Mutualism between co-occurring plant species in South Africa's Mediterranean climate heathland is mediated by birds
Abstract
- Interactions among plant species via pollinators vary from competitive to mutualistic and can influence the probability of stable coexistence of plant species. We aimed to determine the nature of the interaction via flower visitors between Leucospermum conocarpodendron and Mimetes fimbriifolius, two shrubs in the Proteaceae that share many ecological traits and coexist on the Cape Peninsula, South Africa.
- To assess the extent of pollinator sharing we analysed nectar properties and recorded the pollinator fauna, their behaviour and contribution to seed set. To test for competition via interspecific pollen transfer, we recorded the movement patterns of pollinators and quantified pollen loads. To determine the effect of co-flowering on visitation rates we recorded visits in stands that varied in the density of the two species.
- We found that the species produce similar rewards and share pollinating Cape Sugarbirds (Promerops cafer). Interspecific pollen transfer is avoided by placing pollen on different parts of the bird. Both species are visited by nectar-thieving Orange-breasted Sunbirds (Anthobaphes violacea). Insects and autonomous self-pollination contributed little to seed set. Pollinator visits increased with conspecific density in both species, and the slope of the increase was steepest in the presence of high densities of the co-occurring plant species. Nectar thief visits also increased with conspecific density in both species, but the slope declined with increasing density of the co-occurring species.
- Co-occurrence enhanced pollinator visits and alleviated nectar robbing in both plant species, consistent with mutualisms. Mutualism within a trophic level is unusual, but may help to explain the stable coexistence of ecologically similar species.
Introduction
High plant diversity is one of the features that unites the far-flung Mediterranean climate regions of the world (Cowling et al. 1996a). In most of these regions, turnover in species composition between areas is the major contributor to diversity, but species richness within single communities is also high by global standards. In Fynbos, for example, as many as 120 plant species may co-occur in a 1000-m2 plot (Bond 1983). Despite the relatively high richness, communities are typically composed of a small number of growth forms (Slingsby et al. 2014). The co-occurrence of so many apparently equivalent species begs the question of how they can live to together. According to classical niche theory, species can coexist only if they differ in traits that mediate resource partitioning (Chesson 2000; Silvertown 2004). Co-occurring species in Fynbos have been found to differ in mycorrhizal partners (Waterman et al. 2011), in leaf dimensions (Cody 1986), corm diameter (Slingsby & Verboom 2006) and in their strategy for dealing with fire (Yeaton & Bond 1991).
Floral traits are among the ecological traits that may influence species coexistence (Sargent & Ackerly 2008). If two plant species are limited by different pollinators, they can theoretically coexist (Pauw 2013; Song & Feldman 2014; Benadi 2015). If they share a pollination niche, they interact via the shared pollinators and these interactions can vary from competition to facilitation. Competition for visits may occur if there are too few pollinators to go round, and facilitation may occur if pollinators are disproportionately attracted to areas with high floral density (Rathcke 1983).
Apart from competition for pollinator visits, competition may also occur via interspecific pollen transfer (Morales & Traveset 2008). This may negatively affect the recipient via stigma clogging and ovule loss, and the donor via pollen loss. Plants that share a pollinator may reduce this form of competition by placing pollen on different parts of the same pollinator (Armbruster et al. 1994; Muchhala & Potts 2007; Waterman et al. 2011).
Of course flowers attract not only pollinators, but also antagonists such as nectar thieves (Inouye 1980), and these too may influence the probability of stable coexistence. Theoretically, species can coexist if they are limited by different species of antagonists (Chase & Leibold 2003; Chesson & Kuang 2008). When they share larcenists, co-flowering may exacerbate larceny if larcenists are attracted to denser flower patches, resulting in so-called apparent competition (Holt 1977). Alternatively, co-flowering may reduce the negative effect of floral larceny by satiating the larcenist, resulting in facilitation between plant species (Abrams & Matsuda 1996).
