Epiphyte Vanilla relies on birds as long-distance seed dispersers
Abstract
- Angiosperms comprise the most diverse group of land plants. While essentially sessile organisms, flowering plants can disperse their genes through pollen flow and expand their occurrence range by means of seed dispersal. While most orchids are anemophilous, seed dispersal in Vanilla is mediated by vertebrates.
- Here, I investigate processes involved in the attraction and rewarding of seed dispersers of an obligatory epiphytic Vanilla through field observations, analysis of fruit morphology, resource production, fragrance release, and seed viability.
- Dehiscent fruits of Vanilla lindmaniana are attractive exclusively to birds. The fruit cavity contains a mucilaginous substance rich in sugar, fat, protein, and starch, which is consumed by several bird species. The basal cells of the funiculi contain calcium oxalate crystals, which are harmful to mammals. Seed viability testing revealed that seeds germinate after passing through the bird digestive tract. This is the first study describing an obligatorily ornithochorous Vanilla.
- A mucilaginous substance produced by Vanilla fruits is consumed by diurnal birds, corroborating fruit features compatible with ornithochory. The presence of raphides in the funiculi cells also confirms that seed dispersal of V. lindmaniana is mediated exclusively by birds. Ornithochory is pivotal in the effective dispersal of seeds in obligate epiphytic Vanilla, as it ensures that consumed seeds are taken to other phorophytes through avian faeces, allowing gene flow and the colonization of new areas and environments.
INTRODUCTION
Angiosperms are the most diverse group of land plants. While essentially sessile organisms, flowering plants can disperse their genes through pollen flow and expand their occurrence range by means of seed dispersal (Schaefer & Ruxton 2011). Seed movement away from the parent plant has several selective advantages: decreasing competition among offspring and parental individuals and reducing exposure of the diaspores to herbivores and potential pathogens. Furthermore, an efficient seed disperser can minimize the risk of offspring and their plant parents being concomitantly exposed to similar unfavourable conditions, decreasing the chance of extinction (Schaefer & Ruxton 2011). In order to increase the effectiveness of seed dispersal, many zoochorous plants have evolved seeds with hard seed coats embedded in fleshy fruits strongly adapted to endozoochory (Jordano 2000). This is the case in Vanilla, an orchid genus of more than 100 species distributed throughout tropical and subtropical regions. The role of both sclerotic seeds and fleshy fruits in the seed dispersal of Vanilla has been the subject of many investigations (Pansarin 2021, 2024a, 2025a; Pansarin & Suetsugu 2022; Karremans, Bogarín, et al. 2023; Karremans, Watteyn, et al. 2023). While the potential role of insects on seed dispersal of Vanilla has been discussed (see Lozano-Rodríguez et al. 2022), the seeds of Neotropical species are dispersed by vertebrates (Pansarin 2021, 2024a, 2025a; Pansarin & Suetsugu 2022). In fact, Vanilla fruits have evolved several specialized features for attraction (colour and scent) and rewarding (sugar, fat and protein) of vertebrates. In addition, Vanilla seeds also possess sclerotic coats adapted to pass undamaged through the digestive tract of seed dispersers (Pansarin 2021, 2024a, 2025a; Pansarin & Suetsugu 2022; Karremans, Bogarín, et al. 2023; Karremans, Watteyn, et al. 2023). Vanilla species with dehiscent fruits are strongly adapted to dispersal by birds and arboreal mammals (Pansarin 2021, 2025a; Pansarin & Suetsugu 2022), while species with indehiscent fruits are dispersed by terrestrial mammals (Pansarin 2024a). The mesocarp of dehiscent Vanilla fruits commonly contains large amounts of calcium oxalate crystals. Consequently, frugivores access the fruit cavity through the pericarp valves (Pansarin 2021, 2025a; Pansarin & Suetsugu 2022). Seed dispersers are rewarded with the fats and sugars produced by the funiculus and with protein produced by the placenta (Pansarin 2021, 2024a, 2025a; Pansarin & Suetsugu 2022).
