Style polymorphism in Linum (Linaceae): a case of Mediterranean parallel evolution?
Abstract
- Heterostyly is a sex polymorphism that has challenged evolutionary biologists ever since Darwin. One of the lineages where heterostyly and related stylar conditions appear more frequently is Linum (Linaceae). This group is particularly suitable for testing competing hypotheses about ancestral and transitional stages on the evolutionary building up of heterostyly.
- We generated a phylogeny of Linum based on extensive sampling and plastid and nuclear DNA sequences, and used it to trace the evolution of character states of style polymorphism. We also revised available data on pollination, breeding systems, and polyploidy to analyse their associations.
- Our results supported former phylogenetic hypotheses: the paraphyly of Linum and the non-monophyly of current taxonomic sections. Heterostyly was common in the genus, but appeared concentrated in the Mediterranean Basin and the South African Cape. Ancestral character state reconstruction failed to determine a unique state as the most probable condition for style polymorphism in the genus. In contrast, approach herkogamy was resolved as ancestral state in some clades, giving support to recent hypotheses. Some traits putatively related to heterostyly, such as life history and polyploidy, did show marginal or non-significant phylogenetic correlation, respectively. Although pollinator data are limited, we suggest that beeflies are associated with specific cases of heterostyly.
- The consistent association between style polymorphism and heteromorphic incompatibility points to ecological factors as drivers of the multiple evolution of style polymorphism in Linum. Albeit based on limited evidence, we hypothesised that specialised pollinators and lack of mating opportunities drive evolution of style polymorphism and loss of the polymorphism, respectively.
Introduction
The great variation in flowers across lineages has inspired modern plant classification since Linnaeus (1735), as well as the formulation of hypotheses about the causes of extreme angiosperm diversification, otherwise known as Darwin's abominable mystery (Grant & Grant 1965; Stebbins 1970, 1974; see Friedman 2009 and references therein for an historical account of Darwin's views). This floral variation also occurs within species and populations, can be continuous or discontinuous, and often appears associated with geographic variation, which has been important to provide insights on the biotic and abiotic causes of such variation (Herrera et al. 2006; Strauss & Whittall 2006; Gómez et al. 2009).
Discontinuous variation at the population level, that is presence of discrete and modal phenotypes, has been interpreted in the context of population divergence through disruptive selection (Ortiz et al. 2015). However, discontinuous variation sometimes results from negative frequency-dependent selection, as the fitness of one phenotype strongly depends on the abundance of alternative phenotypes. At equilibrium, it is expected to find all phenotypes at the same proportion in the population. Discontinuous variation is better understood when accompanied by gender differentiation. With negative frequency-dependent selection, the success of the uncommon gender is larger than the common gender, as mate availability for the latter is lower (McCauley & Taylor 1997; Dufay et al. 2009). A similar situation can be achieved without gender differentiation (Pannell et al. 2005). Such is the case of reciprocal style polymorphisms, present in some hermaphroditic plants, where floral morphs display styles and stamens in a reciprocal position (Fig. 1) such that pollination and mating occur more often between morphs rather than within morphs, maintaining the frequency of morphs in balance (Barrett 2002).

The most common style polymorphism is heterostyly (Barrett & Shore 2008), for which flowers in populations present two (distyly) or three (tristyly) morphs. This polymorphism has attracted the attention of evolutionists since Darwin (1877) and early geneticists, who soon discovered its apparently simple genetic basis (Bateson & Gregory 1905). Yet, in those early times, it was recognised that most heterostylous species showed the so-called heteromorphic incompatibility system (only crosses between different morphs are compatible, whereas self-fertilisation and within-morph cross-fertilisation is impeded; Darwin 1877; Dulberger 1992). During most of 20th century, heterostyly was used as model system to study the evolution of inbreeding avoidance. Specifically, most of the studies interpreted the evolutionary pathways of heterostyly following the proposals of Mather & de Winton (1941), with important modifications by Baker (1966), ultimately leading to the quantitative model of Charlesworth & Charlesworth (1979). In short, these models predict that reciprocal style polymorphism evolved after the appearance of the incompatibility system, with an ancestral state of non-herkogamous (homostylous) flowers showing high selfing rates and inbreeding depression. These models were challenged by that of Lloyd & Webb (1992a,b), who suggested that the main driving force for establishment of the polymorphism was the promotion of compatible cross-pollination and a decrease in pollen discount (enhanced male fitness, as Darwin himself proposed in 1877). The latter model presumed (i) an independent evolution of sex organ reciprocity and heteromorphic incompatibility system, and (ii) an ancestral condition of an outcrosser with approach herkogamous flowers (i.e. with the stigma protruding above the anthers). This model strongly emphasised the ecological context of pollination: specialised pollinators select for and maintain the style morphs if they are able to place pollen grains on different parts of the body, and legitimately deliver pollen to the opposite stigmas, with minimal pollen loss.
The model of Lloyd & Webb (1992a,b) has progressively gained more support from both micro- and macro-evolutionary studies. Micro-evolutionary analyses have mostly examined the relative rates of pollination and mating between and within morphs in populations (Lau & Bosque 2003; reviewed in Costa 2017). In contrast, macroevolutionary studies on the heterostylous floral syndrome are relatively scarce compared to population-level studies. In this respect, research in some plant groups, such as Narcissus, Lithodora and related genera, Pontederiaceae, Exochaenium, Amsinckia or Primula (Kohn et al. 1996; Schoen et al. 1997; Guggisberg et al. 2006; Pérez-Barrales et al. 2006; Ferrero et al. 2009; Kissling & Barrett 2013; Santos-Gally et al. 2013) have provided strong support to Lloyd & Webb's (1992a,b) ideas. Given that heterostyly is well represented both among lineages of angiosperms (28 families across many orders in both monocots and dicots; Barrett & Shore 2008) and biomes, these studies offer good opportunities to explore ecological and biogeographic correlates of heterostyly in order to infer the conditions that trigger the appearance and maintenance of this polymorphism. For example, heterostyly should be common in plants with specialised pollination, or disadvantageous where outcrossing is at risk, as expected when pollinators are scarce or in highly disturbed environments (Piper et al. 1986). Likewise, it would be unlikely to find heterostyly associated with hybridisation and polyploidy (both associated with self-fertilisation as by-product; Ramsey & Schemske 1998) or with short-lived plants, particularly in annuals, as these typically present higher selfing rates and occur more frequently in disturbed places compared to perennial plants (Barrett 2002).
