Dissecting seed dormancy and germination in Aquilegia barbaricina, through thermal kinetics of embryo growth
Abstract
- Threshold-based thermal time models provide insight into the physiological switch from the dormant to the non-dormant germinating seed.
- This approach was used to quantify the different growth responses of the embryo of seeds purported to have morphophysiological dormancy (MPD) through the complex phases of dormancy release and germination. Aquilegia barbaricina seeds were incubated at constant temperatures (10–25 °C) and 25/10 °C, without pre-treatment, after warm+cold stratification (W+C) and GA3 treatment. Embryo growth was assessed and the time of testa and endosperm rupture scored. Base temperatures (Tb) and thermal times for 50% (θ50) of embryo growth and seed germination were calculated.
- W+C enabled slow embryo growth. W+C and GA3 promoted rapid embryo growth and subsequent radicle emergence. The embryo internal growth base temperature (Tbe) was ca. 5 °C for W+C and GA3-treated seeds. GA3 treatment also resulted in similar Tb estimates for radicle emergence. The thermal times for embryo growth (θe50) and germination (θg50) were four- to six-fold longer in the presence of GA3 compared to W+C.
- A. barbaricina is characterised by a multi-step seed germination. The slow embryo growth during W+C reflects continuation of the maternal programme of development, whilst the thermal kinetics of both embryo and radicle growth after the removal of physiological dormancy are distinctly different. The effects of W+C on the multiphasic germination response in MPD seeds are only partially mimicked by 250 mg·l−1 GA3. The thermal time approach could be a valid tool to model thermal kinetics of embryo growth and radicle protrusion.
Introduction
Requirements for seed dormancy loss and germination are specific for each species and depend on plant provenance (i.e. distribution and habitat) and phylogeny (Finch-Savage & Leubner-Metzger 2006; Baskin & Baskin 2014). Even closely related species, either growing in a variety of habitats (e.g. Vandelook et al. 2009) or co-occurring in a given habitat, may differ in their germination response to pre-dispersal environmental signals (e.g. Daws et al. 2002; Karlsson et al. 2008). Intraspecific variation in germination and embryo growth, among populations⁄ecotypes has also been related to differences in post-dispersal environment, mainly due to habitat (Donohue 2005; Giménez-Benavides et al. 2005; Mondoni et al. 2008).
Multi-step germination, in which testa and endosperm rupture are sequential events controlled by phytohormone balance, is widespread over the phylogenetic tree and has been described for many families, including Ranunculaceae (Hepher & Roberts 1985). In most species the seed-covering layers impose some level of physical constraint to radicle protrusion, which has to be overcome by a decrease in resistance of the surrounding tissue, an increased growth potential of the embryo or a combination of the two (Kucera et al. 2005; Müller et al. 2006). Abscisic acid (ABA) and gibberellic acid (GA) play an important role in a number of physiological processes in plants, including seed germinative growth. For example, in Lepidium sativum and Arabidopsis thaliana seeds, endosperm rupture is promoted by 10 μm GA4+7 and inhibited by about 10 μm ABA (Finch-Savage & Leubner-Metzger 2006; Müller et al. 2006). ABA induces dormancy during maturation, and GA plays a key role in dormancy release and in the promotion of germination, affecting both embryo growth and germination rate (e.g. Chen et al. 2008; Mattana et al. 2012a; Porceddu et al. 2016). Nevertheless, the role of gibberellins in dormancy release is controversial (Bewley 1997); although GA is associated with dormancy release and/or germination, in several species this treatment alone does not completely stimulate germination (Frattaroli et al. 2013; Baskin & Baskin 2014).
Temperature is one of the most important environmental conditions that control germination (Garcia-Huidobro et al. 1982; Probert 2000); it also determines the fraction of germinated seeds in a population and the rate at which they emerge (Heydecker 1977). In non-dormant seeds, the germination response to accumulated temperature has been modelled with a thermal time (θ) approach (Covell et al. 1986; Ellis et al. 1986; Pritchard & Manger 1990; Hardegree 2006). In this model, seeds accumulate units of thermal time (°Cd) to germinate for each percentile, g, of the whole population. When the seeds are subjected to temperatures (T) above the base temperature for germination (Tb), the germination rate increases linearly with temperature to an optimum temperature (To), above which germination rate starts to decrease (Garcia-Huidobro et al. 1982). Thus, in this suboptimal range (To–Tb), germination occurs in the time tg, when the thermal time accumulated has reached a critical value (θg) for percentile g of the population, which can be described as θg = (T−Tb)tg. Intraspecific variation in Tb among populations may be due to different pre-harvest environmental conditions, and related to seed developmental heat sum (Daws et al. 2004). The thermal time approach has also been used to predict seed germination in the field (i.e. Hardegree & Van Vactor 2000; Steadman et al. 2003; Chantre et al. 2009). Recently, the impact of different simulated climate change scenarios on seed dormancy release and germination timing was investigated in Vitis vinifera subsp. sylvestris (Orrù et al. 2012), and used to model the in situ natural regeneration patterns of Rhamnus persicifolia (Porceddu et al. 2013). However, to date there are no specific studies on threshold temperatures and thermal time requirements for embryo growth in morphophysiologically dormant (MPD) seeds. MPD is one of the least understood dormancy classes, but was also proposed to be the ancient class of dormancy (Willis et al. 2014).