Although both mammals and birds have been recorded as seed dispersers in Vanilla, the fruits of some species are consumed exclusively by mammals (Pansarin 2024a, 2025a). In fact, fruits of some species have characteristics that appear to be related to the attraction of specific fruit consumers. For V. chamissonis, while birds and other omnivores are attracted to their indehiscent fruits, only mammalian herbivores (i.e., Dasyprocta azarae) are effective seed disperser of this Vanilla species. This is because the fruits are rich in phenolic compounds, which make them unpalatable to omnivores (Pansarin 2024a). While birds have been recorded as seed dispersers of Neotropical Vanilla (Pansarin & Suetsugu 2022), a seed dispersal system mediated exclusively by birds is still unknown for this orchid genus. In addition, seed dispersal by bats in Vanilla has been indirectly attributed based on molecular evidence (Sierra-Vásquez et al. 2025). Although this topic remains unexplored, the contribution of flying vertebrates appears to be very important for long-distance dispersal in Vanilla. In fact, based on the evidence that some Vanilla species have both insular and continental populations, it is plausible to assume that some kind of long-distance dispersal is involved. In rupicolous or nomadic vine Vanilla, dispersal to islands may potentially occur through the transport of stem fragments by ocean currents. However, in an obligatorily epiphytic Vanilla, the colonization of islands must necessarily occur through flying (i.e., birds or bats) seed dispersers. This appears to be the case for V. palmarum, an epiphytic species on palms, which is supposedly distributed from South America to Cuba (BFG (The Brazil Flora Group) 2018; Soto Arenas & Cribb 2010). However, a recent study revealed that populations distributed throughout the Cerrado, Pantanal, and Amazon rainforest, are actually V. lindmaniana, not V. palmarum. On this basis and in this study, the name V. lindmaniana has been revalidated (Pansarin 2025b). In the present study I investigate seed dispersal of V. lindmaniana, an epiphytic species with fleshy and dehiscent fruits, whose seeds are consumed by birds away from their natural habitat (as V. palmarum: Pansarin 2021). This study is based on populations occurring in the state of Mato Grosso do Sul, Brazil. Here, I investigated: (1) which animals feed on V. lindmaniana fruits in the natural environment; (2) if fruits of V. lindmaniana are dispersed by both mammals and birds in natural areas, as reported in other Neotropical species with dehiscent fruits; (3) the role of fruit content in attraction and rewarding of seed dispersers; (4) whether V. lindmaniana seed dispersal has some kind of specialization, as previously reported for V. chamissonis (Pansarin 2024a); and (5) if the mode of seed dispersal of V. lindmaniana can predict long-distance dispersal and island colonization.
MATERIAL AND METHODS
Study species and sites
This study on the seed dispersal of V. lindmaniana was based on a population of hundreds of individuals occurring in the boundaries of Aquidauana (ca. 20°28′16″S, 55°47′14″W; 150 m a.s.l.), state of Mato Grosso do Sul, central-western Brazil. The climate of the study area is defined as “Aw” (Tropical, with a well-defined dry season; Köppen 1948). The municipality of Aquidauana is in the Pantanal Biome (Coutinho 2016).
Laboratory studies were carried out on plants collected in the field. For that, 10 stem cuttings were collected (ca. 100 cm) from fruiting plants of the studied population. Stem cuttings containing mature fruits were collected in 2018 from plants growing on different phorophytes. Fruits were used as a control in the experiment on seed viability (see below), while the stem cuts were planted in individual pots with pine bark and cultivated in the LBMBP Orchid House at the University of São Paulo (FFCLRP-USP), municipality of Ribeirão Preto (ca. 21°10′S, 47°48′ W, 546 m a.s.l.). The fruits used in the investigation of fruit features were obtained from the plants in cultivation in the LBMBP Orchid House. Fruits were obtained by spontaneous self-pollination (n = 40) from November to December 2023. All analyses were based on mature fruits.
