Pollen and stigma size changes during the transition from tristyly to distyly in Oxalis alpina (Oxalidaceae)
Abstract
- Pollen and stigma size have the potential to influence male fitness of hermaphroditic plants, particularly in species presenting floral polymorphisms characterised by marked differences in these traits among floral morphs. In this study, we take advantage of the evolutionary transition from tristyly to distyly experienced by Oxalis alpina (Oxalidaceae), and examined whether modifications in the ancillary traits (pollen and stigma size) respond to allometric changes in other floral traits. Also, we tested whether these modifications are in accordance with what would be expected under the hypothesis that novel competitive scenarios (as in distylous-derived reproductive system) exert morph- and whorl-specific selective pressures to match the available stigmas.
- We measure pollen and stigma size in five populations of O. alpina representing the tristyly–distyly transition.
- A general reduction in pollen and stigma size occurred along the tristyly–distyly transition, and pollen size from the two anther levels within each morph converged to a similar size that was characterised by whorl-specific changes (increases or decreases) in pollen size of different anthers in each floral type.
- Overall, results from this study show that the evolution of distyly in this species is characterised not only by changes in sexual organ position and flower size, but also by morph-specific changes in pollen and stigma size. This evidence supports the importance of selection on pollen and stigma size, which increase fitness of remaining morphs following the evolution of distyly, and raises questions to explore on the functional value of pollen size in heterostylous systems under pollen competition.
Introduction
The fate and success of pollen is one of the main factors affecting male fitness and evolution of the plant reproductive system. Mating and reproductive systems influence the amount of resources destined for pollen production and how the pollen is presented within the flower to improve the likelihood of a pollen grain reaching a stigma, germinating and fertilising an ovule (Harder & Routley 2006). The quantity and quality of pollen reaching the stigmatic surface has been shown to influence siring success through pollen competition in a number of species (Mulcahy 1979; Cruzan 1990; Harder 1998; Marshall & Evans 2016; McCallum & Chang 2016). For example, the interaction between pollen grains and the stigmatic tissue (papillae length and stigma depth) has been suggested to have an important role by influencing pollen recognition and the probabilities and timings of both pollen germination and the arrival of pollen tubes to the stylar tissue (Edlund et al. 2004; Cruden 2009). In this sense, pollen size has been considered a key attribute to understand different steps in this interaction (Williams & Mazer 2016). In addition, the stigmatic environment and the length of the style have been conceived as selective barriers encouraging pollen competition by limiting pollen attachment to stigmatic papillae, or by increasing the distance between the site of pollen deposition and the targeted ovules (Lankinen & Skogsmyr 2001; Edlund et al. 2004; Williams 2008; Cruden 2009).
The finding of significant and positive correlations between pollen size, papillae length, stigma depth and style length across several species of angiosperm, has been interpreted as evidence of their functional role during pollination (Williams & Rouse 1990; Torres 2000; Cruden 2009). A possible explanation for this is that the rates of pollen germination and pollen tube growth through the style are expected to be under strong selective pressure, because differences in these attributes will ultimately influence paternal reproductive success (Cruzan 1990; Walsh & Charlesworth 1992; Lau & Stephenson 1993; Aizen & Raffaele 1998; Marshall & Evans 2016). Pollen size could have a functional role in pollen competition because of its relation to the amount of pollen reserves stored in the pollen coat (soluble carbohydrates like sucrose, glucose or fructose; Carrizo García et al. 2015, 2016; Paccini & Dolferus 2016), and because pollen size could be related to temperature and dehydration rates that increase pollen longevity (Ejsmond et al. 2011, 2015; Carrizo García et al. 2016). Therefore, pollen size and nutrient storage in the exine can affect pollen germination and initial pollen tube growth even before entering the style during the ‘autotrophic’ stage (Stephenson et al. 2003; Cruden 2009).