With this background in mind, we studied the pollination biology of two very similar Proteaceae shrub species that typically co-occur in Fynbos communities on the Cape Peninsula, South Africa (Fig. 1). Leucospermum conocarpodendron and Mimetes fimbriifolius share many vegetative features, mode of seed dispersal and fire regeneration strategy, but their pollination biology has not previously been studied (Midgley et al. 1998). We combined nectar analysis, differential pollinator exclusion experiments, pollinator observations and pollen load data from captured pollinators to answer the following questions: (i) do L. conocarpodendron and M. fimbriifolius offer similar floral rewards; (ii) do they share pollinators and/or nectar thieves; (iii) is seed production dependent on pollinator visits; (iv) is pollen placed on the same part of the pollinator; (v) do pollinators move between plant species potentially transferring pollen; and (vi) what is the effect of conspecific and heterospecific density on pollinator and nectar thief visitation rates? A decrease in pollinator visitation rate with increasing heterospecific density would be consistent with competition (−), whereas an increase would be consistent with facilitation (+). Conversely, a decrease in nectar thief visitation rate with increasing heterospecific density would be consistent with facilitation, whereas an increase would be consistent with competition. Both competition and facilitation can be one-sided, or may occur in both directions. Mutualism is two-sided facilitation (++; Bruno et al. 2003).

Material and methods
Study site and species
This study was conducted in a high diversity Mediterranean climate heathland on Red Hill, Cape Peninsula, South Africa (34°10′32.4′′ S; 18°23′56.8′′ E; Cowling et al. 1996b; Pauw & Johnson 1999). Here, the vegetation has three strata: at ~0.5 m there is a diverse layer of fine-leafed shrubs and geophytes among which are bird-pollinated Erica (Ericaceae) and Chasmanthe (Iridaceae) species; at ~1 m, sclerophyllous Leucadendron laureolum (Proteaceae) is dominant; at 2 m the stratum is composed only of the two study species, Leucospermum conocarpodendron subsp. viridum Rourke (hereafter L. conocarpodendron) and Mimetes fimbriifolius Salisb. Ex Knight. Both species are in the Proteaceae and have small ranges, with M. fimbriifolius confined to the Cape Peninsula and L. conocarpodendron extending slightly beyond.
An earlier study of these two prominent species exemplified them as ecological equivalents (Midgley et al. 1998). They occur interspersed and are so similar that they are difficult to distinguish when not flowering (Fig. 1). Their leaves are broad, sclerophyllous, hairy and tipped with extra-floral nectaries (Zachariades & Midgley 1999). Both are unusual in that their aboveground parts generally survive fires and sprout new growth from buds protected under thick bark (Midgley et al. 1998). This strategy gives them the height advantage over the other species in the community, all of which either recover from seeds or resprout from underground organs. Both species recruit a small number of seedlings from large, ant-buried seeds that germinate in response to intense fires (Bond et al. 1990).
The flowers of the two species share many traits, such as large size, but there are also key differences. L. conocarpodendron inflorescences possess ~60 flowers with bright yellow, incurved styles that act as pollen presenters (Fig. 1b). M. fimbriifolius inflorescences (alternatively, conflorescences) are composed of separate headlets in multiple whorls. Each headlet consists of four to seven flowers with long, straight pollen presenters and enclosed in a tube formed by bracts (Fig. 1c). The adaxial bract is pink and typically extends beyond the pollen presenters to form a hood. Although flowering overlaps broadly, M. fimbriifolius peaks earlier (September) than L. conocarpodendron (October), and M. fimbriifolius has a longer flowering window (May–February) than L. conocarpodendron (July–January; A. G. Rebelo, unpublished data).
Although no detailed studies exist, floral morphology suggests that both species are bird-pollinated (Rebelo 1987). In contrast with the large number of bird-pollinated plants, there are few specialist nectar-feeding bird species in the Cape Floristic Region (Rebelo 1987). On the Cape Peninsula, these are the Cape Sugarbird (Promerops cafer L., 37 g), Malachite Sunbird (Nectarinia famosa L., 18 g), Orange-breasted Sunbird (Anthobaphes violacea L., 10 g) and Southern Double-collared Sunbird (Cinnyris chalybea L., 8 g; Geerts & Pauw 2009). The Cape Sugarbird is a member of the Promeropidae and is equipped with a brush-tipped tongue; the smaller Sunbirds are in the Nectariniidae and have tubular tongues (Pauw 1998). Additional opportunistic nectar feeders include Cape White-eyes (Zosterops virens Sundevall, Zosteropidae, 11 g). It is common for plant species to be predominantly pollinated by only one of the available bird species (Geerts & Pauw 2009).
Necarivorous bird abundance is low in the first few years after fire (Geerts et al. 2012). The last large fire at the study site occurred in January 2008, giving sufficient time for birds to return.