Fruit features
Studies on morphology and anatomy were performed on 30 mature fruits (3 fruits per plant) of V. lindmaniana. Characteristics, such as size, colour, maturation time, dehiscence, and fragrance release, were recorded. In addition, the size and shape of seeds were observed. Remaining fruits (n = 10) were maintained attached to the inflorescences and monitored for 1 year from the beginning of ripening to check whether when they fall to the ground they might be dispersed by terrestrial vertebrates, as reported for V. chamissonis (Pansarin 2024a). I also performed a histochemical investigation of V. lindmaniana fruits (including the mucilaginous substance) to identify the main classes of compounds produced. Ripe fruits at the beginning of fruit dehiscence were transversally sectioned by hand and the following histochemical procedures performed: Sudan IV for total lipids (Sass 1951), Fehling's reagent for reducing sugars (Purvis et al. 1964), 1% iodine solution for starch grains (Johansen 1940), Xylidine Ponceau for total proteins (O'Brien & McCully 1981), tannic acid/ferric chloride for total mucilage (Pizzolato 1977), and 2% (w/v) aqueous solution of Ruthenium red for acid mucilage (Kraus & Arduin 1997). Appropriate controls (slides with no coloration) were performed simultaneously in each case. Slides were examined under a Leica DM500 light microscope, and the images were captured with a Leica ICC50 video camera attached to a PC using IM50 image analysis software.
Sugar concentration in the mucilaginous substance of V. lindmaniana fruits were measured with a Bellingham & Stanley (series Eclipse) hand-held refractometer (Dafni 1992). Sugar concentration measurements were made on 30 fruits (10 inflorescences; 10 plants) at the beginning of fruit dehiscence.
Fruit scent
Fragrance analyses of four ripe V. lindmaniana fruits (one extraction per fruit) were performed on plants collected in the municipality of Aquidauana, Mato Grosso do Sul, Brazil, and maintained under cultivation at the LBMBP Orchid House.
Volatile organic compounds (VOCs) of V. lindmaniana fruits were collected using the dynamic headspace collection technique (pumping air through adsorbent polymers) adapted for use in the field, combined with gas chromatography coupled to mass spectrometry (Shimadzu QP2010 GC–MS). Ripe fruits of V. lindmaniana were bagged in a polyester bag secured with cotton thread. Using a small portable electric air pump, the fragrance-laden atmosphere, including the volatiles surrounding the fruits, was drawn through an ORBO adsorption tube [PoraPak Q (50/80) 150/75 mg, ORBO -1103] for 7–8 h. Simultaneous extractions from empty polyester bags (controls) were carried out to identify possible contaminants in the samples. Subsequently, the adsorbed scent was recovered by HPLC elution with ca. 1 mL ethyl acetate. The eluate was collected in a vial with a 500 μL insert and kept under refrigeration (−20°C) prior to injection into the GC. The volatile sample was injected into a gas chromatograph (2010A; Shimadzu, Tokyo, Japan) coupled to a quadrupole mass spectrometer (QP2010, Shimadzu) using an RTX-5MS capillary column (30.0 m length, 0.25 mm internal diameter, 0.25 μm film thickness) and helium (75.4 kPa) as a carrier gas at a constant flow of 1.3 mL.min−1. Injection was performed in the split mode, with a split ratio of 5.0. The oven temperature started at 50°C and increased linearly to a maximum temperature of 220°C at a rate of 3°C min−1. An electron ionization mass spectrometry (EI-MS) detector was operated at ion source temperatures of 250°C. The global run time was recorded in full scan mode (35–400 m/z mass range) at a scanning rate of 0.30 scan s−1.
The resulting data were processed using GCMS Solution software (Shimadzu), and fruit VOCs were identified by screening NIST08, Wiley 7, FFNSC1.3, and Adams libraries (Adams 2007) for comparison of MS spectra. Furthermore, the Kovats Retention Index (RI) was calculated for each VOC using data obtained by injection of a homologous set of n-alkanes (C9–C22) according to the Kovats formula (Robards et al. 1994). Similarities between mass spectra combined with RI were used for the identification of fruit VOCs. The relative abundance of each VOC was estimated from the proportion of peak areas of total ion current (TIC) chromatograms.