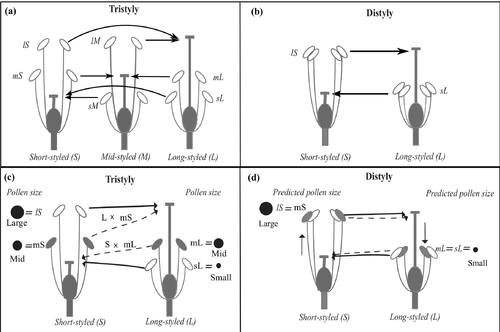
In species with floral polymorphisms such as heterostyly, pollen and stigma size are some of the most important ancillary features reinforcing differences among floral morphs and the function of this reproductive system (Dulberger 1992; Costa et al. 2016). Heterostylous species are characterised by a sexual polymorphism, where sexual organs are positioned in a reciprocal manner between floral morphs or types (reciprocal herkogamy) and populations can have two (distyly) or three (tristyly) floral morphs (Ganders 1979). This polymorphism is generally associated with a heteromorphic incompatibility system (HIS), precluding seed production from self- and same-morph crosses (Fig. 1). Crosses between anthers and stigma positioned at the same level are compatible, and thus are able to produce seeds (legitimate crosses sensu Darwin 1877; Fig. 1a,b). Tristyly is characterised by three floral morphs (short-, mid- and long-styled morphs) differentiated by the relative position of the stigma in relation to two whorls of anthers (Fig. 1a), while in the case of distyly only two morphs occur in populations (long- and short- styled morphs; Fig. 1b). The heterostylous polymorphism is generally associated with morph-specific ancillary traits such as the size and shape of stigmas and stigmatic papillae, and the number and size of pollen grains. Long anthers of the mid- and short-styled floral morphs produce large pollen grains that pollinate long styles, while anthers at the mid and low positions produce intermediate and small pollen grains that pollinate mid and short styles, respectively (Dulberger 1992). The observed correspondence of heterostylous traits between floral morphs (e.g., ovules of short-styled plants, which also have smaller stigmas, are fertilised with small pollen grains coming from short-level anthers of the long- or mid-styled morphs) supports the expectation that ancillary traits and the heterostylous floral polymorphism have been adjusted via selection to increase the efficiency of legitimate pollen transfer, pollen capture and fertilisation, and the avoidance of sexual interference (Darwin 1877; Barrett & Glover 1985; Webb & Lloyd 1986; Barrett 1998; Bianchi et al. 2000, Costa et al. 2016). If pollen and stigma size play a role in pollination and fertilisation success, either through the match between pollen and stigma or via pollen competition through pollen germination and tube growth at different style lengths, selection on the original conditions maintaining tristyly, such as morph frequencies and incompatibility reactions, could eventually lead to changes in the phenotypic expression of these traits in response to changes in the mating environment. Hence, more studies characterising within- and among-morph variation in pollen size and stigma depth are needed to understand their evolutionary potential in response to the breakdown of tristyly.
The evolutionary transition from tristyly to distyly is a relatively common phenomenon among heterostylous systems, and has been considered as an ideal scenario to analyse changes in the heterostylous syndrome related to disruptions in the expected morph frequencies and incompatibility reactions (Charlesworth & Charlesworth 1979; Ganders 1979; Weller 1986; Barrett 1992 and references therein). The evidence demonstrates that the evolution of distyly in some tristylous species is accompanied by adjustments in floral morphology that involve changes in the length of sexual organs and/or corolla traits (Eckert & Barrett 1994; Li & Johnston 2001; Sosenski et al. 2010; Santos-Gally et al. 2013). However, few studies have analysed the evolution of ancillary traits such as pollen and stigma size in heterostylous species under evolutionary transition, even though these are key attributes for fertilisation success (Eckert & Barrett 1994; Li & Johnston 2001; Manicacci & Barrett 1995; Costa et al. 2016). In this study, we take advantage of the evolutionary lability of a heterostylous species to analyse changes in pollen and stigma size during the tristyly–distyly transition (Ornduff 1972; Weller 1992; Weller et al. 2007). Although phenotypic changes in pollen traits can be expected due to direct selection to match the stigmatic and stylar environment or via pollen competition, changes in pollen and stigma size could also result from their allometric relationship with other floral traits adjusted through natural selection during the transition, increasing the morphological match among floral morphs in a new distylous competitive environment.