Nectar properties
To determine whether the species offered similar rewards for pollinators, we measured nectar volume and concentration from newly opened flowers in the field using capillary tubes (Blaubrand, Wertheim, Germany) and a handheld refractometer (Eclipse; Bellingham & Stanley, Basingstoke, UK). Nectar was kept on filter paper and sugar composition was determined by gas chromatography. Samples (N = 5) were reconstituted in 80 ml methoxyamine hydrochloride (30 mg·ml−1 in pyridine) and incubated in an oven for 2 h at 30 °C. After 2 h, samples were derivatised with 140 ml MSTFA (N-methyl-N-trimethylsilyltrifluoroacetamide) for 1 h at 37 °C. We injected 1 μl of each of the derivatised samples onto a gas chromatography column.
Pollination experiments
Many Proteaceae that conform to the bird pollination syndrome are nevertheless effectively pollinated by insects, or capable of autonomous self-pollination (Schmid et al. 2015). To determine the importance of pollinators versus autonomous self-pollination for seed production, we bagged inflorescences with fine gauze netting to exclude all pollinators. To determine the relative importance of birds versus insects for seed production, we ‘caged’ inflorescences to exclude bird visitors, while still allowing access to insects. Cages were constructed from wire mesh with hexagonal openings with a maximum diameter of 2.9 cm. In a third treatment, we tested for pollen limitation of seed set by supplementing natural pollination with outcrossed pollen using a paintbrush. All treatments were compared to open control inflorescences. We selected four inflorescences on each of ten individuals per species, such that each individual received all four treatments (bagged, caged, supplemented, open). Once flowers began to wilt, inflorescences were enclosed in mesh bags for ~50 days to capture seed production. The effect of each treatment on seed set relative to the control was compared with a Wilcoxon signed rank tests in R (R Core Team 2016).
Visitor observations
We observed bird behaviour with binoculars and recorded visitation rate on five warm, clear days from 2 August to 12 October 2011. Observations were conducted in 32 semi-circular plots with a radius extending from the observer to a distance of 20 m (i.e. 628 m2). Plots were selected to span a range of M. fimbriifolius and L. conocarpodendron inflorescence densities. Observations were recorded in 30-min periods between 06:30 h and 11:00 h to coincide with the peak in bird activity. We recorded the species identity of each floral visitor, the number of visits observed, the visitor's position (perched on top, or below the inflorescence) and whether contact was made with the pollen presenter. A visit is defined as a single probe of the bill. By recording whether pollen presenter contact was made during the visit, we hoped to estimate visit quality, but we are aware that visitation rate may not be a good proxy for pollination rate, nor ultimately for seed set and population growth rate (Price et al. 2008). Bird species were categorised into functional groups (pollinators or thieves) based on whether or not they habitually made contact with pollen presenters.
Interspecific pollen transfer
To determine whether birds potentially transferred pollen between the focal plant species, we observed their patterns of movement and located pollen on captured individuals. To test whether birds move randomly between species or exhibit floral constancy, we observed bird movement patterns in a stand consisting of three L. conocarpodendron and three M. fimbriifolius plants in peak flower. A bird had a 40% random expectation of moving between conspecific individuals because the plant from which the bird departs is excluded (Heystek et al. 2014). We compared observed movements with expected using a Pearson's Chi-squared test.
We captured birds with mist nest over 3 days (10/10, 11/10, 18/10/2011). Using equal effort, pollen samples were taken from the crown and throat region of captured birds with fuchsin gel cubes of the same size (Beattie 1971). Samples were melted onto slides and pollen grains were counted using compound light microscopy. L. conocarpodendron pollen grains are larger isosceles triangles with concave sides; M. fimbriifolius pollen grains are smaller equilateral triangles with straight sides. A Wilcoxon signed rank test was used to test for differences in pollen load size on the crown versus the throat.
Density dependence of visitation rate
To understand how the number of visits observed by pollinators and thieves was influenced by conspecific and heterospecific density we constructed generalised linear models with the number of visits per 30 min by each functional group as dependent variables and L. conocarpodendron and M. fimbriifolius inflorescence density and their interaction as predictor variables. The same model was run for visits (by pollinators or thieves) to L. conocarpodendron and M. fimbriifolius, giving a total of four models that differed only in the dependent variable. The models had a Poisson error structure and a log link function. All analyses were done in R (R Core Team 2016).