Seeds of Ranunculaceae species can exhibit morphological (MD) and MPD (Baskin & Baskin 1994, 2014; Walck et al. 1999; Cho et al. 2016). Aquilegia sp. pl. seeds have linear underdeveloped embryos (sensu Baskin & Baskin 2007) and stratification of the seeds at 3–5 °C for 2–4 weeks is recommended before sowing for germination (Ellis et al. 1985). Mattana et al. (2012b) reported MPD for Aquilegia barbaricina and A. nugorensis, which could be broken more efficiently by a combination of warm and cold stratifications.
The aims of our investigations on A. barbaricina seeds were to: (i) identify the phases of germination in MPD seeds; (i) individually characterise the thermal requirements for embryo growth, dormancy release and germination in MPD seeds; and (iii) assess the intraspecific variability on embryo growth and seed germination based on two distinct populations.
Material and Methods
Study species
Aquilegia barbaricina Arrigoni & E.Nardi (Ranunculaceae) is a rhizomatous perennial herb that branches underground and has stems 30–60-cm long (Arrigoni & Nardi 1977). The fruits are erect capsules that produce dark trigonal seeds, each with a linear underdeveloped embryo. This species is endemic to the Gennargentu and Supramontes regions of central–eastern Sardinia where the plant grows from 800 to 1400 m a.s.l. in wet woodlands, meadows and stream margins, mainly occurring on siliceous substrates and secondarily on limestone ones (Garrido et al. 2012). The plants flower and fruit centre on May and July, respectively. The species is included in the IUCN Red Lists ( http://www.iucnredlist.org), it is classified as Critically Endangered (Fenu et al. 2011) and also as one of the 50 most endangered plants of the Mediterranean islands (de Montmollin & Strahm 2005).
Seed lot details
Seeds of A. barbaricina were collected directly from plants in riparian woods of Alnus glutinosa at the time of natural dispersal in early summer 2011 in two different populations in central–eastern Sardinia (Italy), specifically in Rio Correboi (RC; Villagrande Strisaili, OG) and in Rio Olai (RO; Orgosolo, NU; see Table 1).
| locality | population code | region | coordinates (UTM–Datum WGS84) | elevation range (m a.s.l.) | aspect | date of collecting | mean seed mass (mg ± SD) |
|---|---|---|---|---|---|---|---|
| Rio Correboi (Villagrande Strisaili, OG) | RC | Gennargentu |
N 40°03′ E 09°20′ |
1190–1300 | E–NE | 29/06/2011 | 1.26 ± 0.06 |
| Rio Olai (Orgosolo, NU) | RO | Supramontes |
N 40°07′ E 09°22′ |
948–970 | NE | 28/06/2011 | 1.40 ± 0.05 |
Germination tests
Three replicates of 20 seeds each per condition were sown in July 2011, on the surface of 1% agar water in 60-mm diameter plastic Petri dishes. Dishes were incubated in the light (12 h of irradiance) at a range of germination temperatures (10, 15, 20, 25 and 25⁄10 °C). In the alternating temperature regime, the light period coincided with elevated temperature. Further replicates were given a dormancy-breaking treatment consisting of a warm (W = 25 °C for 3 months) followed by a cold stratification (C = 5 °C for 3 months), before being incubated at the range of germination temperatures (Table 2). This pre-treatment was chosen on the basis of the findings of Mattana et al. (2012b). Three extra replicates of 20 seeds each were also sown on the surface of 1% agar water with 250 mg·l−1 GA3 and incubated in the light (12 h light) at the range of germination temperatures.