Populations of Oxalis alpina (Rose) Knuth (Oxalidaceae section Ionoxalis) located in the Sonoran Sky Islands range from tristylous isoplethic (equal morph frequencies) to tristylous anisoplethic and distylous isoplethic populations, characterised by the gradual loss of the mid-morph, thus encompassing the whole evolutionary sequence in the tristyly–distyly transition (Weller et al. 2007). Modifications in the heteromorphic incompatibility system disrupting the tristylous equilibrium seem to initiate the evolutionary transition to distyly (Weller et al. 2007; Weber et al. 2013). These modifications represent a gradual increase in the compatibility between two formerly incompatible crosses involving the long- and short-styled morphs (crosses between short stigmas and pollen from mid anthers of the long floral morph (S × mL) and between long stigmas and mid anthers of the short-styled floral morph (L × mS) (Kutaka et al. 2011; Weber et al. 2013; Fig. 1c). The loss of the mid-morph and, therefore of the target stigmas of pollen from the mid-anther whorls of long- and short-styled plants, is accompanied by morphological modifications in their flowers (Sosenski et al. 2010). First, a 22% reduction in flower size was observed in 12 populations along the tristyly–distyly transition, including the overall mean of corolla size and sexual organs in the three floral morphs (Sosenski et al. 2010). Second, rearrangements in the position of sexual organs produced an increase in the reciprocity between long- and short-styled plants (reciprocity being the degree of convergence between the two anther whorls within each morph, and between styles and anther whorls among morphs; Sosenski et al. 2010; Fig. 1d). Third, increased reciprocity between long and short morphs also augmented the efficiency of pollen transfer by 30% in both modified tristylous and distylous populations in comparison with ancestral tristylous populations (Baena-Díaz et al. 2012). These modifications increased the ability of pollen from mid-level anthers of short- and long-styled morphs to reach the corresponding long and short styles more effectively. Consequently, the loss of the mid-styled morph and the morphological rearrangements observed among populations of O. alpina have the potential to modify the match between pollen and stigma and possibly the arena for pollen competition by merging pollen from two distinct origins (mL and sL pollen in short stigmas, mS and lS pollen in long stigmas from different parents; Fig. 1a,b) on stigmas adapted to deal with only one pollen type. Hence, morph-specific and whorl-specific changes in both pollen attributes (pollen size) and stigma traits (receptivity, stigmatic environment, stigma depth, style length) are expected as a result of this new selective scenario, via selection of individuals presenting variations in any of these attributes, increasing the proportion of fit pollen (Torres 2000; López et al. 2006; Lankinen & Madjidian 2011).
If the ancillary traits (pollen and stigma size) are important in determining the outcome of pollination, and ultimately affect siring success in O. alpina, then they should express predictable adjustments throughout the tristyly–distyly transition in order to improve pollination and fertilisation success in distylous-derived populations. This study seeks to evaluate whether pollen and stigma size change during the tristyly–distyly transition and how these changes relate to allometric responses in other floral traits. To this end, we tested the following predictions: (i) because flower size shows a significant reduction through the tristyly–distyly transition (Sosenski et al. 2010) and because floral traits in tristyly are associated with S and M loci (Lewis & Jones 1992; Barrett & Shore 2008), an associated reduction in pollen size and stigma depth is also expected during the evolution of distyly due to allometric correlations among floral traits. (ii) Considering that the evolution of distyly in this species is related to the convergence in length of two anther levels of long and short morphs, pollen size of the two anther whorls in the long-styled flowers (sL and mL) and two anther whorls in the short-styled flowers (mS and lS) should converge to approximately the same size within flowers of each morph to match the stigmas of the derived distylous phenotype (Fig. 1d). (iii) Because of the incompatibility modifications and the increase in pollen transfer between the remaining floral morphs (long and short) during the tristyly–distyly transition described above, we expect that if pollen from two different whorls (differing in size) is transferred to a same stigma, one type of pollen grain would be exposed to a potential advantage/disadvantage when competing with the other type of pollen, because of the match to the stigmatic environment (i.e., papillae size) or because pollen size has an effect on pollen germination and pollen tube growth through the amount of reserves stored in the pollen grain. Hence, a relatively larger phenotypic adjustment is expected in pollen grains under competitive disadvantage due to a lack of match to the stigma size or style length. Pollen from mid-level anthers of both long- and short-styled plants (mS and mL) should have higher adjustment in relative sizes, reducing the difference in pollen from short and long anthers of their respective morph. (iv) We also expect that changes in stigma size will follow the overall reduction in flower size.