Results
Nectar properties
Leucospermum conocarpodendron produced 9.7 ± 1.72 μl (N = 5) of nectar per flower with a 18.2 ± 1.67% sugar concentration (w/w, N = 5). The sugar consists almost entirely of fructose (73.71 ± 0.22% (N = 5) and glucose (26.14 ± 0.29%, N = 5) with small traces of sucrose (0.14 ± 0.071%, N = 5) and no xylose (N = 5). An inflorescence in full flower contained 57 ± 1.41 flowers (N = 5), giving a total of about 553 μl per inflorescence. M. fimbriifolius produced 28.7 ± 4.7 μl (N = 5) of nectar per headlet, which translates into 4.36 ± 0.70 μl (N = 5) per flower. Sugar concentration was 22. 5 ± 0.49% (w/w, N = 5) and sugars consist almost entirely of fructose (74.73 ± 1.05%, N = 5) and glucose (25.13 ± 0.10%, N = 5) with small traces of sucrose (0.12 ± 0.13%, N = 5) and no xylose (N = 5), confirming earlier findings (Nicolson & Van Wyk 1998). In full flower an inflorescence contains 121.6 ± 10.78 flowers (N = 5) giving a total of about 530 μl per inflorescence.
Pollination experiments
For L. conocarpodendron, we found a significant difference in seed production between open (mean = 4.5 ± 2.42) and bird-excluded (caged) inflorescences (mean = 0.9 ± 0.94; P < 0.001, V = 6, N = 10, Wilcoxon signed rank test). Autonomous (bagged) seed production was also significantly lower than open pollination (mean = 0.6 ± 0.82; P < 0.001, V = 0, N = 10, Wilcoxon signed rank test). Pollen supplementation did not significantly increase seed production (mean = 4.8 ± 1.83; P = 0.719, V = 23.5, N = 10, Wilcoxon signed rank test). Results for M. fimbriifolius were similar. There was a significant difference between seed set in open (mean = 3.8 ± 1.60) and bird-excluded (caged) inflorescences (mean = 0.1 ± 0.30; P < 0.001, V = 0.5, N = 10, Wilcoxon signed rank test), and between open and bagged inflorescences (mean = 0 ± 0; P = 0.006, V = 0, N = 10, Wilcoxon signed rank test). Pollen supplementation did not significantly increase seed production (mean = 4.5 ± 1.57; P = 0.095, V = 2, N = 10, Wilcoxon signed rank test).
Visitor observations
Among the visitors to L. conocarpodendron, Cape Sugarbirds and Malachite Sunbirds perched on the apex of the inflorescence and foraged downwards, contacting pollen presenters on their crowns. Orange-breasted Sunbirds, Southern Double-collared Sunbirds and Cape White-eyes foraged from underneath inflorescences and failed to contact pollen presenters (Table 1). Cape Sugarbirds were the only effective pollinators observed at M. fimbriifolius. They sit on the bracts at the apex of the inflorescence and contacted pollen presenters with their throats while foraging downwards. Orange-breasted Sunbirds, Southern Double-collared Sunbirds and Cape White-eyes forage from underneath inflorescences and failed to contact pollen presenters. Hymenoptera were occasionally seen foraging on L. conocarpodendron and M. fimbriifolius but were never seen contacting pollen presenters or collecting pollen. Since Cape sugarbirds and Malachite sunbirds are the only visitors to contact pollen presenters we grouped the remaining bird species into one category (nectar thieves). Malachite Sunbirds were very infrequent; including them with Cape Sugarbirds (pollinators) did not make a qualitative difference to the analysis results, so we excluded them from the main analyses.
| Cape Sugarbird | Malachite Sunbird | Orange-breasted Sunbird | Southern double-collared Sunbird | Cape White-eye | |
|---|---|---|---|---|---|
| L. conocarpodendron | 0.359 (98) | 0.035 (100) | 0.113 (0) | 0.012 (0) | 0.003 (0) |
| M. fimbriifolius | 0.212 (100) | 0 | 0.182 (1) | 0.005 (0) | 0.007 (0) |
Interspecific pollen transfer
We found that Cape sugarbirds move frequently between plant species. Of 33 recorded transitions, 17 were conspecific compared to 13.2 predicted (χ2 = 36.167, df = 33, P = 0.333). In a larger sample, some constancy has been detected (Schmid et al. 2016a), but the fact remains that sugarbirds move frequently between species, potentially transferring pollen.