| condition | embryo growth measurements | ||
|---|---|---|---|
| code | description | number of measurements | timing |
| 0 | Control | 5 | After 15, 30, 60, 90 and 120 days |
| W+C | 3 months, 25 °C (W) →3 months, 5 °C (C) | 13 | After 15, 30, 60 and 90 days during warm (W), 15, 30, 60 and 90 days during cold (C), and 15, 30, 60 and 90 and 120 days after sowing for germination |
| GA3 | GA3 (250 mg·l−1) in the germination medium | 5 | After 15, 30, 60, 90 and 120 days |
Germination was defined as visible radicle emergence. Germinated seeds were scored three times a week. During the germination tests, seeds with a split seed coat were scored, and the time from seed coat splitting to endosperm rupture was monitored daily in 15 seeds for each treatment and investigated population. Germination tests lasted for 1–4 months. When no additional germination had occurred for 2 weeks, a cut-test was carried out to estimate the viability of the remaining seeds; soft seeds being considered non-viable. The final germination percentage was calculated as the mean of three replicates (±1 SD), on the basis of the total number of filled potentially competent seeds.
Embryo measurements
Embryo growth was assessed at different times, during the above-described conditions and germination temperatures by measuring ten seeds at each sample interval (see Table 2). Seeds were cut in half under a dissecting microscope and images of embryos acquired using a Zeiss SteREO Discovery.V8, with an objective Achromat S 0.63x, FWD 107 mm (Carl Zeiss MicroImaging, Germany) at 6.3× magnification, coupled to a Canon (Power shot G11) digital camera. Embryo and seed lengths were measured using the image analysis software ImageJ 1.41ᴏ (National Institutes of Health, Bethesda, MA, USA). Seed length was measured excluding the seed coat. The initial embryo length was calculated through measuring 20 randomly selected seeds before the start of the experiments. The embryo length of seeds with a split seed coat, but no radicle protrusion (i.e. critical embryo length), was determined for 20 randomly selected seeds and used as a surrogate for embryo length for seeds that had germinated before measurements (Vandelook et al. 2007).
Thermal time analyses
 (1)
(1)An average (±1 SD) of the x-intercept among percentiles was calculated for the suboptimal temperature range (10–20 °C) to establish the base temperature for germination (Tbg) for each treatment (Ellis et al. 1986; Pritchard & Manger 1990). Linear regression equations were recalculated for each percentile, but constrained to pass through Tbg (Hardegree 2006). A comparison of regressions was then made between this model and one in which the Tbg were allowed to vary for all percentiles, and the best estimate was considered to be that which resulted in the smallest residual variance (Covell et al. 1986). Thermal time (θg, °Cd) estimates were then calculated separately as the inverse of the suboptimal regression equations (Covell et al. 1986; see equation 1).
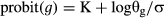 (2)
(2) (3)
(3) (4)
(4)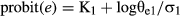 (5)
(5)
Statistical analysis
Generalised linear models (GLMs) were used to compare embryo length, period of the endosperm rupture phase, final germination percentages and base temperature (Tb). Then, significant differences within each condition were analysed by a post-hoc pair-wise comparisons t-test (with Bonferroni adjustment). GLMs with a log link function and quasi-Poisson error structure were used for analysing embryo length, rate of endosperm rupture and Tb values, while a GLM with a logit link function and quasi-binomial error structure was used for analysing germination percentages. Quasi-Poisson and quasi-binomial error structures and F tests with an empirical scale parameter instead of chi-squared on the subsequent anova were used in order to overcome residual over-dispersion (Crawley 2007). All statistical analyses were carried out with R version 2.14.0 (R Development Core Team 2011).
Results
Embryo growth
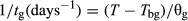
The GLM analysis detected no statistically significant differences (P > 0.05) between initial embryo length and final embryo length measured at the end of W+C, and did not highlight statistical differences (P > 0.05) among populations. The mean initial embryo length was 0.029 ± 0.006 mm for seeds of both populations (Fig. 1). During the W+C pre-treatment the embryo length increased slowly over time (Fig. 1). The mean embryo lengths after 90 days (i.e. at the end of the warm treatment) were ca. 0.040 mm for RO and ca. 0.037 mm for RC, and after 180 days (i.e. at the end of the W+C) these values increased to ca. 0.049 and ca. 0.044 mm for RO and RC, respectively (Fig. 1). Although final values at the end of W+C were not statistically different (P > 0.05 with GLM) from the initial embryo length, the linear regressions showed positive and significant relationships between the embryo length and time of the treatment (r2 = 0.56, P < 0.0001 for RO; r2 = 0.42, P < 0.0001 for RC), highlighting continuous and stable growth through W+C (Fig. 1).