Material and Methods
Study species and populations
Populations of the heterostylous O. alpina included in this study were from the Sonoran Sky Islands of the southwest USA and northwest Mexico. In this region, tristylous and distylous populations of O. alpina represent different stages in the evolutionary transition between tristyly to distyly. Among-population variation in floral morph frequencies, modifications in the incompatibility reactions and changes in floral morphology characterise this transition (Weller et al. 2007; Sosenski et al. 2010; Baena-Díaz et al. 2012).
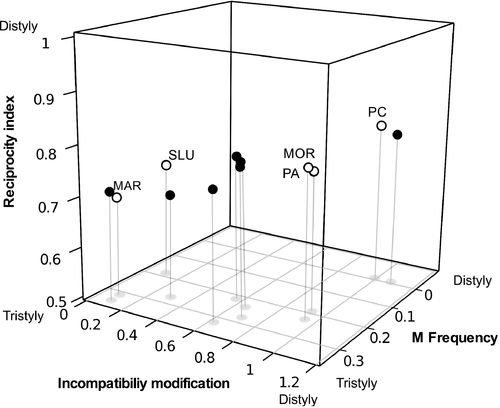
The five O. alpina populations included in this study are described in detail elsewhere (Weller et al. 2007), and were chosen to represent the entire range of variation in the tristyly–distyly evolutionary pathway. The Mariquita population (MAR) represents the ancestral tristylous condition: equal frequencies of long-styled (L), mid-styled (M) and short-styled (S) floral morphs, reciprocal herkogamy and unmodified tristylous incompatibility reactions (typically only legitimate crosses yield substantial numbers of seeds; Weller et al. 2007), although some mid-styled individuals are moderately self-compatible (Weller et al. 2007; Weber et al. 2013). The San Luis (SLU) population is also tristylous and close to the ancestral tristylous condition with reciprocal herkogamy, and incompatibility reactions are for the most part unmodified (a few individuals show incompatibility modifications; Kutaka et al. 2011; hereafter in this paper SLU will be denoted ‘unmodified’). Pinos Altos (PA) and Morse Canyon (MOR) are two populations that represent and intermediate state in the distyly–tristyly transition because they possess completely modified incompatibility and floral morphology of long- and short-styled plants resembling that of distyly, but still maintain a high frequency of mid-styled individuals (Fig. 2). Finally, the Pinery Canyon (PC) population has a distylous reproductive system in which the mid-styled morph is absent, possesses a completely modified incompatibility system and flowers from each morph show the highest convergence in length of the two anther whorls (although positioned very closely together, two anther levels are still distinguishable in each morph). These five populations also show a reduction in flower size as the mid-styled morph decreases in frequency (Sosenski et al. 2010).
Because we were interested in pollen changes associated with incompatibility modifications between short- and long-styled plants, we only took samples of pollen from these floral morphs. Bulbs from each population were collected in the field and grown in 2008 under controlled conditions at the UC Irvine greenhouse to reduce environmentally induced variation in pollen attributes.
Pollen size
Pollen was collected independently from the two whorls of anthers of one newly opened flower from each of 20–30 plants per morph per population. All five anthers in each whorl were collected, dried and kept in paper within 1.5-ml microcentrifuge tubes. To avoid pollen contamination between anther levels, petal and sepals were carefully removed using fine forceps before pollen was harvested. Lower-level anthers were collected first to avoid contamination with pollen from the upper-level anthers.
Pollen was mounted dry on microscope slides, covered with a cover slip and sealed with nail polish. Pollen photographs were taken with a Zeiss microscope (40×) and measured with the Axio Vision software (Axio Vision rel. 4.7.1; Zeiss Imaging Solutions). Polar and equatorial lengths were measured for each of 30 pollen grains of the two anther levels. Between 6,000 and 9,000 pollen grains were measured (30 pollen grains × 2 anther levels × 20–30 plants per morph × 5 populations).
Because Oxalis pollen grains are ellipsoid in shape, equatorial and axial lengths were analysed with principal components analysis (PCA) to find the best estimate of pollen size and the first PC (PC1) was interpreted as the pollen size.