Seed dispersers
Field data for seed dispersers from natural populations of V. lindmaniana were obtained over a period of 31 days, from 14–28 July 2023, to 10–25 August 2023, using two 12 MP camera traps equipped with infrared triggers (Bushnell Trophy Cam; Bushnell Outdoor Products, Kansas, USA) to sample the interactions of biotic dispersers with V. lindmaniana fruits. Cameras were installed in distinct focal fruiting plants in the population. Each camera was active for 24 h day−1, for a total of 744 h camera−1. Cameras were moved each 7–15 days, where a new fruiting plant was targeted. Visitors interacting with V. lindmaniana fruits were considered as seed disperser when they consumed and transported seeds through immediate ingestion (endozoochory). During observations with the motion cameras, high resolution images were taken with a Sony FDR-AX100 4 k Handycam attached to a 12 V 60 Ah battery and a 200 W power inverter 12 V DC to 127 V AC. During the observations (2023 fruiting period), faeces of seed dispersers were also collected and examined under a stereomicroscope and light microscope for the presence of Vanilla seeds. Faeces were collected from the leaf litter below phorophytes of V. lindmaniana. Seed dispersers were identified with the help of a field guide for Brazilian birds (Sick 1993).
Seed viability
In vitro germination tests were carried out to determine whether the seeds collected from seed disperser faeces were viable. Here, the procedures were similar to those described to check the seed viability of V. chamissonis and V. pompona (Pansarin 2024a, 2025a). Vanilla lindmaniana seeds were isolated from disperser faeces under a binocular stereomicroscope, while a group of non-scarified seeds (500 seeds, 10 fruits, 10 plants) were used as control. Both scarified and intact seeds (control) were disinfected with a 10% sodium hypochlorite solution for 10 min, then washed three times in autoclaved distilled water. The seeds were then inoculated under a Veco laminar flow into 500 mL flasks previously sterilized and filled with 40 mL of ½ Murashige & Skoog (1962) medium (pH adjusted to 5.8 ± 0.5) and autoclaved at 120°C for 20 min. Each flask (n = 10 per treatment) received ca. 50 seeds. The flasks were maintained under a 40 W LED light for 16 h day−1 at a room temperature of 25–27°C and air humidity of 60–65%. The occurrence of germination and seedling development was determined daily over a period of 1 year. After the period of incubation, the plants were transferred to collective pots, with sphagnum and pine bark. The number of seedlings recorded in the experiments after chemical scarification (seeds collected from avian faeces) and control (non-scarified seeds) were compared using a t-test for independent samples. The analyses were performed using Statistica v. 7.0 software (Statsoft Inc 2004).
RESULTS
Fruit features
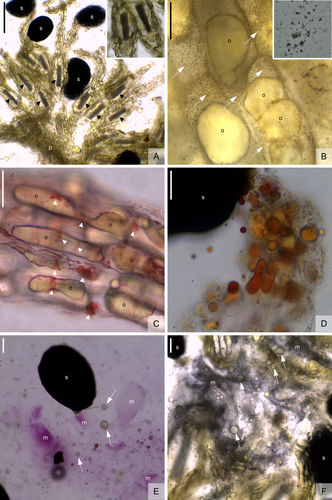
The fruits of V. lindmaniana ripened 9 months after self-pollinations. Fruits were fleshy, oblong to clavate, and measured 5.4–6.7 × 1.1–2 cm (Fig. 1A,B). The epicarp of ripe fruits transitioned from green to yellowish, turning black at the open valves (Fig. 1A,B). The fruit cavity contained thousands of sclerified ovoid seeds 0.5 mm long (Fig. 1A,C) connected to the placentae by long funiculi. All fruits analysed were basipetally dehiscent (Fig. 1A–D). Ripen fruits did not detached from the infructescence within the period of 1 year from the start of maturation (Fig. 1C). Fruit dehiscence occurred through two of three carpel suture lines, throughout two dehiscence splits made by thin-walled cells (Fig. 1D). The dehiscence line is adjacent to a chlorenchymatic tissue that is limited internally by a vascular bundle and externally by a triangular-shaped sclerenchyma (Fig. 1D). Both mesocarp and endocarp had few layers of parenchymal cells. While idioblasts were absent in the mesocarp, a large cell at the base of the funiculus presented raphides (Fig. 2A). Tests with Fehling's reagent showed a positive result for sugar in the placenta funiculi and mucilaginous substance, as demonstrated by the presence of brown precipitates (Fig. 2B). The tests with Xylidine Ponceau showed the presence of protein in the funiculi and placentae, as demonstrated by reddish dots inside the cells (Fig. 2C). The tests with Sudan IV showed a positive reaction for fats in the placentae and funiculi, as revealed by orange dots (Fig. 2D). The funiculi were rich in mucilage, as reactions were positive for Ruthenium red (Fig. 2E) and acid tannic/FeCl3 (Fig. 2F), respectively. The sugar concentration of the mucilaginous substance in V. lindmaniana fruits was 14%–32% (mean 23.2 ± 5.3).