The GLM analysis highlighted statistically significant differences (P < 0.001) on embryo lengths for the ‘treatment’ factor, whereas no statistical differences were found for one-way analysis of ‘population’ and ‘temperature’ factors and for all their interactions (Table 3). Incubation temperatures had a statistically significant effect (P < 0.001) on final embryo length with respect to the initial embryo length, or that calculated at the end of W+C pre-treatment (Fig. 2A). Temperatures in the control (0) had no effect on embryo growth, and the small differences between initial embryo lengths were due to the elapsed period from the initial to final (120 days) measurements (Fig. 2A). The mean critical embryo lengths calculated on seeds incubated at different germination conditions after W+C pre-treatment with a split seed coat but without endosperm rupture (as well as no radicle protrusion) were 0.115 ± 0.020 mm and 0.117 ± 0.023 mm for RO and RC populations, respectively (see Fig. 2A). In both populations, values obtained at the end of W+C and during GA3 treatment showed values similar to critical embryo length, while at the end of the control test they were similar to initial embryo length (Fig. 2A).
| df | deviance | resid. df | resid. Dev | F | P (>F) | |
|---|---|---|---|---|---|---|
| Embryo length (mm) | ||||||
| Null | 298 | 5.2642 | ||||
| Treatment | 2 | 3.1617 | 296 | 2.1025 | 244.1995 | <2e−16*** |
| Population | 1 | 0.0010 | 295 | 2.1015 | 0.1549 | 0.6942 |
| Temperature | 4 | 0.0445 | 291 | 2.0570 | 1.7179 | 0.1462 |
| Treatment: Population | 2 | 0.0023 | 289 | 2.0548 | 0.1738 | 0.8406 |
| Treatment: Temperature | 8 | 0.0824 | 281 | 1.9724 | 1.5905 | 0.1275 |
| Population: Temperature | 4 | 0.0112 | 277 | 1.9612 | 0.4322 | 0.7853 |
| Treatment: Population: Temperature | 8 | 0.0315 | 269 | 1.9297 | 0.6088 | 0.7702 |
| Rate of endosperm rupture (days) | ||||||
| Null | 283 | 64.390 | ||||
| Treatment | 1 | 12.1639 | 282 | 52.226 | 104.7452 | <2.2e−16*** |
| Population | 1 | 0.3706 | 281 | 51.855 | 3.1910 | 0.0752 |
| Temperature | 4 | 20.3480 | 277 | 31.507 | 43.8047 | <2.2e−16*** |
| Treatment: Population | 1 | 0.9061 | 276 | 30.601 | 7.8028 | 0.0056** |
| Treatment: Temperature | 4 | 2.4064 | 272 | 28.195 | 5.1804 | 0.0005*** |
| Population: Temperature | 4 | 0.6684 | 268 | 27.527 | 1.4388 | 0.2215 |
| Treatment: Population: Temperature | 4 | 0.4923 | 264 | 27.034 | 1.0599 | 0.3768 |
| Germination (%) | ||||||
| Null | 89 | 5098.6 | ||||
| Treatment | 2 | 3640.4 | 87 | 1458.2 | 445.2532 | <2.2e−16*** |
| Population | 1 | 55.4 | 86 | 1402.8 | 13.5411 | 0.0005*** |
| Temperature | 4 | 206.9 | 82 | 1196.0 | 12.6505 | 1.584e−07*** |
| Treatment: Population | 2 | 149.2 | 80 | 1046.8 | 18.2441 | 6.461e−07*** |
| Treatment: Temperature | 8 | 457.8 | 72 | 589.0 | 13.9978 | 3.024e−11*** |
| Population: Temperature | 4 | 165.5 | 68 | 423.5 | 10.1215 | 2.501e−06*** |
| Treatment: Population: Temperature | 8 | 167.1 | 60 | 256.4 | 5.1091 | 7.146e−05*** |
- ** P < 0.01, *** P < 0.001

Testa and endosperm rupture
Seeds exhibited a multi-step germination that followed embryo growth, such that there was a delay between testa rupture, following embryo elongation and exposure of the endosperm, and endosperm rupture, when the radicle emerged (Fig. 2B). Statistically significant differences (P < 0.001) were found on estimated rate of endosperm rupture, for ‘treatment’ and ‘temperature’ factors, while no statistical difference (P > 0.05) was detected for the ‘population’ factor. A statistically significant difference (P < 0.01) was found for the interactions treatment × population and treatment × temperature, and no statistically significant differences (P > 0.05) were detected for the interactions population × temperature and treatment × temperature × population (Table 3). After W+C treatment, the mean time from testa to endosperm rupture (i.e. radicle protrusion) decreased with increasing temperature, ranging from just over 6 days at 10 °C to just under 2 days at 20 °C for RO and RC populations (Fig. 2B). At 25 °C and at 25/10 °C this time interval was around 4 days and 2 days, respectively, for both RO and RC populations (Fig. 2B). In contrast, the time from testa to endosperm rupture in the GA3 treatment was slower than after the W+C treatment (Fig. 2B). This was most evident in seeds of RC population, with intervals of 22 days at 10 °C and ca. 5 days at 25 °C (Fig. 2B).