Stigma length along the tristyly–distyly transition
To evaluate whether changes in pollen size were related to female traits, we measured the length of the stigmatic tissue on three to five stigmas per plant (we measured the length of the stigmatic lobes, from the boundary where the style begins to the tip of the stigma). We could not measure papillae size, although the length can vary between floral morphs of Oxalis (Rosenfeldt & Galati 2009). We took photographs of the flowers with a Zeiss Stereo Discovery V8 zoom microscope during summer 2009. These measurements were only taken in populations SLU, PA, MOR and PC, not in MAR as material was not available.
Data analysis
To test our first prediction of the reduction in pollen size along the tristyly–distyly transition, we used a general linear mixed model and the lmer function in R (Bates 2012, 2015). Scores from the first principal component (PC1) of pollen size estimated over all populations were used as the response variable. Population (pop), anther type and the interaction pop × anther type were included as fixed effects. The population term used here is a continuous measure of the position a population has in the tristyly–distyly transition (it stands for the average level of incompatibility modification in a population and is negatively correlated with the frequency of the mid morph; see Weller et al. 2007 for details). The model also included a random term with a nested structure (anther type nested within plant and morph) due to the naturally nested structure of the data, where each floral type presents a unique combination of two anther types within a flower. The significance of fixed effects was tested through comparing different models with and without a given effect using a likelihood ratio test (Zuur et al. 2009).
To test our second prediction on whether pollen size converges between the two anther types of a given morph as the evolution of distyly proceeds, we used a linear model including the difference in mean pollen size between the two anther types (from the overall PC1) as the response variable, and the degree of incompatibility modification, morph and their interaction as independent variables.
Because we hypothesised that morph-specific and whorl-specific selective pressures were operating on pollen size during the tristyly–distyly transition, due to a mismatch with the targeted stigma, we focused on the relative changes pollen size experienced once we accounted for the potential effects of the reduction in flower size. Accordingly, we performed PCA in each population to standardise the data to a population mean of zero and eliminate the effect of the flower size reduction. The first principal component (Standardised_PC1) accounted for 81.7%–84.5% of the variation and was used as an estimate of pollen size. The new standardised PC1 scores were used as the response variable in a mixed model that included incompatibility modification (pop), anther type and the interaction as fixed effects, and anther type nested within plant and morph as random effect. Because we were interested in determining whether the magnitude and sign of the slopes (changes in pollen size along the tristyly–distyly transition) differed among anther types, independent contrasts were obtained using the lmer function in R. Contrasts within populations were done to estimate within-population differences in pollen size between anther types.
Finally, we estimated changes in stigma size along the tristyly–distyly transition using a linear model that included morph, degree of incompatibility modification for each population (pop, a continuous measure of the position a given population has in the tristyly–distyly transition) and the interaction as fixed factors. Contrasts were done to compare differences within and between populations. All analyses were done with R program, version 2.13 (R Development Core Team 2008).
Results
Changes in pollen size along the tristyly–distyly transition
As predicted, mean pollen size was reduced along the tristyly–distyly transition, as shown by the significant effect of incompatibility modification on PC1 (χ2(1) = 23.107, P < 0.0001, estimate = −0.677). The anther type effect and the interaction with population effects were also significant (anther type: χ2(3) = 273.44, P < 0.0001; pop x anther type: χ2(3) = 25.83, P < 0.0001).
Variation in pollen size among anther types within each population showed that, as expected for the typical tristylous polymorphism, the MAR population showed differences in pollen size between anther types, with long anthers (lS) producing larger pollen grains than mid (mS and mL) and short anthers (sL) (Fig. 3b). In the other three tristylous populations, SLU (essentially unmodified), PA and MC (with complete incompatibility modifications), pollen size from the mid anthers showed significant modifications and diverged between the floral morphs along the tristyly–distyly transition (Fig. 3b). The distylous population PC expressed the maximum divergence in pollen size between mid anthers of the two floral morphs (mS > mL; Fig. 3b). Accordingly, this population also exhibited the highest level of convergence in pollen size and stamen length within each morph (Fig. 3a).