Heterostyly in Linaceae was first reported in the seminal works of Darwin (1864, 1877) and Hildebrand (1864). In particular, Darwin's experimental and observational work on Linum grandiflorum and L. perenne was influential to infer the function of the polymorphism. Later, it was suggested that other genera in the family could include distylous and tristylous species (Lloyd et al. 1990; Thompson et al. 1996). After Darwin′s work, geneticists used species of Linum to study the inheritance of heterostyly, and showed that style polymorphism and heteromorphic incompatibility appeared linked (Lewis 1943; Dulberger 1992; Lewis & Jones 1992; Ushijima et al. 2012). Furthermore, the stability of heterostyly as a trait has been valuable for taxonomists, who used it as a binary character (‘heterostylous’ versus ‘homostylous’) in identification keys and diagnoses (e.g. Ockendon & Walters 1968; Ockendon 1971; Martínez-Labarga & Muñoz-Garmendia 2015; Ruiz-Martín et al. 2015). Thus, taxonomic descriptions have been valuable to characterise species and conduct evolutionary reconstructions of the trait (McDill et al. 2009). However, Linum is a highly diverse genus with a wide geographic distribution, in which the diversity of stylar conditions is much greater than previously reported (J. Ruiz-Martín, unpublished data; Darwin 1877; Heitz 1980; Armbruster et al. 2006). Most of the taxonomic diversity appears in the Mediterranean and, surprisingly, the morphological variation on the types of polymorphism and other associated traits remain to be explored. Thus, Linum represents an excellent study system for testing macro-evolutionary hypotheses and correlates with heterostyly.
The specific aims of our study were: (i) to generate an updated phylogeny of Linum, including lineages and infra-generic taxa recognised in taxonomic studies, (ii) to estimate divergence times in order to date events of evolutionary significance for the polymorphism, (iii) to reconstruct ancestral states for stylar condition and other related traits, (iv) to estimate the significance of correlated evolution between style polymorphism and those other traits across the phylogeny, and (v) to integrate all these results in a geographic and ecological context, in order to infer the conditions under which heterostyly most likely evolved. Ultimately, we wished to validate current evolutionary models of heterostyly.
Material and methods
Floral measurements and categorisation
Previous work reported that style polymorphism in Linum concentrates mostly in the Mediterranean Basin and South Africa (McDill et al. 2009). Thus, we concentrated our field sampling efforts in these regions (although other regions were also explored), and retrieved information about the type of polymorphism from published sources. We collected up to 100 flowers from 50 populations of 50 taxa of Linum (Table S1), and preserved flowers in 70% ethanol for morphological measurement in the laboratory. Linum flowers have five styles and five stamens, each of them reaching similar heights (we conducted a pilot study to assess within-flower variation in the position of anthers and stigmas, and found that it was nearly negligible; results not shown). Anther and stigma heights were measured as the distance from the base of the ovary to the top of the organ. All measurements were taken from digital images of the lateral view of flowers with petals removed, using ImageJ (Rasband 2008). Images were taken using a stereomicroscope (Zeiss Stemi-2000) with attached digital camera (Zeiss Axiocam). Data for the remaining Linum species and outgroups were collected from the literature (see Table S1).
We classified flowers of style polymorphic species as L-morph when the stigmas were positioned above the anther whorl, and S-morph when the stigmas were below the anther whorl. Style polymorphism includes two morphs (distyly and stigma height dimorphism) or three morphs (tristyly and stigma height trimorphism); here we refer to stigma height polymorphism as the discrete variation in stigma height but not in anther height, a condition related to heterostyly (Barrett et al. 2000). Species with populations with only one floral morph were named monomorphic and classified as follows: homostylous (no apparent separation between sexual organs), approach or reverse herkogamous (stigmas placed above or below the anther whorl) and horizontal herkogamous (anther–stigma separation along the horizontal plane of the flower). This classification was based on extensive flower measurements and the frequency distribution of sex organ heights among population (J. Ruiz-Martín, unpublished data). It is important to highlight that most taxonomic references classify style polymorphism as heterostylous (sometimes discriminating distyly from tristyly) or homostylous; the latter referring to any style monomorphic condition, regardless of the relative position of anthers and stigmas (see description above). This distinction is critical for testing models of evolution of heterostyly in relation to the ancestral stylar condition (true non-herkogamous homostyly in Charlesworth & Charlesworth 1979 versus approach herkogamy in Lloyd & Webb 1992a). Hence, we characterised those species that could not be sampled in the field using the quantitative information provided in taxonomic descriptions (e.g. approach or reverse herkogamous when no overlap was reported between stamen and style length, otherwise homostylous).
We included other biological traits of species putatively related to style polymorphism, and gathered information from the literature on life history (i.e., annual or perennial), chromosome number, breeding system, pollinators, ancillary traits (polymorphism in size and form of pollen grains and/or stigma papillae) and genetic control of polymorphism (see Table S1 for references).