Fruit scent
Fruits were scentless. No VOCs were detected in the GC–MS analyses performed on fruits of V. lindmaniana.
Seed dispersers
The records with motion cameras revealed eight avian species as seed dispersers of V. lindmaniana (Fig. 3A–F, Table S1, Video S1). Birds were commonly attracted to the ripe fruits (Fig. 3A–F, Table S1, Video S1). Sometimes the birds tried to open the immature fruits from the dehiscence zone (Fig. 3D), and from the abscission layer between perianth and ovary (Video S1). All bird species attracted to the V. lindmaniana fruits consumed and dispersed the seeds (Fig. 3A–F, Table S1, Video S1). The most abundant seed dispersers of V. lindmaniana were the birds Tangara sayaca (13 visits; Fig. 3B, Video S1) and Icterus pyrrhopterus (11 visits; Fig. 3E–F, Video S1) in the study area (Table S1). Visits by birds were documented during daytime. In all cases, the visitors accessed the seeds and the sugar-rich mucilage using the fruits' dehiscent valves (Fig. 3A–F). None of the seed dispersers consumed the fruit mesocarp. The avian faeces collected in the study area contained V. lindmaniana seeds (Fig. 3D).

Seed viability
Of the seeds sampled from bird faeces, 32.2 ± 5.2% (N = 161) germinated, while 32.8 ± 5% (N = 164) of the intact seeds germinated. Development of protocorms was recorded from 5 to 7 months of incubation in seeds collected from bird faeces and 9 months after inoculation in seeds that did not undergo acid scarification (Fig. S1). No significant difference was recorded between the group of seeds collected from the bird faeces and the control (i.e., non-scarified seeds; P > 0.005).
DISCUSSION
While the seeds of some Vanilla species are dispersed by both mammals and birds, V. lindmaniana relies exclusively on frugivorous birds as seed dispersers. In fact, V. lindmaniana fruits show many characteristics that support dispersal by birds, such as the absence of odour and a viscous and sugar-rich resource. The lack of scent in fruits of the bird-dispersed V. lindmaniana, may be a plesiomorphic trait, since this species, in addition to V. palmarum and V. bicolor, emerges in a basal position within the Neotropical clade with non-membranaceous leaves as sister to the “aromatic Vanilla clade”. The thick-leafed Neotropical clade is sister of the entire clade, i.e., Asian/African/Caribbean clade, whose species produce scentless fruits (Pansarin & Suetsugu 2022; Pansarin & Menezes 2023). In addition, fruits of V. lindmaniana present funiculi cells with raphide idioblasts that are also an adaptation for ornithochory. The presence of raphides is mainly associated with protection against herbivory (Konno et al. 2014). Species of Vanilla with dehiscent fruits commonly have a mesocarp with copious amounts of raphides (Pansarin 2021, 2025a; Pansarin & Suetsugu 2022). Consequently, mammalian frugivores access the fruit cavity through both pericarp valves (Pansarin & Suetsugu 2022; Pansarin 2025a). This is because mammals need to chew before swallowing, and crystals of calcium oxalate can be released into their mouth, throat and oesophagus, with consequent physical damage (Naude & Naidoo 2007). In addition, oxalic acid is toxic for mammals (Naude & Naidoo 2007). In the case of V. lindmaniana, despite absence of crystals of calcium oxalate in the fruit pericarp, the production of raphides by the basal cells of the funiculus reveals that seed dispersal of this Vanilla species is strongly adapted to non-chewing animals. In fact, the seeds of V. lindmaniana are dispersed exclusively by birds. This is related to avians swallowing the food whole, which is then macerated in their gizzard (Witty et al. 2010). Birds also have resistance to the soluble oxalic acid, as this is mixture of calcium oxalate salts and the organic acids in the plant. The acids present in the gizzard dissolve the calcium oxalate crystals, which are then expelled by the kidneys (Tremaine et al. 1985; Verkoelen & Romijn 1996). My data also show a stable mutualistic relationship between birds and V. lindmaniana, as their seeds are only consumed by birds. Some investigations have revealed that seed ingestion by birds ensures reproduction, as the fruit pulp that are usually germination inhibitors, is removed when seeds pass through the gastrointestinal tract (Carlo et al. 2003). In addition, the experiment on seed viability revealed that scarified seeds germinate in a shorter time than those that have not passed through the bird digestive tract. This is because the gastric acid scarifies and softens the hard seed coat, breaking dormancy and enhancing seed germination after avian evacuation (Meyer & Witmer 1998). The removal of the pulp in the bird gastrointestinal trait also improves gas exchange between the seeds and the external medium, further enhancing seed germination (Meyer & Witmer 1998; Lobova et al. 2009). In addition, these phenomena are crucial for the synchronization of biological processes related to seed germination, being critical in maintenance of the integrity of plant populations and the diversity of Neotropical forests (Howe & Smallwood 1982).