Seed germination
The GLM highlighted statistical effects (P < 0.05) on seed germination for all applied factors, as well as for all their interactions (Table 3). While no seeds germinated during the control (0) and very low percentages (ca. 1% for RO and ca. 6% in RC) were detected at the end of C during W+C pre-treatment, seeds germinated to >50% both after W+C and during GA3 treatments in each population (Fig. 2C). Statistically significant differences (P < 0.001) among temperatures were detected within each treatment, except for seeds of RC treated with GA3 (P > 0.05), where the germination range was from ca. 52% (at 10 °C) to ca. 80% (at 25 °C; Fig. 2C). GA3-treated seeds of the RO population germinated from ca. 12% (at 10 °C) to ca. 62% (at 20 °C; Fig. 2C). After W+C, high germination was observed at 25 °C (88 ± 6%) for RO, and at 15 °C (81 ± 12%) for RC (Fig. 2C).
Thermal time approach on embryo growth
The GLM analysis (Table 3) did not show statistically significant differences (P > 0.05) in embryo growth between populations. Therefore, a combined population response dataset was used to evaluate embryo thermal requirements, ascribing this characteristic to the species level. Seeds that germinated after W+C and during GA3 treatments showed differences in both critical embryo length rate (1/te) and cardinal temperatures (Fig. 3). Based on embryo length rate responses for each 10th percentile (from 10% to 90%) of seeds that reached the critical embryo length, it was possible to estimate the mean base temperature (Tbe) in the suboptimal temperature range for W+C and GA3, and the mean ceiling temperature (Tce) in the supra-optimal temperature range, and subsequently the optimal temperature for embryo growth (Toe) for W+C (Fig. 3). Linear regressions for the different percentiles of suboptimal temperature range for W+C were calculated passing through 5 °C, which corresponds to an embryo growth rate equal to 0, the value obtained at the end the W+C pre-treatment (see Fig. 1). The obtained regression lines were then constrained to pass through the common value of Tbe. For the supra-optimal temperature range, linear regressions were constrained to pass through the common value of Tce. Linear regressions for the different percentiles for GA3 were constrained to the common value of Tbe. These models showed higher values of r2 for all of the linear regression equations than the model where Tbe and Tce varied for each percentile. Average Tbe were 5.20 ± 0.60 °C and 5.30 ± 2.56 °C for W+C and GA3 treatments, respectively (Fig. 3), without statistically significant differences among treatments (P > 0.05). Average Tce for W+C was 29.52 ± 2.37 °C, and the average Toe was 15.00 ± 1.02 °C (Fig. 3), whereas in GA3 treatment Toe may be assumed to be ≥25 °C (Fig. 3).
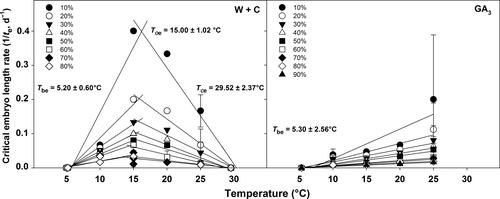
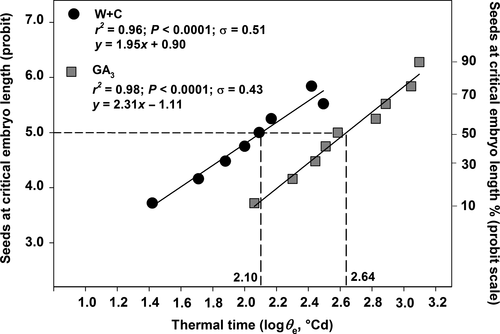
Figure 4 shows the relationship between log thermal time (θe) and percentages of seeds that reached the critical embryo length expressed in probits, calculated according to equation 2. The relationship between log θe and probit critical embryo length had better residual sums of square (0.1420 for W+C and 0.1228 for GA3) and r2 (0.95 and 0.97 for W+C and GA3, respectively) than when expressed on a linear scale (data not shown). Thermal time required for 50% of seeds to reach the critical embryo length (θe50) was longer for the GA3 with a value of 2.64 log °Cd compared to the W+C-treated seeds with a value of 2.10 log °Cd. However, seeds of W+C and GA3 that reach the critical embryo length showed a very similar σ value (0.51 and 0.43 °Cd, respectively; Fig. 4).