Given the lack of a comprehensive monograph for species identification on Linum, we followed the most recent and comprehensive taxonomic treatment for regions with high species diversity in the genus: Yusepchuk (1949), Davis (1967), Ockendon & Walters (1968), Rogers (1981), Greuter et al. (1984), Yılmaz & Kaynak (2008) and McDill et al. (2009).
Phylogeny and divergence times
Sampling
A total of 103 samples from 93 species or subspecies of Linum were included as in-group, representing the five taxonomic sections. Two or three samples from different localities were included for nine Linum species with taxonomic doubts to test for monophyly. In addition, samples from eight species representing closely related genera (Anisadenia, Cliococca, Hesperolinon, Hugonia, Radiola, Reinwardtia, Sclerolinon and Tirpitzia; McDill et al. 2009) were included to evaluate if Linum is a monophyletic genus. Three species from closely related families (Hypericum perforatum from Hypericaceae, Viola pubescens from Violaceae and Humiria balsamifera from Humiriaceae) were also included as out-group (Table S1).
Fifty-five leaf samples from 48 species or subspecies of Linum were collected during field trips (vouchers stored at SEV herbarium; Table S1), whereas leaves from an additional 18 taxa were obtained from herbaria collections (SEV, MA and E; Table S1). The DNA sequences from the remaining 29 species of Linum, eight of Linaceae and three from other families were directly downloaded from the GenBank database and previously published sources (see Table S1 for species and references). Two taxa were sampled in the field and obtained from herbaria.
DNA extraction, PCR and sequencing
Total genomic DNA was extracted using DNEasy Plant Minikit (QIAGEN, BIO Laboratories, Carlsbad, CA, USA). One nuclear DNA region, ITS (internal transcribed spacer, and three plastid DNA regions, NADH dehydrogenase subunit F (ndhF) gene, maturase K (matK) gene and trnL-F spacer, were amplified, purified and sequenced. PCR amplification was performed following McDill et al. (2009) with minor modifications. Products were purified using ExoSAP-IT (USB, Cleveland, OH, USA). Sequencing reactions were performed using the ABI BigDye® Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific, MA, USA) in Macrogene Europe Laboratory (Amsterdam, The Netherlands).
Phylogenetic analyses
Sequences from the four DNA regions were aligned separately using MaffT 6.0 FFT-NS-I (Katoh & Toh 2008) as implemented in Geneious Pro™ 5.3 (Kearse et al. 2012). The resulting alignments were manually revised. Putative homoplasic regions were detected and removed from the alignments using GBlock v0.91b (Castresana 2000). Incongruence between DNA regions was discarded and the four DNA regions were combined in a single matrix (2,900 bp).
Bayesian inference analysis was performed using Markov chain Monte Carlo (MCMC) as implemented in MrBayes 3.0b4 (Huelsenbeck & Ronquist 2001). The best-fitted model of DNA evolution for each DNA region was selected from the analysis in ModelTest 3.06 (Posada & Crandall 1998). GTR + G + I was selected for ndhF and matK regions and GTR +G for trnL-F and ITS regions. To avoid over-parameterisation, we combined the three plastid regions in a matrix and analysed them together using GTR +G +I model. Two independent analyses of four Metropolis-coupled Markov chains were run for 10 million generations. After a burn-in of 25%, the remaining trees (15,000) were used to construct a majority-rule consensus tree using posterior probability values as a measure of clade support. Phylogenetic analyses were performed using CIPRES Science Gateway version 3.3 portal (Miller et al. 2010).
Analyses of divergence times
The four DNA regions were combined in a single partition (using GTR + G + I as DNA model of evolution). Analyses were conducted using three independent MCMC runs of 120 million generations each, using Yule process as tree model and relaxed clock log normal as clock model, as implemented in BEAST v1.4.8 (Drummond & Rambaut 2007). Run convergence and burn-in were assessed in Tracer 1.6 (Rambaut & Drummond 2007). Trees from the three independent runs were combined using LogCombiner 1.4.8 (10% of burn-in). Maximum clade credibility trees were calculated with TreeAnnotator 2.3.2 using a posterior probability limit of 0.95, maximum clade credibility tree and the mean heights options.
Two calibration points were used: (i) a secondary calibration based on the age of the stem node of Linaceae, which is the Malpighiales crown node (Bell et al. 2010). Specifically, a normal distribution with a mean of 93.5 MYA (95% CI, 88–97 MYA) was used as recommended for secondary calibrations. (ii) A log-normal distribution with mean = 0, SD = 1.0 and zero offset = 33.9 for the crown node of genus Linum (which includes genera Cliococca, Hesperolinon, Radiola and Sclerolinon). This last calibration point accounts for the oldest Linum fossil. This is a pollen grain from Ebro River Basin (33.9–37.2 Ma, Late Eocene; Cavagnetto & Anadón 1996). Analyses of times of divergence were performed using CIPRES Science Gateway version 3.3 portal (Miller et al. 2010) and the cluster located in the Andalusian Scientific Information Technology Center (CICA, Seville, Spain).