Several basal orchid genera have seeds with hard coats adapted to endozoochory (e.g., Suetsugu 2020; Pansarin 2021, 2025a; Zhang et al. 2021; Pansarin & Suetsugu 2022). Although, for most of these genera, the mechanism of seed dispersal is still unknown; besides Vanilla, ornithochory also occurs in the Asian Cyrtosia (Suetsugu et al. 2015). This suggests that ornithochory may be more widespread in orchids than currently known. Particularly in Vanilla, many species with non-aromatic fruits have both continental and insular populations (Andriamihaja et al. 2020), corroborating that long-distance seed dispersal mediated by birds could be involved. In both obligatorily epiphytes, V. lindmaniana and V. palmarum, since vegetative dispersal via sea currents is unlikely, island colonization must have occurred through long-distance dispersal events mediated by birds. In fact, ornithochory plays an important role in the colonization of new areas by plant species. Many species dispersed by birds occupy vast areas and show either continuous or disjunct populations, suggesting that they disperse seeds over long distances (Viana et al. 2016). Long-distance seed dispersal is a fundamental process by which plants disperse their genes away from the parent plants, expanding their range of distribution (Martínez-López et al. 2020). Birds allow plants to colonize different habitats and establish new populations, including on islands (Viana et al. 2016).
Although V. palmarum has been reported in Cuba, a recent study found that this species is distributed throughout northeastern Brazil, while V. lindmaniana is distributed throughout the Pantanal, Cerrado, and Amazon biomes (Pansarin 2025b). Whereas migration from the Atlantic Forest and Caatinga biomes occurs, most birds that migrate from South America to the Caribbean islands leave from the Amazon (e.g., Somenzari et al. 2018). In this sense, it is most likely that the plants of V. lindmaniana in Cuba originated from South American populations of V. lindmaniana, given the diversity of birds, including frugivores, that migrate from that biome to Caribbean islands. In any case, the decision on species boundaries in Vanilla must be based on multiple data sources (e.g., Pansarin & Menezes 2023; Pansarin 2024b). Integrative studies are needed to confirm the origin of the plants that colonized the Caribbean islands. Apart from the source of seeds dispersed to islands, this study provides the first evidence for long-distance seed dispersal mediated by birds in Vanilla. Furthermore, although several bird species are involved in the seed dispersal of V. lindmaniana, this study shows that there is some specialization, since the seeds of this Vanilla species are not consumed by mammals, nor by invertebrates. Although some studies show a multimodal seed dispersal system (Karremans, Bogarín, et al. 2023; Karremans, Watteyn, et al. 2023), the seed dispersal of some Vanilla species is based on target mutualism (Pansarin 2024a). Here, my data show ornithochory appears pivotal in the effective dispersal of seeds in obligately epiphytic Vanilla, as it ensures that consumed seeds can be taken to other phorophytes through avian faeces, allowing gene flow and the colonization of new areas and environments.
ACKNOWLEDGEMENTS
The author thanks Silvia R.M. Pedro for help with fragrance analyses. I am also grateful to ICMBIO for permission to collect (Protocol SISBIO number 35178-1). This research was supported by the São Paulo Research Foundation – FAPESP (Grant 2018/07357-5) and by CNPq (Productivity Research Grant 301773/2019-0).
CONFLICT OF INTEREST STATEMENT
The author declares no conflicts of interest.