Leucospermum conocarpodendron pollen was deposited more often on the Cape Sugarbird's crown than throat feathers (P = 0.031, V = 0, N = 6, Wilcoxon signed rank test; Table 2). In contrast, M. fimbriifolius pollen was found only on the Cape Sugarbird's throat (P = 0.036, V = 21, N = 6, Wilcoxon signed rank test; Table 2). L. conocarpodendron and M. fimbriifolius pollen on Orange-breasted Sunbirds and Cape White-eyes (Table 2) is likely the result of being showered with pollen that was dislodged from pollen presenters in the process of stealing nectar from below the inflorescence and is therefore unlikely to be transferred to stigmas.
| visitor | N | L. conocarpodendron pollen grains | M. fimbriifolius pollen grains | ||
|---|---|---|---|---|---|
| crown | throat | crown | throat | ||
| Cape Sugarbird | 6 | 761.5 (18–6038) | 264 (5–1912) | 0 | 27.5 (3–143) |
| Orange-breasted Sunbird | 10 | 1.5 (0–15) | 2 (0–7) | 0 (0–17) | 0 |
| Malachite Sunbird | 2 | 5 (4–6) | 1 (0–2) | 0 | 0 |
| Cape White-eye | 1 | 2 | 0 | 0 | 0 |
Density dependence of visitation rate
For all four of the models the deletion of the interaction term increased the AIC score, indicating that the response of birds to the density of one plant species depended on the density of the other plant species.
The number of pollinating Cape Sugarbird visits to L. conocarpodendron increased significantly with conspecific density, but the rate of increase was greatest at high densities of co-occurring M. fimbriifolius (significant positive interaction term; Table 3, Fig. 2a, Fig. S1). Similarly, Cape Sugarbird visits to M. fimbriifolius increased steeply with increasing conspecific density only in the presence of high densities of L. conocarpodendron (significant positive interaction term; Table 3, Fig. 2b, Fig. S2). Thus, for both plant species the number of visits by pollinating Cape Sugarbird is positively impacted by increasing density of the co-occurring species, but this effect is more strongly observed in M. fimbriifolius than in L. conocarpodendron.
| focal species | predictor variable | visitor | estimate | SE | z-value | P-value* |
|---|---|---|---|---|---|---|
| L. conocarpodendron | L. conocarpodendron density | Sugarbirds | 0.00524 | 0.00026 | 20.56 | <0.001 |
| L. conocarpodendron | M. fimbriifolius density | Sugarbirds | −0.00251 | 0.00150 | −1.504 | 0.133 |
| L. conocarpodendron | L. conocarpodendron × M. fimbriifolius density | Sugarbirds | 0.00002 | <0.00001 | 2.557 | 0.011 |
| L. conocarpodendron | L. conocarpodendron density | Thieves | 0.00239 | 0.00048 | 4.947 | <0.001 |
| L. conocarpodendron | M. fimbriifolius density | Thieves | −0.00243 | 0.00240 | −1.011 | 0.312 |
| L. conocarpodendron | L. conocarpodendron × M. fimbriifolius density | Thieves | −0.00002 | 0.00001 | −1.400 | 0.161 |
| M. fimbriifolius | L. conocarpodendron density | Sugarbirds | −0.00409 | 0.00182 | −2.249 | 0.025 |
| M. fimbriifolius | M. fimbriifolius density | Sugarbirds | −0.00391 | 0.00185 | −2.112 | 0.035 |
| M. fimbriifolius | L. conocarpodendron × M. fimbriifolius density | Sugarbirds | 0.00006 | 0.00002 | 2.994 | 0.003 |
| M. fimbriifolius | L. conocarpodendron density | Thieves | −0.00608 | 0.00367 | −1.807 | 0.071 |
| M. fimbriifolius | M. fimbriifolius density | Thieves | 0.00969 | 0.00191 | 5.077 | <0.001 |
| M. fimbriifolius | L. conocarpodendron × M. fimbriifolius density | Thieves | −0.00009 | 0.00004 | −2.155 | 0.031 |
- *P-values < 0.05 are indicated in bold.
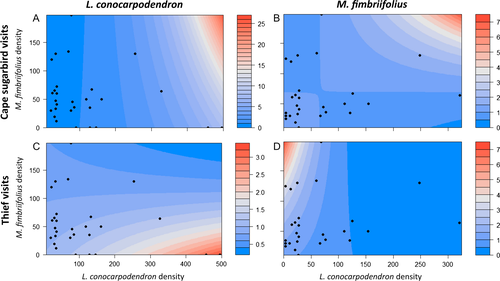
The number of visits by nectar-thieving birds to both plant species increases significantly with conspecific density, as might be expected, but in this case the rate of increase was greatest at low densities of the co-occurring species (negative interaction terms; Table 3, Fig. 2c, d; Fig. S3–S4). This interactive effect of heterospecific density is significant for M. fimbriifolius, but not for L. conocarpodendron for which the effect size is also lower. Thus, for both plant species, the number of visits by nectar-thieving birds is negatively impacted by increasing density of the co-occurring species, but the effect of L. conocarpodendron on M. fimbriifolius is stronger than in the reverse direction.