Ancestral state reconstruction
We used maximum likelihood approaches to reconstruct the ancestral states of the stylar polymorphism in Linum, implemented in R (R Development Core Team 2015). We performed the analyses on the BEAST Bayesian phylogenetic tree obtained from ITS and chloroplast DNA regions. This tree was pruned to remove tips when the information on character state was unavailable. Because we included more than one sample for eight species, we also pruned the additional samples for the same species in the case of monophyly. Outgroup species and Hugonia busseana (Linaceae) were also pruned. Character ancestral state was estimated for each internal node of the tree using the re-rooting method of Yang et al. (1995) provided as a function in the package ‘phytools’ (Revell 2012), where conditional probabilities are calculated for the root node (which is the same as the marginal state reconstruction for that node) and consecutively moves the root to each node in the tree. First, just to compare results with former studies based on a simple binary codification (McDill et al. 2009), we reconstructed ancestral states to understand the evolution of monomorphic versus polymorphic states. The former included any of the states without within-population differentiation in morphs, with or without herkogamy; the latter included any of the style polymorphisms found. Second, we considered for the analysis of ancestral state reconstruction only relevant states to the two competing hypotheses of the evolution of heterostyly (Charlesworth & Charlesworth 1979; Lloyd & Webb 1992a). Thus, we formed five state groups: (i) monomorphic homostyly (ancestral state proposed by Charlesworth & Charlesworth 1979); (ii) monomorphic approach herkogamy (ancestral state proposed by Lloyd & Webb 1992a); (iii) monomorphic reverse herkogamy, which is the alternative state to monomorphic approach herkogamy; (iv) style polymorphism, including conventional distyly, three-dimensional distyly, stigma-height dimorphism and trimorphism; and (v) monomorphic horizontal herkogamy. The last is not considered in any of the models, but it was found in some species and we were interested in determining its evolutionary pathway. Finally, because the most common ancestor of Linum (genus Tirpitzia) presents two monomorphic and one heterostylous species (Suksathan & Larsen 2006), we reconstructed ancestral states for Linum codifying the genus Tirpitzia first as monomorphic and second as heterostylous.
Phylogenetic correlations
To test the evolutionary correlations between stylar polymorphism and life history, and stylar polymorphism and polyploidy in Linum, we performed Pagel's (1994) binary character correlation test implemented in the package ‘phytools’ (Revell 2012) in R (R Development Core Team 2015). We performed the analyses on the same tree used for ancestral reconstruction analysis. The tree was pruned to include species for which information on stylar morph (monomorphic versus polymorphic), life history (perennial versus annual) and polyploidy (diploid versus polyploid) was available. The method applies a continuous-time Markov model of trait evolution that calculates the likelihood of discrete trait data under two models of evolution: one in which the traits are allowed to evolve independently of one another on the phylogenetic tree, and one in which they evolve in a correlated fashion (dependent model). The independent and dependent models can be compared by means of a likelihood ratio test, calculated as 2(log[likelihood (dependent model)] – log[likelihood (independent model)]). Significance of the difference in log likelihoods is based on a χ2 distribution with four degrees of freedom (four parameters are estimated in the independent model and eight are estimated in the dependent model). The parameters of the model of trait evolution are the values of the transition rates between the four possible character state combinations in a model of correlated evolution.
Results
Style polymorphism and other traits
Table S1 includes detailed information on species traits. From field sampling or from bibliographic sources we obtained information for 85 Linum species or subspecies, and 11 outgroup species. Our data includes 60% of species number (141) of Linum, as recorded in The Plant List (2013). Detailed quantitative data of flower measurements are still unpublished, and here we summarise the main results (see Table S1). Within Linum, 44 (47.3%) species presented some kind of style polymorphism, 41 (44.1%) were monomorphic and eight (8.6%) lacked sufficient information to ascertain the stylar condition. Style polymorphic species were mostly distylous (two morphs), but we identified deviations from typical distyly in some species, which we describe here. Armbruster et al. (2006) reported a new type of distyly in the western Mediterranean endemic L. suffruticosum, showing high reciprocity, in three dimensions: on the vertical axis of the flower (flowers are from either L- or S-morph), on the radial axes (flowers have either outer stamens and inner styles or vice versa) and on the longitudinal axis of each sex organ (anthers and stigmas are twisted to inner or outer side of the flower; Fig. 1). Information provided by Darwin (1877) on L. grandiflorum indicates that the species displays stigma-height dimorphism, that is styles are either long or short, but stamens are not perfectly in a reciprocal position to stigmas. In the literature, we also found that Heitz (1980) mentioned some populations of L. perenne as having similar stigma-height dimorphism as in L. grandiflorum. Finally, L. hirsutum represents an interesting case resembling trimorphism; in our survey, we observed two anther levels and three style lengths in three populations sampled, but our sample size was limited as to completely ascertain it (J. Ruiz-Martín, unpublished data). Given the paucity of these unconventional cases of polymorphism, all of them were pooled as style polymorphism for the analysis of ancestral state reconstruction and correlated evolution, and their particular position along the tree is discussed below.
Monomorphic species or subspecies of Linum were also variable: non-herkogamous homostyly was observed in 16 species, approach herkogamy in 19 species, reverse herkogamy in three species and horizontal herkogamy also in three species.
We found information on breeding system in only 19 species. Twelve species were reported as self-incompatible and seven species as self-compatible; the former were all style polymorphic whereas the latter were all monomorphic. All self-incompatible species presented a typical heteromorphic incompatibility system. We found data on ancillary traits (any heteromorphism on pollen size or colour, exine sculpturing, stigma width, stigmatic papillae) for eight taxa, all of them being distylous.
With regard life form, 27% of Linum species in our sample were annual and 73% perennial (Table S1). We found reports on chromosome numbers in 50 taxa, with 23 being style polymorphic and 27 monomorphic. Ten out of the former and three out of the latter showed variation in the level of polyploidy (different counts of the whole chromosome set; Table S1). A particularly noteworthy case is that of L. suffruticosum, with a polyploid series from diploid to decaploid (Nicholls 1986; A. Afonso, personal communication).
