotic pathways (Chia et al. 2000). Fumarate can be synthesized from malate by fumarate hydratase, and the reverse reaction with loss of water can convert fumarate into malate (Chia et al. 2000). Conversion of malate to pyruvate via NAD(P)-malic enzyme in the mitochondria or cytosol is under the control of fumarate. When fumarate accumulates, the conversion of malate to pyruvate is facilitated (Igamberdiev et al. 2001). Finally, pyruvate, as well as entering the tricarboxylic acid (TCA) cycle, can be converted to phosphoenolpyruvate (PEP), which is then carboxylated to oxaloacetate (OAA) and reduced to malate, which is accumulated in the vacuole in CAM plants during the night (Igamberdiev and Eprintsev 2016). Osmond et al. (1988) observed a labeling of [1-13C] malate by virtue of label randomization between C-4 and C-1 by fumarase during the dark period in K. daigremontiana. At the beginning of the dark period, a decrease in fumarate content was observed in K. daigremontiana leaves, while the malate content showed an increase during this time. In this way, as previously observed for K. daigremontiana, fumarate could contribute to increasing the accumulation of malate into the vacuole of K. laxiflora, which would explain the high nocturnal malate accumulation observed in this species. On the other hand, K. delagoensis showed a higher nocturnal accumulation of citrate into the vacuole, compared with fumarate. For this Kalanchoë species, the nocturnal citrate storage could be a way to generate reducing power at night and survive under severe environmental conditions. Lüttge (1988) showed that citric acid accumulation is more energetically favorable than malic acid. In addition, citrate represents a way to generate reducing power at night without causing a loss of carbon, as 3 mol NADH is generated per mol of formed citrate (Lüttge 1988, Freschi et al. 2010b, Pereira et al. 2013). Furthermore, the greater storage of citrate at night in K. delagoensis plants kept under 2.5 mM nitrate could be another alternative to generate reducing power from citrate synthesis for NR activity, as previously observed for the CAM bromeliad species, Tillandsia pohliana (Freschi et al. 2010b). In this way, a higher CAM expression in K. delagoensis compared with K. laxiflora, could also be due to the high nocturnal citrate accumulation into the vacuole, which may help to increase NR activity by providing reducing power to this enzyme and consequently increase the production of NO by NR, which could be a signal to increase CAM expression in K. delgoensis leaves.
This study has shown that higher nitrate concentrations (5 mM) decrease CAM expression in K. laxiflora and K. delagoensis. Studies have previously reported that NO3− could inhibit the tonoplast ATPase (Smith et al. 1984). Therefore, Kalanchoë plants kept under a high nitrate concentration (5 mM) would not allow the movement of organic acids and protons into the vacuole, because this nitrate concentration seems to be sufficient to decrease ATP-dependent proton transport and, consequently, decrease CAM expression. However, low nitrate concentrations seem to have no inhibitory effect on the V-ATPase activity. Brüx et al. (2008) reported that A. thaliana plants grown in the presence of 1 mM nitrate did not reduce V-ATPase activity. In K. laxiflora and K. delagoensis, the highest ATP-dependent proton transport was observed in the plants kept under 2.5 mM nitrate. Thereby, the nitrate inhibitory effect on V-ATPase activity seems to be directly related to nitrate concentration, as well as dependent upon the species. Thereby lower nitrate concentration (2.5 mM) does not seem to inhibit V-ATPase activity compared with higher nitrate concentration (5 mM) in these Kalanchoë species used in this study. In regard to proton and organic-acids transport into the vacuole, White and Smith (1989) reported very similar ATP- and PPi-dependent proton transport rates in K. daigremontiana, and these rates were higher in the presence of fumarate compared with malate. In K. laxiflora and K. delagoensis, a significantly higher ATPase-dependent proton transport in the presence of fumarate or malate was observed over PPiase-dependent transport. Moreover, fumarate was more effective than malate and citrate in all of the treatments, performed on both species, in supporting ATP- or PPi-dependent vesicle acidification, indicating a preferential selectivity of the vacuolar anion channel for fumarate over malate in K. laxiflora and K. delagoensis. However, this does not imply that more fumarate will be accumulated than malate in the vacuole. As was shown in our study, there is not more fumarate than malate available in the cytosol, restricting the fumarate capable of being accumulated in the vacuole of both Kalanchoë species. For PPi-dependent vesicle acidification, in K. laxiflora, fumarate was more effective than the other two organic acids tested in all of the treatments. In K. delagoensis, fumarate was more effective than citrate in all of the treatments, but it was only more effective than malate in 2.5 mM ammonium and in higher nitrate and ammonium concentrations. ATP-dependent proton transport in K. delagoensis, in the presence of fumarate or malate, was significantly higher than in K. laxiflora.