Thermal time approach on seed germination
The Tbg for the RO population were 6.85 ± 0.26 °C for W+C and 8.43 ± 1.53 °C for GA3 treatment, while for the RC population it was 5.34 ± 1.38 °C and 5.42 ± 0.26 °C for W+C and GA3 treatment, respectively (Fig. 5). These values were statistically different (P < 0.01) according to the GLM, and a post-hoc pair-wise comparisons t-test highlighted that the difference was determined by the Tbg value of GA3-treated seeds belonging to the RO population (Fig. 5). For each treatment on both populations, the linear regressions were re-calculated for each percentile, constraining them to pass through the mean Tbg (Fig. 5). This model led to no differences in residual sum of squares compared with when Tbg was allowed to vary for each percentile, and showed highest values of r2 for all of the linear regression equations (r2 > 0.91 for RO W+C, r2 > 0.58 for RC W+C, r2 > 0.88 for RO GA3 and r2 > 0.57 for RC GA3).
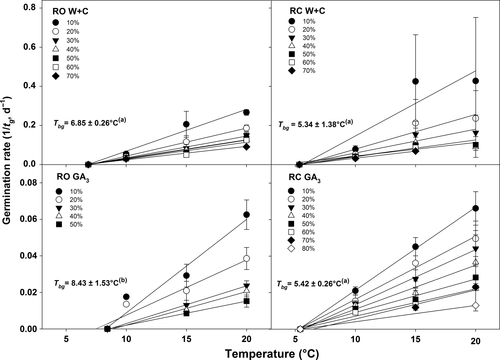
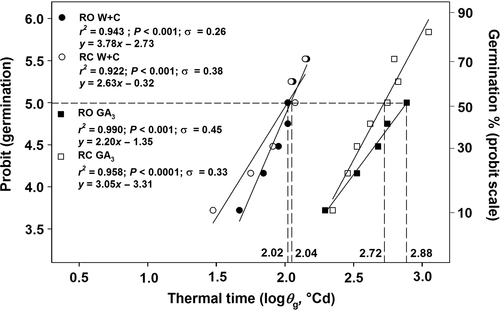
Figure 6 shows the relationship between log thermal time (θg) and germination expressed in probits, calculated according to equation 1. The relationship between log θg and probit germination had better residual sums of squares both in W+C pre-treated seeds (0.1349 and 0.1851 for RO and RC populations, respectively) and in the GA3-treated seeds (0.0098 and 0.1477 for RO and RC populations, respectively) than when expressed on a linear scale (data not shown). Thermal time required for 50% of germination (θg50) was longer for the GA3-treated seeds (2.88 and 2.72 log °Cd for RO and RC, respectively) compared to the W+C pre-treated seeds (2.04 and 2.02 log °Cd for RC and RO, respectively; Fig. 6). In addition, GA3-treated seeds of RO had a larger σ value (0.45 log °Cd) than seeds belonging to the RC population (0.33 log °Cd) and of those W+C pre-treated seeds (0.38 log °Cd and 0.26 log °Cd for RC and RO populations, respectively; Fig. 6).
Discussion
Seed dormancy and multiple phases to the completion of seed germination
The embryo in seeds of A. barbaricina is small at dispersal (0.03-mm long) and must grow before radicle emergence. Therefore, these seeds would be classified as morphologically dormant (MD) following the Baskin & Baskin (2014) dormancy classification system. Generally, if embryos have only MD, growth is completed in a relatively short period, and seeds germinate within about 4 weeks (Baskin & Baskin 2014). A. barbaricina seeds of each population did not germinate without any treatment, even after 120 days. Nonetheless, embryos in seeds subjected to W+C (25 °C, followed by 5 °C) grew internally by about 50% to ca. 0.04–0.05 mm (Fig. 1). The rate of change in embryo length was about 0.00009 mm·day−1. After warm followed by cold stratification or GA3 treatment, seeds started to germinate (radicles emerged) at all tested temperatures, due to a rapid increase in embryo growth to ca. 0.12 mm. This second phase of internal growth of the embryo is about 800× faster (ca. 0.07 mm·day−1) than during W+C treatment. This suggests two distinctly different physiological responses of the embryo during treatment.