The current information on the pollination biology of Linum species is scarce. Beeflies from the genus Usia (Bombyliidae) seem important pollinators in some distylous species from the Mediterranean Basin. Distylous L. pubescens was almost exclusively pollinated by U. bicolor in the eastern Mediterranean (Johnson & Dafni 1998; Gibbs 2014). Armbruster et al. (2006) observed that L suffruticosum was also almost exclusively pollinated by several Usia beeflies, whereas other flies and bees visited flowers but did not function as effective pollinators. Our own observations in additional populations of L. suffruticosum confirmed that Usia beeflies are the main pollinators, as well as in the distylous western Mediterranean L. tenue, and to a lesser extent L. viscosum and L. narbonense (unpublished data). In contrast, monomorphic European Mediterranean L. tenuifolium was visited by a wide array of pollinators, including mostly bees and, to a lesser extent, flies (but not beeflies) of different size (see Fig. 1). Monomorphic L. bienne was reported to be visited by large Bombylius spp. beeflies (Boesi et al. 2009), which often hover over flowers to collect nectar, rather than crawl down to the bottom of the flower, as observed in smaller Usia (Johnson & Dafni 1998; Armbruster et al. 2006). Its close relative, the monomorphic L. usitatissimum (cultivated flax) appeared to be visited mostly by bees (Ssymank et al. 2009). Finally, Kearns & Inouye (1994) reported that North American monomorphic L. lewisii received visits by 25 species from nine families of flies and 19 species from four families of different orders, with very different body size, pollination efficiency, visit rate and frequency across populations.
Phylogenetic reconstruction based on Bayesian inference
The analyses of the three plastid (rbcL, matK and trnL-F) and nuclear (ITS) regions recovered congruent topologies under Bayesian criteria (data not shown), thus a consensus tree is shown (Figure S1). Inferred trees were partially congruent with taxonomic subgeneric classification of Linum (sections) as already shown by McDill et al. (2009). Whereas the genus Linum was paraphyletic, as core Linum included the genera Cliococca, Hesperolinon, Screrolinom and Radiola, the family Linaceae was monophyletic. The topology recovered by MrBayes (Figure S1) showed two main clades, similar to what was found by McDill et al. (2009). The first clade, Clade A, was mainly formed by sections Linum and Dasylinum, mainly from Eurasia. Specifically, a species from section Linum, L. stelleroides from China, is sister to two main clades, Clade A1, formed by most of the species from section Dasylinum, and the second clade, Clade A2, formed by most of the species from section Linum (including also some species from section Dasylinum). The second main clade, Clade B, was formed by the other genera included in core Linum and the remaining sections (Linopsis, Syllinum and Cathartolinum). Specifically, Radiola is sister to two main clades, Clade B1, formed by genera Cliococca, Hesperolinon and Scleronlinon and section Linopsis from North and South America and South Africa, and the second clade, Clade B2, formed by sections Linopsis (excluding the species from America and South Africa), Syllinum and Cathartolinum, and with a distribution mainly in Europe, Mediterranean Basin and western Asia.
Times of diversification
The topology of the maximum credibility tree inferred from BEAST (Fig. 2) analyses was highly congruent with the majority rule consensus tree inferred from MrBayes. The divergence time for crown node of Linaceae was 61.35 (MYA) (95% CI: 44.48–84.62; Fig. 2). The crown node of core Linum was dated back to 35.37 MYA (95% CI: 33.95–43.31). The crown node of Clade A was dated back to 30.38 (95% CI: 23.65–38.59). The crown node of Clade A1 was about 10.62 MYA (95% CI: 5.62–17.42) and the crown node of Clade A2 was about 21.89 MYA (95% CI: 15.2–28.67). The crown node for Clade B was dated back to 19.7 MYA (95% CI: 11.48–29.49). Finally, the crown node of Clade B1 was about 9.02 MYA (95% CI: 5.58–29.49) and the crown node of Clade B2 was about 14.67 MYA (95% CI: 8.95–22.06).
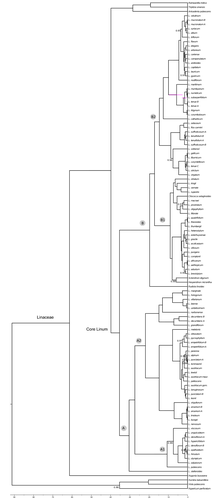
Evolutionary pathways of style polymorphism and phylogenetic correlations
Binary reconstruction (monomorphism versus polymorphism)
There were no significant differences when Tirpitzia was coded as polymorphic or monomorphic. Equivocal ancestral state reconstruction of the most common ancestor of Linum and core Linum (Clade A, Clade A1, Clade A2, Clade B and Clade B2; Figure S2) precludes inference whether the evolution of heterostyly derived from the monomorphic or polymorphic condition. However, within particular clades in the genus it is possible to infer some trends. In Clade A1, there is a transition from polymorphism to monomorphism, although this is not significant (see L. seljukorum). Within Clade A2, three clear and significant transitions from polymorphism to monomorphism were inferred (see L. leonii, L. pallescens and L. lewisii). The transitions from monomorphic to polymorphic state are also inferred in this clade (see L. grandiflorum and L. narbonense) but they were not significant. The most recent common ancestor of Clade B1 is clearly inferred as monomorphic, with two significant transitions to polymorphism (see South African L. comptonii and L. heterostylum). Within Clade B2, transitions from polymorphism to monomorphism and from monomorphic to polymorphic states were not clear.