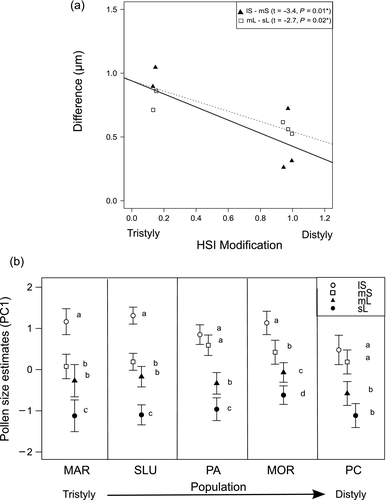
As predicted, pollen size converged along the tristyly–distyly transition, as the mean difference in pollen size between the two anther whorls from each floral morph decreased as incompatibility modification increased (pop: F(1) = 13.24, P = 0.0108; Fig. 3a). No significant effect was found for morph and its interaction with the degree of incompatibility modification (morph: F(1) = 0.005, P = 0.94; pop x morph: F(1) = 2.16, P = 0.19).
After standardising the pollen size in each population to control for differences in flower size among populations (Standardised_PC1), the linear mixed model revealed no differences in the average pollen size among populations (pop: χ2(1) = 3.119, P = 0.0773). However, pollen size was significantly affected by anther type (χ2(3) = 285.01, P < 0.0001) and by the interaction between incompatibility modification and anther type (pop x anther type: χ2(3) = 40, P < 0.0001), indicating that the main pollen size changes correspond to anther type specific adjustments along the tristyly–distyly transition. In particular, pollen from anthers positioned at the lowest level in both morphs (sL and mS, those producing smaller pollen) increased their relative size along the tristyly–distyly transition (pop effect for sL: χ2(1) = 11.342, P < 0.001; pop effect for mS: χ2(1) = 11.678, P < 0.001). In contrast, pollen size from long anthers of short-styled plants (lS) showed a significant reduction along the evolutionary transition (lS: χ2(1) = 13.968, P < 0.0001), while no change in pollen size was detected for pollen from mid anthers of long-styled plants (mL: χ2(1) = 0.0105, P = 0.918).
Changes in stigma size
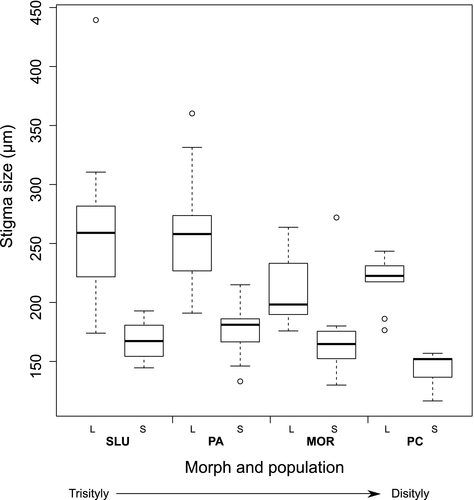
Stigma size also changed with floral morph and population incompatibility modification (morph: F(1) = 71.203, P < 0.0001; pop: F(1) = 4.947, P = 0.028; Fig. 4). Within each population long-styled plants had larger stigmas than short-styled plants (Fig. 4), and the two floral morphs showed a reduction in stigma size along the tristyly–distyly transition. The interaction of morph x pop was not significant (F(1) = 2.736, P = 0.101).
Discussion
Significant modifications of pollen and stigma size were observed along the tristyly–distyly transition in O. alpina. Such modifications produced a dimorphism in pollen size associated with the presence of two floral morphs (short- and long-styled plants) after the loss of the mid morph. As predicted, we observed an overall reduction in pollen and stigma size, parallel to the previously observed reduction in flower size during the transition to distyly (Sosenski et al. 2010). This reduction of the flower was also accompanied by the convergence of pollen size between the two anther levels of the remaining morphs (long and short), increasing their reciprocity (Fig. 3a). When excluding the flower size effect, results from this study revealed that changes in pollen size during the evolution of distyly were both morph- and whorl-specific, and partially supported our expectation of larger adjustments in pollen from mid anthers to match the new stigmatic environments. Pollen grains of the mS changed significantly, while no significant adjustments were observed for mL pollen. Surprisingly, pollen from sL whorls increased in size, while the larger pollen from the lS whorl decreased (Fig. 3b).