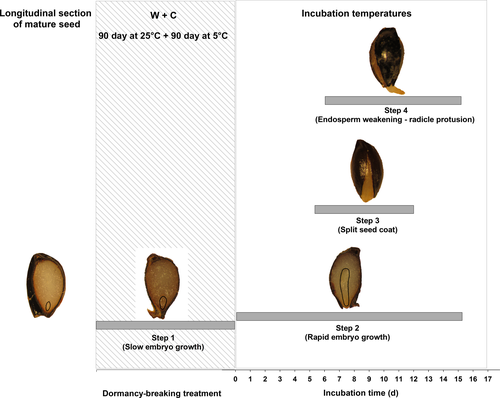
This study confirmed the presence of multi-step seed germination in the Ranunculaceae, involving the need for embryo growth within the seed before emergence, as previously reported by Hepher & Roberts (1985) for Trollius ledebouri. The classical conceptual model of the imbibition process distinguished three principal phases: phase I, marking a rapid uptake of water due to the low water potential of the seed; phase II, in which the water content does not change substantially in the intact seed prior to radicle protrusion; and phase III, when rupture of the covering layers (testa and endosperm) allows growth of the collet and the protruded radicle becomes visible (Bewley 1997). Recently, Toorop (2015) proposed a new dormancy-dependent conceptual model for multiphasic imbibition of seeds in which the classical phase II is split into three sub-classes: phase IIA is identical to the classical phase II; phase IIB is associated with testa rupture; and the transition between phase IIC and phase III indicates the endosperm rupture and radicle protusion. However, for seeds with underdeveloped embryos such as those of A. barbaricina, a multi-step seed germination can be described, with at least four well recognisable phases after imbibition: (I) the embryo grows slowly inside the seed; (II) the embryo grows rapidly inside the seed; until the (III) seed coat splits and (IV) the endosperm weakens allowing the radicle protrusion (Fig. 7). Accordingly to the conceptual model for multiphasic imbibition proposed by Toorop (2015), the phases III and IV detected in this work for A. barbaricina correspond to the phases from IIB to III. More recently, multiphasic sequential germination steps (i.e. embryo growth, testa rupture, endosperm rupture – radicle emergence) including the epicotyl–plumule emergence event, were also identified for P. corsica seeds (Porceddu et al. 2016).
It is known that the inhibitory effect of ABA is counteracted by gibberellin and that endosperm rupture is under the control of an ABA–gibberellin antagonism (Koornneef et al. 2002; Leubner-Metzger 2003; Kucera et al. 2005; Weitbrecht et al. 2011). In A. barbaricina, GA3-treated seeds had longer mean time courses for the transition from testa rupture to endosperm rupture, compared to W+C stratified seeds. This suggests that GA3 does not substitute completely for the beneficial effects of temperature pretreatment when considering kinetics of the germination process.
Overall, germination in A. barbaricina is a complex, multi-step process that involves the phased completion of embryo development and phased emergence. In non-dormant seeds (i.e. after warm + cold treatment), the critical embryo length is reached <2 days after a shift in temperature, the seed coat splits and the radicle protrudes by ca. 6 days, with significant overlap among all the phases during a period of ca. 16 days (Fig. 7). This overlap suggests that the seed coat starts to split when the embryos are still growing within the seed, before they reach their ‘critical length’ for germination and that radical protrusion immediately follows the split of the seed coat (Fig. 7). We next attempted to quantify and compare some key steps in this complex germination response.
Thermal thresholds for embryo growth and seed germination
The base temperature for embryo growth (Tbe) of non-dormant seeds of A. barbaricina was approximately 5 °C both in W+C-stratified and GA3-treated seeds. For W+C pre-treated seeds, it was possible to calculate all cardinal temperatures, with optimal temperature for embryo growth of ca. 15 °C and a ceiling temperature of ca. 29 °C. Base temperature for germination (Tbg) varied from ca. 5 to 7 °C in W+C stratified seeds, and from 5 to 8 °C for GA3-treated seeds, depending on the provenance. Considering that no seeds of A. barbaricina germinated without treatment at the tested constant temperatures, a Tb ≥ 25 °C (i.e. the highest temperature tested) may be hypothesised for dormant seeds of the two investigated populations. However, this should be confirmed through incubating seeds without pre-treatments at higher temperatures. A similar trend was detected in seeds of V. vinifera subsp. sylvestris (Orrù et al. 2012).