Five-state reconstruction
There were no significant differences when Tirpitzia was coded as polymorphic or monomorphic. Again, equivocal ancestral state reconstruction of the most common ancestor of Linum precludes sound inference (Figure S3). The most recent common ancestor of core Linum, Clade A, Clade A1, Clade A2, Clade B and Clade B2 is equally likely to have presented homostyly or polymorphic state. Within Clade A, clear and significant transitions from polymorphism to homostyly (see L. leonii and L. pallescens; also see L. seljukorum although it was not significant) and from polymorphism to approach herkogamy (see L. lewisii) were inferred. Also within Clade A, transitions from homostyly to polymorphic state (see L. grandiflorum and L. narbonense) and, to approach herkogamy (see L. hologynum) were inferred, although they were not significant. The most recent common ancestor of Clade B1 is approach herkogamy with four possible transitions inferred: to horizontal herkogamy (see He. micrantum and L. tenuifolium), to polymorphism (see L. comptonii and L. heterostylum), to reverse herkogamy (see L. littorale and L. prostratum) and to homostyly (see S. digynum). Reconstruction of shallower nodes of Clade B2 inferred clear and significant transitions from polymorphic state to reverse herkogamy (see L. nodiflorum), to horizontal herkogamy (see L. tenuifolium) and to homostyly (see L. corymbulosum, L. trigynum clade; only marginally significant). Also within Clade B2 a transition from homostyly or from polymorphic state to approach herkogamy was inferred (see L. volkensii).
Trait correlations
There was marginal support for the correlation between presence of stylar polymorphism and perennial life history of species. Our results indicated that a dependent model of evolution between life history and stylar polymorphism provided a marginally significant better fit to the data than an independent model (difference between likelihood ratio = 9.136, P = 0.057). For the set of 50 species where we were able to obtain data on chromosome number, there was no significant correlation between presence of stylar polymorphism and polyploidy (difference between likelihood ratio = 3.646, P = 0.456).
Discussion
Linaceae include one of the largest diversities of style polymorphisms, with homostyly and different types of herkogamy, stigma-height dimorphism and trimorphism, distyly and tristyly, and Linum seems to display most of this diversity. This allows testing of evolutionary models for traits where specific transitions are predicted, as proposed by Charlesworth & Charlesworth (1979) and Lloyd & Webb (1992a). In particular, Lloyd & Webb's (1992a) model challenged the formerly prevalent ideas represented by Charlesworth & Charlesworth (1979), and proposed an alternative ancestral condition (approach herkogamy, instead of homostyly) to heterostyly. Interestingly, Hugonia within Linaceae was one of the study cases that inspired the new model (Lloyd et al. 1990), which was later confirmed as tristylous (Thompson et al. 1996; Meeus et al. 2011). Trimorphism is here also reported in Linum, widening the variety of stylar conditions in the genus.
Although the variation in Linum inspired Darwin to interpret the adaptive significance of heterostyly (Darwin 1877), it is surprising that the wide variation of stylar conditions in the genus has been rarely explored. In our study, we wished to validate current evolutionary models, for which we generated an updated phylogeny, incorporated the stylar conditions, and explored trait correlates to throw light on the plausibility of the alternative models. As discussed below, our results failed to ascertain clearly the ancestral condition in the genus, which precluded supporting any of the competing models, with the exception perhaps of the South African clade, which supported the Darwinian model of Lloyd & Webb (1992a). The additional information gathered for other traits was limited, and precluded statistical analyses to incorporate the evolutionary significance of breeding systems, pollination biology and biogeography of species for this purpose. However, life history provided plausible explanations for the presence of style polymorphism. Our main result is that, with the available data, both models could explain parts of the evolution of heterostyly in Linum.
Phylogeny, divergence times and geographic ranges
We confirmed taxonomic aspects that deserve further work (e.g. the inclusion of four Linaceae genera resulted in the paraphyly of Linum, and the non-monophyly of some sections; see McDill et al. 2009; McDill & Simpson 2011). Despite our sampling efforts almost duplicated sampling in previous systematic work (McDill et al. 2009) and included a larger proportion of Linum species, and that some of the DNA regions used were different, we obtained similar results to those previously reported by McDill et al. (2009) and McDill & Simpson (2011), making the phylogeny reported here valuable for testing evolutionary hypotheses.
In our study we found that, unlike species from other geographic regions, the South African species, which all belong to the section Linopsis, formed a well-supported monophyletic clade. In addition, the South African clade turned to be closely related to the American clades, rather than the Euroasiatic clades from the same section. This result has important implications for evolutionary interpretations because none of the surveyed American Linum species present stylar polymorphisms, while species in section Linopsis in Eurasia do. In our analyses, we were interested in estimating the sequence of divergence dates leading to clades present in the Mediterranean Basin and South Africa, the latter being the only region with style polymorphic Linum species outside the Mediterranean Basin. Thus, it is remarkable that the South African clade separated from its monomorphic sister American clade in the late Miocene, about 9 MYA. In contrast, its closest Mediterranean clade, which includes members of section Linopsis and section Syllinum (with mostly western and eastern Mediterranean species, respectively), diverged much earlier (in middle Miocene, > 14 MYA). Mediterranean clades include many style polymorphic species, whereas American clades did not include style polymorphism. By the time the clades split, continents were already separated, particularly Africa and the Americas. Thus, episodes of long-distance dispersal should be invoked or, alternatively, massive extinctions of connecting clades in Africa, which would not have left a living or fossil trace. These episodes are coincident with last Antarctic glaciation and sharp decrease in temperature in southern Africa (Linder 2005). A thorough biogeographic analysis incorporating explicit palaeo-geographic settings would be necessary to ascertain the most likely scenario.