Our results showed that the observed reduction in flower size along the tristyly–distyly transition in O. alpina (Sosenski et al. 2010) had an overall allometric effect on pollen and stigma size by reducing their mean and potentially explaining the reduction in large pollen from the long anther of the short-styled morph (lS). Although variation in flower size and shape has been found in other tristylous species (Barrett et al. 2004; Barrett & Hodgins 2006; Hodgins & Barrett 2008; Santos-Gally et al. 2013), and some have associated corolla attributes to sexual organ reciprocity (Thompson & Dommee 2000; Ferrero et al. 2011; Keller et al. 2012), there is no clear pattern on how the corolla attributes relate to ancillary traits like pollen size and stigmatic features. In this sense, results from the present study contribute to understanding evolution of the integrated heterostylous phenotype during the tristyly–distyly transition.
Reciprocity between sexual organs of different floral morphs in heterostylous systems has proven to have adaptive value because it increases outcrossing through legitimate pollen transfer, avoids selfing and reduces interference between sexual functions (e.g., Mulcahy & Caporello 1970; Cesaro & Thompson 2004; Baena-Díaz et al. 2012; Keller et al. 2014; Zhou et al. 2015). Also, it has been hypothesised that the ancillary traits, including pollen and stigma attributes, should match this reciprocity to increase the efficiency of pollen transfer and fertilisation success (Lewis 1975; Lloyd & Webb 1992; McKenna 1992; Cruzan & Barrett 1996). We found a strong convergence in pollen size between the two anthers within a morph during the transition from tristyly to distyly, thus increasing the reciprocity in pollen size between morphs and supporting the idea that pollen traits also contribute to the likelihood of fertilisation success. We also detected a very strong correspondence between pollen size, style length and stigma size in the unmodified tristylous populations (MAR and SLU; Fig. 3b) and more markedly in the distylous population (PC), corresponding to findings in many other tristylous and distylous species (Ganders 1979; Dulberger 1992; Massinga et al. 2005; Ferrero et al. 2011 and references therein) and corroborating the findings of Weller (1979) in O. alpina. The work of Costa et al. (2016) showed that the marked variation in pollen size and stigmatic papillae size is very important in promoting disassortative mating through a morph-specific match. Moreover, Manicacci & Barrett (1995) found, in Eichornia paniculata, that variants of the mid-styled plants, with an elongated stamen from the short whorl (selfing variant), presented larger pollen size compared to the mid-styled plants without the elongated anther, and that this larger pollen had higher success in pollination. The results of the present study support previous findings on the importance of ancillary traits in heterostylous species, and represent the first evidence of specific adjustments in pollen traits during the evolutionary transition from tristyly to distyly in O. alpina as a response to the loss of a floral morph, incompatibility modifications and further changes in position in the sexual organs.
After controlling for the reduction in flower size to detect non-allometric changes, we found that the relative changes in pollen size were morph- and whorl-specific following the previously described morphological changes associated with the evolution of the distylous syndrome in O. alpina (Weller et al. 2007, Sosenski et al. 2010; Baena-Díaz et al. 2012). Furthermore, we found variation in the correlations between pollen size, anther and style length between the long- and short-styled morphs and between populations (Table S1). If selection on the floral traits promotes non-random mating between floral types in heterostylous species, we could expect strong correlations between style length, stigma size, anther lengths and pollen size within and between floral morphs, maintaining high probability of compatible pollen transfer that will be reinforced by the match to the stigmatic environment and the action of pollen competition (Lewis & Jones 1992; Sarkissian & Harder 2001; Ferrero et al. 2011). Therefore, once a floral morph is lost, pollen adjustments should be conditioned to maintain the adaptive value of the reciprocity between floral morphs in the distylous populations while maximising pollen competitive ability. Despite a positive relationship between pollen size and style length in many plant species (Plitmann & Levin 1983; Williams & Rouse 1990; Torres 2000; Aguilar et al. 2002; López et al. 2006; McCallum & Chang 2016), the relationships of pollen size to other floral traits, including stamen length and anther size, should be further explored to understand how this correlation relates to pollination efficiency and fitness.