Constraining the linear regressions of each percentile for germination through the mean Tb resulted in an improvement of the residual sum of squares and r2 values. Therefore, Tb for embryo growth and for germination can be used to describe the whole population response in A. barbaricina seeds, as previously reported for other species (e.g. Covell et al. 1986; Ellis et al. 1987; Pritchard & Manger 1990; Orrù et al. 2012; Porceddu et al. 2013). The best model was obtained by fitting germination expressed in probit and log-normal (log °Cd) rather than normal distributed thermal times (°Cd), as previously reported for other herbaceous (Covell et al. 1986; Ellis & Butcher 1988) and woody (Pritchard & Manger 1990; Porceddu et al. 2013) species. Also, regarding the thermal times of embryo growth rate, the best model was obtained by fitting the values in probit and log-normal (log °Cd) compared to when normal-distributed, confirming that this methodology increases the goodness of fit of the model.
Seeds of A. barbaricina varied in their thermal time estimates to reach θ50, depending on treatment. W+C pre-treated seeds had the lowest θ50 values (2.10 log °Cd; i.e. 128 °Cd) for embryo growth compared to GA3-treated seeds (2.64 log °Cd; i.e. ca. 440 °Cd). The same trend was detected also for germination (radicle emergence), with θ50 values of 2.03 log °Cd (110 °Cd) for W+C stratified seeds and ca. 2.80 log °Cd (ca. 650 °Cd) for GA3-treated RC and RO seeds. The four- to six-fold longer thermal times for embryo growth (θe50) and germination (θg50) in GA3- compared to W+C-treated seeds suggests that GA3 treatment only partially mimicks/replaces the W+C pre-treatment in seeds of this species.
There is now considerable evidence for negative linear relations between association θg50 and Tbg in a broad range of species within life forms (Dürr et al. 2015). Yet in A. barbaricina such a relationship appears to vary with pretreatment, as removal of seed dormancy by GA3 and W+C results in the same threshold temperature for germination (Tbg) but different germination thermal times (θg50), with GA3-treated seeds being much slower to grow. Whilst long-standing dormancy classification systems rely heavily on fixed time intervals for the germination process (see Baskin & Baskin 2014), our work emphasises the importance of using thermal time kinetics to dissect the various stages of the germination process.
The analysis carried out in this study showed that in A. barbaricina the thermal requirements for embryo growth did not vary among populations, while for seed germination these were different between populations. Embryo growth could be strictly related to the seed biology of the species, whereas germination could be more related to the habitat of provenance of the species. Copete et al. (2014) reported that in seeds of Narcissus eugeniae (Amaryllidaceae) belonging to two different populations and tested in both near-natural and laboratory conditions, the embryo growth showed a similar pattern, while radicle emergence did not begin simultaneously. Intraspecific germination differences among populations of a species can arise due several factors, such as light, moisture and temperature (Gutterman 1992; Fenner & Thompson 2005), and can be interpreted as being an adaptation to specific habitat (Meyer et al. 1995, 1997), as detected in this study for A. barbaricina.
Conclusions
The results indicate that A. barbaricina is characterized by multi-step seed germination. The slow embryo growth during W+C treatment reflects the continuation of the maternal programme of development that was punctuated by seed dispersal, while the thermal kinetics of both embryo growth and radicle protrusion after the removal of physiological dormancy are distinctly different. The thermal time model developed in this work allowed us to identify the thermal threshold (Tb and θ50) requirements of embryo growth and seed germination of this species. The beneficial effects of W+C treatment on the multi-phasic germination response in MPD seeds is only partially mimicked by 250 mg·l−1 GA3 treatment, having a similar controlling influence on base temperature for embryo growth and germination but not on rate processes. This attempt to model thermal requirement for embryo growth using a thermal time approach was confirmed through the morphological observations. This model could be applied in those species whose seeds also have a morphological component of dormancy (MD and MPD), could be a valid tool to model thermal kinetics of embryo growth and radicle protrusion, and may also be useful to predict seedling emergence in the field.
Acknowledgements
We gratefully acknowledge Sardinia Regional Government for financial support to Marco Porceddu PhD scholarship (P.O.R. Sardegna F.S.E. Operational Programme of the Autonomous Region of Sardinia, European Social Fund 2007-2013 – Axis IV Human Resources, Objective l.3, Line of Activity l.3.1.). CCB is supported by the ‘Provincia di Cagliari – Assessorato Tutela Ambiente’. The Royal Botanic Gardens, Kew, receives grant in-aid from Defra, UK.