Evolution of style polymorphism in Linum (models test)
Previous work in Linum (McDill et al. 2009) provided a plausible reconstruction of pathways of heterostyly and ‘homostyly’ (including all types of monomorphic conditions). Our findings were similar to those previously reported in terms of phylogenetic relationships (Figure S2) but the interpretation of evolution of style polymorphism is different. Specifically, we were unable to determine the most likely ancestral stylar condition in the genus, which could be either style polymorphic and monomorphic (our terms). The variability of stylar conditions in Linaceae and in Linum (Ganders 1979; Lloyd et al. 1990; Thompson et al. 1996; Suksathan & Larsen 2006; McDill & Simpson 2011) combined with the inferred high transition rates among character states, and long branches arising from the root of the phylogeny may explain this lack of resolution. An analysis at the family level would probably provide a better resolution of the ancestral condition. Despite lack of resolution at the basal stage, we detected several events of independent evolution of the polymorphism through the evolutionary history of Linum. Although some clades were integrated by mostly monomorphic or polymorphic species, any of these conditions appeared secondarily lost, even in pairs of sister species. For example, loss of polymorphism was detected in L. seljukorum–L. pubescens, L. leoni–L. punctatum, L. lewisii–L. pallescens, L. tenuifolum–L. suffruticossm, L. corymbulosum–L. trigynum. In addition, polymorphic species evolved repeatedly in mostly monomorphic clades, as shown by the species pairs L. grandiflorum–L. decumbens, L. comptoni–L. pungens, L. heterostylum–L. esterhuysenae. Particularly dynamic on evolutionary grounds was Clade B2 (Figure S2), especially its western Mediterranean subclade, including species from L. virgatum to L. setaceum. This clade includes L. suffruticosum s.l. (López González 1979; Martínez-Labarga & Muñoz-Garmendia 2015), with a special case of three-dimensional reciprocity (Armbruster et al. 2006), L. tenue, a polyphyletic species with substantial morphological variation in NW Africa (J. Arroyo and J. Ruiz-Martín, personal observations), as well as a recently named new distylous species, L. flos-carmini (Ruiz-Martín et al. 2015), different from its sister homostylous L. setaceum. All this variation clearly reflects that further work is required in these taxa and geographic ranges.
Perhaps one of the most remarkable outcomes is the independent evolution of heterostyly in two South African species within a clade integrated by 14 species. In his taxonomic review, Rogers (1981) suggested that heterostyly appeared in South Africa independently from its occurrence in the Mediterranean Basin and nearby regions, which was later supported by McDill et al. (2009), and confirmed here. Our population sampling allowed us to confirm the presence of distyly in L. comptonii and L. heterostylum. Because the South African Linum clade was monophyletic and closely related to the monomorphic clade of American Linum species, the independent evolution of the polymorphism is thus fully supported. Unlike American species, all South African Linum species, except L. thurnbergi, are restricted to the Mediterranean-type climate of the Cape Floristic Region (CFR) (Rogers 1981). Thus, the presence of style polymorphism restricted to Mediterranean climates (CFR and the Mediterranean Basin) points to an apparent case of parallel evolution linked directly or indirectly to climate. In other Mediterranean climate regions of the world the number of Linum species is much lower.
After characterisation of monomorphism as homostyly and different types of herkogamy (Figure S3), the only clade within Linum with certainty in the ancestral condition was the South African clade. Here, the Lloyd & Webb (1992a) model was fully supported, with approach herkogamy as ancestral condition. Interestingly, approach herkogamy is widespread in this South African clade. In contrast, approach herkogamy is uncommon in other clades (e.g. L. hologynum, L. lewisii and L. volkensii) whereas homostyly appears frequently. This homostyly is secondary, derived from a polymorphic condition, and probably associated with shifts towards selfing to increase reproductive assurance (see e.g. L. corymbulosum and L. trigynum or L. leonii). Such shifts have been reported in other style polymorphic groups (Schoen et al. 1997; Guggisberg et al. 2006; Mast et al. 2006; Pérez-Barrales et al. 2006; Kissling & Barrett 2013; Santos-Gally et al. 2013). More detailed information on the breeding system of the species would confirm this hypothesis.
Other stylar conditions are scarcer. Reverse herkogamy, a necessary phenotype in an intermediate step for the establishment of style polymorphism in any model, was detected in the Mediterranean L. nodiflorum and the two South American sister species L. littorale and L. prostratum. Surprisingly, reverse herkogamy appeared in these species as derived monomorphic condition. This transition has been reported in Exochaenium in the Gentianaceae (Kissling & Barrett 2013), although the mechanisms remains unclear that might favours the selection of monomorphic reverse herkogamy. Horizontal monomorphic herkogamy was detected in two Linum species, L. kingii and L. tenuifolium, and in two closely related genera, Hesperolinum and Radiola, which are placed within Linum. This condition might result from selection to avoid self-pollination, as in the self-compatible L. tenuifolium (Nicholls 1986; see Fig. 1). Finally, it was not possible to include an evolutionary reconstruction of stigma-height dimorphism, as it is an unusual condition in Linum, only present in L. grandiflorum and perhaps L. perenne (Heitz 1980). This condition has been reported as an intermediate and unstable state towards heterostyly (Lloyd & Webb 1992b; but see Barrett & Harder 2005), which is consistent with its unclear ancestral/derived condition. This evolutionary lability has been reported for stigma-height dimorphism in some Boraginaceae (Ferrero et al. 2009).
Correlated evolution and trait associations
Few studies have attempted to investigate correlations between style polymorphisms and other traits in an explicit phylogenetic context, and these have focused on associations with other floral traits (e.g. corolla size and form: Santos-Gally et al. 2013; Kissling & Barrett 2013). In our study, we investigated the association between style polymorphism and life history (annual versus perennial). This association is expected (Dulberger 1992) because pollination of style polymorphic plants is often specialised (Darwin 1877; Lloyd & Webb 1992a; Lau & Bosque 2003), and short-lived plants, especially annuals, are more sensitive to loss of these pollinators or pollinator uncertainty, and shifts to selfing are common. Our results showed that style polymorphism occurs more frequently among perennial than annual species, although the association was only marginally significant. However, we only gathered data for a subset of species, and data on breeding systems from more species would be particularly valuable here. Despite the limitations, this result suggests that reproductive assurance is probably important in annual species, and most likely plays a role against maintaining style polymorphism.