It is known that the size of the stigmatic papillae is related to pollen capture, adhesion and hydration, and a relationship between stigma depth and pollen size has been found (Cruden & Lyon 1985; Edlund et al. 2004; Cruden 2009). Such evidence suggests that pollen must fit in its stigmatic environment, particularly in heterostylous species, where papillae traits are polymorphic (Dulberger 1975; Li & Johnston 2001). In the genus Oxalis there is no variation in the shape of the papillae but marked variation in size according to the morph (Rosenfeldt & Galati 2009). Our results show marked differences in stigma size between long- and short-styled morphs, and an overall reduction in stigma size along the tristyly to distyly transition that could be associated with the observed reduction in pollen size, including the reduction in pollen from the long anthers in the short-styled plants (lS; Fig. 4).
The breakdown of specific incompatibility reactions, and subsequent adjustments in sexual organ reciprocity and pollen flow (Weller et al. 2007; Sosenski et al. 2010; Baena-Díaz et al. 2012), modified the features of pollen grains competing in a given stigma/style (pollen from long and mid anthers from different individuals of short-styled plants competing on the styles of long-styled plants, and pollen from short and mid anthers of long-styled plants competing on short styles), thus we predicted a functional adjustment in pollen size to these new competitive scenarios. Predictions of this study were based on the assumption that pollen size should match the corresponding stigma to increase their competitive advantage during fertilisation, thus a larger change was expected for pollen of mid anthers. However, we found that the smaller pollen grains from the shorter anthers in each morph (mS in the short and sL in the long floral morph) increased their size significantly, while no change was detected for pollen from mid anthers in the long-styled plants (mL). These results could also be explained through the hypothesis that pollen size has a functional relationship with pollen germination and pollen tube growth to increase their competitive ability through the amount of pollen reserves and substance in the pollen coat (Stephenson et al. 2003; Cruden 2009; Carrizo García et al. 2015, 2016). Under this hypothesis, smaller pollen grains with fewer reserves should be under competitive disadvantage and a relatively larger phenotypic adjustment would be expected. Although a handful of studies have shown that pollen size is positively related with siring success (Anderson & Barrett 1986; Quesada et al. 1995; Aizen & Raffaele 1998; Marshall & Evans 2016; McCallum & Chang 2016), the functional role of this associations remains controversial (Cruden & Miller-Ward 1981; Cruden & Lyon 1985; Cruzan 1990; Young & Stanton 1990; Young et al. 1994), More experimental studies exploring the functional relationship between stigma and papillae size, style length, pollen size, pollen germination and siring success, together with the correlations with floral traits, should be done to obtain a better understanding of the selective pressures acting on pollen attributes and their relationship to the evolution of reproductive and mating systems.
Overall, results from this study highlight the importance of ancillary traits (pollen and stigma size) in heterostylous systems like that of O. alpina and the complex relationship between these traits and other floral attributes, particularly during the evolutionary transition from tristyly to distyly. Results of this work showed that although pollen size followed allometric changes, additional morph- and whorl-specific changes suggest that the selective patterns on pollen size could be related to pollen competition. Our findings open questions about the functional value of pollen size in relation to the stigma–style environment. Further exploration of these associations in heterostylous systems will be very valuable to understand selective patterns on pollen traits.
Acknowledgements
We thank Ruben Pérez-Ishiwara for assistance with logistics and laboratory work and Weigang Yang for taking care of the plants; Roberto Munguía and Santiago Benitez for statistical advice; Judith Márquez-Guzmán, Daniel González-Tokman and J.F. Ornelas for comments and discussion on the manuscript; and JMG for providing laboratory facilities. This paper constitutes part of the doctoral thesis of FB at the Posgrado en Ciencias Biológicas of the National Autonomous University of México (UNAM). FB acknowledges the scholarship and financial support provided by the National Council of Science and Technology (CONACyT) and UNAM. This research was supported by grants from the University of California Institute for Mexico and the United States (UC MEXUS), CONACyT (47858-Q), the National Autonomous University of México (PAPIIT IN221210) and the National Science Foundation (grant DEB-0614164). FB, CAD and JF planned and designed the research. FB performed experiments and analysed the data. SGW collected plant material and contributed materials and analysis tools. PS contributed data on flower size and analysis. FB, JF, PS and CAD wrote the manuscript. All authors discussed the results and commented on the manuscript.