Discussion
Leucospermum conocarpodendron and M. fimbriifolius flower together, secrete large volumes of dilute, hexose-dominated nectar and employ the same pollinator, the Cape Sugarbird. The much smaller Sunbirds, Cape White-eyes and honeybees do not contact the pollen presenters of either species, but do thieve nectar (Table 1). Bird exclosure experiments demonstrated the importance of birds relative to insects: in both species, seed set per inflorescence was at or near zero when only insects were allowed access to flowers and autogamous seed set was negligible.
Sugarbirds move frequently between the two plant species, but interspecific pollen transfer might be reduced by separation of the pollen loads on the body of the pollinator. L. conocarpodendron pollen is placed predominantly on the crown feathers whereas M. fimbriifolius pollen is placed only on the throat feathers (Table 2). Despite this trend, a substantial amount of L. conocarpodendron pollen occurs on the throat feathers, from where it can be transferred to M. fimbriifolius, but pollen transfer in the reverse direction is unlikely to occur.
Our observations show that pollination biology can be added to the long list of ecological traits that L. conocarpodendron and M. fimbriifolius have in common (Midgley et al. 1998). In the case of pollination, however, similarity may lead to mutualism rather than competition. The mutualism occurs via two different routes, one involving pollinators, the other nectar thieves. A mutualism via pollinators may occur because Cape Sugarbirds concentrate on nectar-dense areas of the landscape and the addition of nectar-producing heterospecific individuals enhances visitation rate (Table 3, Fig. 2a, b). The mutualism via nectar thieves may occur because Sunbirds are satiated at high floral densities with the result that their negative effect is diluted across two plant species (Table 3, Fig. 2c, d). Size differences are probably important in determining the difference in behaviour between pollinators and thieves. The larger Sugarbirds have larger nectar requirements and, in addition, may displace the smaller thieves from dense stands (Skead 1967; Wooller 1982; Geerts & Pauw 2009; Schmid et al. 2016b).
Whereas hundreds of studies demonstrate competition for pollinator visits (reviewed in: Mitchell et al. 2009), relatively few have found positive interactions between pollinator-sharing plant species (Moeller 2004; Duffy & Stout 2011; Liao et al. 2011). Mutualism, i.e. positive interactions in both directions, has been detected in even fewer cases (Waser & Real 1979; Thomson 1981). Nevertheless, our work is preliminary. The effect of co-occurrence on seed set and ultimately on population growth rate remains to be investigated.
Whereas classical mutualisms, such as pollination and seed dispersal, occur between trophic levels, recent studies in diverse systems are uncovering mechanisms whereby competing species in the same trophic level can nevertheless be engaged in mutualism (Bruno et al. 2003; Crowley & Cox 2011). Examples of mutualism among competitors now include cooperative hunting among fish species (Bshary et al. 2006), mutualisms among plants via shared mycorrhizal fungi (Bever 2002), mutualism between ant species via shared ant-plants (Lee & Inouye 2010), mutualism between anemone fish species via shared anemones (Schmitt & Holbrook 2003) and the mutual amelioration of abiotic conditions in clump-forming plants (Nunez et al. 1999). Simultaneously, new models are exploring how mutualism may facilitate coexistence of competitors (Zhang 2003; Feldman et al. 2004; Johnson & Amarasekare 2013). For example, Bastolla et al. (2009) showed that mutualism among plant species via shared pollinators can theoretically enhance the number of coexisting plant species in a pollination network.
The demonstration of potential mutualism between plant species sharing a pollinator helps to explain the frequent occurrence of pollination guilds consisting of several plant species that share a pollinator species and a syndrome of floral traits (Fenster et al. 2004). In the Cape Floristic Region, for example, there are about 80 Proteaceae species that conform to the bird-pollination syndrome, and plots with a diameter of 500 m contain up to eight of these (Heystek & Pauw, unpublished data). The possible existence of positive, rather than negative, interactions among these plant species via their shared flower visitors makes it easier to understand floral trait convergence, especially when it is accompanied by divergence in morphological traits that determine pollen attachment site on the body of the pollinator (Brown & Kodric-Brown 1979; Pauw 2006).
Acknowledgements
We would like to thank Phoebe Barnard and Anina Heystek for mist-netting birds, SANParks for permits, and two anonymous reviewers for their comments.