An important trait associated with breeding system and thus with style polymorphism is polyploidy. In principle, if polyploidy is associated to selfing it should be negatively correlated with style polymorphism. The available evidence is mixed across angiosperms, ranging from lack of association to heterostyly being frequent among diploids (Naiki 2012). As shown for Primula, heterostyly is negatively associated to allopolyploidy (Guggisberg et al. 2006), probably due to breakdown of heterostylous supergenes by recombination after hybridization (Lewis & Jones 1992). Most of hybridisation between Linum species is homoploid (Seetharam 1972; Muravenko et al. 2003; Yurkevich et al. 2013), which does not promote breakdown of heterostyly. We were unable to detect a significant correlation between polyploidy and heterostyly in our data set of 50 species of Linum. It is possible that this data set includes mostly polyploidy series of autopolyploids, which do no promote either breakdown of heterostyly (Naiki 2012). This is well illustrated by the closely related L. tenuifolium and L. suffruticosum. Linum tenuifolium is monomorphic and diploid across its wide range in Europe and western Asia (Nicholls 1986). In contrast, L. suffruticosum, with three-dimensional distyly (Fig. 1; Armbruster et al. 2006), displays a polyploid series from diploidy to decaploidy (Nicholls 1986; A. Afonso, unpublished data) across its western Mediterranean range.
In the limited data set available, all self-incompatible species display heteromorphic incompatibility with typical ancillary traits, whereas all self-compatible species are monomorphic, with no intermediate cases being reported. Thus, the independent evolution of presence and type of self-incompatibility and style polymorphism proposed by Lloyd & Webb (1992a) is not supported.
A possible role of pollinators in the evolution of style polymorphisms in Linum?
One of the most insightful predictions made by Lloyd & Webb (1992a) stated that pollinators are critical for the selection of style polymorphisms. Pollinators need to fit tightly with flowers and contact anthers and stigmas with specific body parts to legitimately transfer pollen between morphs. This involves precise shapes of flowers and behaviours of pollinators. At present, the scarcity of pollinator data on Linum precludes explicitly testing this hypothesis across the genus. However, studies on the pollination ecology of some species offer interesting insights. This has been approached in L. pubescens (eastern Mediterranean range, section Dasylinum, Clade A1 in Figure S3; Johnson & Dafni 1998) and L. suffruticosum (western Mediterranean, section Linopsis, Clade B2 in Figure S3; Armbruster et al. 2006), both almost exclusively pollinated by Usia beeflies (Bombyliidae), with U. bicolor in L. pubescens and two species of different size in L. suffruticosum. Armbruster et al. (2006) described that the three-dimensional reciprocity in L. suffruticosum allows separation of the placement of pollen from L and S flowers on the ventral and dorsal parts of the Usia body, respectively. Those authors interpreted this as the combination of the Usia behaviour with the three-dimensional reciprocity probably increasing legitimate pollinations between style morphs (Fig. 1). Usia species seem to commonly visit other Mediterranean distylous Linum species (Du Merle & Mazet 1978; and personal observations). Interestingly, Usia is a truly Mediterranean genus, with its highest species diversity in the southern Iberian Peninsula, northwest Africa and Anatolia (Gibbs 2011, 2014), regions with the largest diversity of Linum species. Whether heterostyly in Linum is restricted in the Northern Hemisphere to the Mediterranean Basin due to its tight association with Usia flies, is a challenging hypothesis that deserves further insight.
We lack information on the pollination ecology of heterostylous Linum species in the CFR of South Africa, which prevents us making strong inferences about the causes of the independent evolution of heterostyly there. Although Usia is not present in the CFR, fly pollination in South Africa is common (Johnson 2010), and it would not be surprising if other Bombyliidae or other fly families behave similarly to Usia. Interestingly, the recent description of three-dimensional reciprocity in a group of tristylous CFR Oxalis species (Oxalidaceae; Turketti et al. 2012), with similar arrangement of stamens and styles to that described in L. suffruticosum and similar flower morphology (i.e. funnel-like corollas), confirms the suggestion of Armbruster et al. (2006) that perhaps this kind of polymorphism is not so unusual, and closer examinations of sexual whorl arrangement and pollinator fit can help identifying new examples, providing additional support to the Darwinian view on the function and evolution of heterostyly. This would suggests that ecological adaptations, perhaps mediated by pollinators, rather than phylogenetic conservatism is probably the main driver for the evolution of the stylar polymorphism. Ultimately, this would provide further evidence in support to the Darwinian pollinator hypothesis for the evolution of heterostyly.
Acknowledgements
This study forms part of a PhD project of JRM, who received a fellowship from MINECO (FPI: BES-2008-003946). This study was funded by MINECO grants (CGL2013-45037-P, CGL2010-11379-E, CGL2009-12565, CGL2006-13847-CO2-01). RSG was recipient of a post-doctoral contract from the Andalusian regional government (excellence grant P09-RNM-5280) and from the University of Seville. RPB had a post-doctoral contract of the “Juan de la Cierva” programme, and ME had a post-doctoral contract of MINECO. Many people helped in collecting or locating populations, particularly: J.J. Aldasoro, M. Benavent, Y. Bouchenak-Khelladi, A. de Castro, S. Gómez-González, J. A. Mejías, P. Peñalver, S. Moreno A. Pérez and Ross Turner. Blanca Arroyo, Yuval Sapir and Ross Turner provided some photographs for Fig. 1 and Jordi Bosch identified bees on Linum tenuifolium flowers. Ana Afonso, Silvia Castro and Joao Loureiro provided valuable information on Linum chromosome numbers. We thank the Andalusian Scientific Information Technology Center (CICA, Seville, Spain) for providing computational resources.