The contribution made by PPiase and V-ATPase in the vacuolar proton transport reported for Mesembryanthemum crystallinum showed a large difference, because the V-ATPase activity was about nine times higher than the PPiase when these plants were CAM induced by salinity stress. Bremberger et al. (1988) suggested that tonoplast ATPase is a more important enzyme than H+-PPiase in driving the nocturnal accumulation of organic acids in this CAM plant. Our results corroborate this study, as we observed a higher proton transport in the vacuole in the presence of ATP rather than PPi for both Kalanchoë species. Besides a higher nocturnal organic acids accumulation in K. delagoensis compared with K. laxiflora, the quinacrine fluorescence-quenching experiments for both species showed that the permeability of the tonoplast membrane for the anions used in this study appears to be higher in K. delagoensis than in K. laxiflora. This result is consistent with a higher nocturnal organic acids accumulation into the vacuole of K. delagoensis compared with the accumulation into the vacuole of K. laxiflora. Although it has not been evaluated in these Kalanchoë species, it is important to raise the questions of the effects of anions concentrations and carbohydrate supply on the organic acids accumulation into the vacuole. Lüttge et al. (2000) observed the influence of concentration and combination of different ions on the proton transport rates in Kalanchoë daigremontiana. An increase of malate concentration from 20 to 50 mM caused a significant increase in H+ transport rate. In addition, this same study showed that the addition of a chloride anion after adding a malate anion to the tonoplast vesicles reduced the relative rates of H+ transport compared to when malate was presented without chloride (Lüttge et al. 2000). Other compounds, such as storage carbohydrates, also affect the acids accumulation into the vacuole. Chen and Nose (2004) showed that starch-degrading CAM species exhibited a higher V-PPiase activity compared with monosaccharide-degrading CAM species, which seem to show a preference for V-ATPase to the energization of the vacuole. The starch-degrading CAM species are able to generate malate by phosphorylase activity (PPiase) and through this enzyme they can save energy (ATP). However, the effects of anions concentrations and carbohydrate supply on the organic acids accumulation into the vacuole have not been studied in K. delagoensis and K. laxiflora. Therefore, more studies are necessary to better understand how they affect CAM photosynthesis in relation to proton and anion transport rates as well as organic acids accumulation into the vacuole in these Kalanchoë species.
In conclusion, this study has provided evidence that both ATP- and PPi-dependent proton transport rates are influenced by inorganic nitrogen sources (simultaneous or individual occurrence) by their concentrations (2.5 or 5 mM) and by the Kalanchoë species itself. Moreover, we have shown, to our knowledge for the first time in Kalanchoë species, that there is a slight ATP- and PPi-dependent proton transport in the absence of any anion, that can be considered as a background, and also that there is a preference for ATPase rather than PPiase to the proton transport in K. laxiflora and K. delagoensis. Finally, our data indicate that a low nitrate concentration (2.5 mM) increases the nocturnal organic-acids accumulation, increases ATP- and PPi-dependent proton transport into the vacuole in both K. laxiflora and K. delagoensis, and is responsible for the highest CAM expression in both Kalanchoë species whereas a higher nitrate concentration (5 mM) decreases the amount of organic-acids accumulated during the night, and also seems to have an inhibitory effect on ATP-dependent proton transport into the vacuole in both K. laxiflora and K. delagoensis. In addition, this study has shown a higher CAM expression in K. delagoensis compared with K. laxiflora. This could be due to a higher nocturnal citrate accumulation into the vacuole as observed to K. delagoensis rather than K. laxiflora. The higher citrate levels may help to increase NR activity by providing reducing power to this enzyme and consequently increase the production of NO by NR. In this way, NO could be a signal to increase CAM photosynthesis in K. delagoensis in the presence of 2.5 mM nitrate.
Author contributions
P. N. P conducted all of the experiments. H. M., J. A. C. S. and P. N. P. conceived the project. J. A. C. S. assisted in developing experimental protocols and H. M. supervised the experiments. P. N. P. wrote the article with contributions from the other authors. All authors read and approved the manuscript.
Acknowledgements
The authors acknowledge Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (99999.001109/2014-06) for the scholarship awarded to Paula Natália Pereira, Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (2011/50637-0), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (309504/2014-7) for their financial support to Helenice Mercier, SPRINT – University of Oxford and the Biotechnology and Biological Sciences Research Council (UK) for a Sparking Impact Award to J. A. C. S.




