Phylogeny determines flower size-dependent sex allocation at flowering in a hermaphroditic family
Abstract
- In animal-pollinated hermaphroditic plants, optimal floral allocation determines relative investment into sexes, which is ultimately dependent on flower size. Larger flowers disproportionally increase maleness whereas smaller and less rewarding flowers favour female function. Although floral traits are considered strongly conserved, phylogenetic relationships in the interspecific patterns of resource allocation to floral sex remain overlooked. We investigated these patterns in Cistaceae, a hermaphroditic family.
- We reconstructed phylogenetic relationships among Cistaceae species and quantified phylogenetic signal for flower size, dry mass and nutrient allocation to floral structures in 23 Mediterranean species using Blomberg's K-statistic. Lastly, phylogenetically-controlled correlational and regression analyses were applied to examine flower size-based allometry in resource allocation to floral structures.
- Sepals received the highest dry mass allocation, followed by petals, whereas sexual structures increased nutrient allocation. Flower size and resource allocation to floral structures, except for carpels, showed a strong phylogenetic signal. Larger-flowered species allometrically allocated more resources to maleness, by increasing allocation to corollas and stamens.
- Our results suggest a major role of phylogeny in determining interspecific changes in flower size and subsequent floral sex allocation. This implies that flower size balances the male–female function over the evolutionary history of Cistaceae. While allometric resource investment in maleness is inherited across species diversification, allocation to the female function seems a labile trait that varies among closely related species that have diversified into different ecological niches.
Introduction
Patterns of resource allocation constitute a fundamental strategy in the ecology and evolution of plants. As individuals deal with limited resource availability, allocation to a specific structure or function constrains investment to other structures (Lloyd 1980; Aragón et al. 2009). It follows that allocation shows traits and reproductive strategies that maximise fitness (de Jong & Klinkhamer 2005). In animal-pollinated plants, flower production requires sizeable amounts of biomass and nutrients appropriately distributed among the different structures and functions to achieve reproduction (Cruden & Lyon 1985; Méndez & Traveset 2003; Campbell et al. 2011). For example, larger corollas significantly increase pollinator attraction and resulting pollen transfer among individuals, whereas sepals or additional green structures act as carbon sources and provide protection to developing fruits and seeds (reviewed in Case & Ashman 2005). Otherwise, sexual structures are especially costly in terms of nutrients (i.e. N and P) due to high protein content related to pollinator rewards, pollen tube growth, fertilisation and subsequent resource supplies to set fruit (Roulston et al. 2000; Case & Ashman 2005).
For hermaphrodites, classical theory predicts that allocation to male and female functions is dependent on the respective gender fitness gain curve (Charnov 1982). Resource allocation to floral structures should optimise fitness gains via male and female functions, and this will depend on the shape of the gain curve for each sex. Therefore, shape of the fitness gain curve would determine optimal resource allocation. For example, increasing resource allocation to pollen production with high pollen–ovule ratios and secondary allocation to pollinator attractiveness with increased corollas favours the quantity of mating opportunities for the male function (Bell 1985; Cruden & Lyon 1985; Charnov & Bull 1986). In contrast, higher resource allocation to carpels together with lower investment in pollinator attractiveness indicates a bias towards femaleness by optimising offspring quality through higher investment in fruit and seed production (Lloyd 1980; Charlesworth & Charlesworth 1981). Consequently, patterns of sexual expression in animal-pollinated hermaphroditic plants usually depart from strict equisexuality (Charnov & Bull 1986; Campbell 2000).
A diverse range of ecological and evolutionary factors, such as plant size (e.g. Méndez & Traveset 2003), breeding and mating systems (e.g. Campbell et al. 2011) or environmental variation (e.g. Parachnowitsch & Elle 2004; Zhao et al. 2008) mediate differential allocation to floral gender in hermaphroditic plants. From a macroevolutionary perspective, interspecific changes in patterns of resource allocation to floral phenotypic or functional gender and secondary structure expression are finally mediated by changes in flower size (i.e. allocation to structures for pollinator attraction and pollen-to-ovule ratios: Bell 1985; Goodwillie et al. 2010). Hence, flower size-dependent allometric scaling in investment of resources to floral sex and structures would be expected. Evidence in this sense would provide support to arguments based on the gender fitness gain curve, which would in turn potentially explain the pattern of increased maleness with flower size. Several studies have focused on allometric relationships between allocation to floral structures and gender bias with flower size, but they were carried out only within a particular species (Ushimaru & Nakata 2001; Méndez & Traveset 2003; Parachnowitsch & Elle 2004; Summers et al. 2015).
A next logical but overlooked step is to ascertain whether allocation to floral functional gender disproportionally differs with interspecific variation in flower size among related species. Phylogenetic analyses revealing species relationships are required to understand floral trait evolution and diversity (Dodd et al. 1999; Ackerly 2009; Gómez et al. 2015). Species in a given clade might show high similarity for any conservative trait with strong phylogenetic signal, whereas labile traits might diverge even in closely related species that have diversified into different ecological niches (Blomberg & Garland 2002; Ackerly 2009). Floral traits are commonly thought to be strongly adaptive and conserved as flowers are directly involved in the first stages of reproduction that determine fitness (Armbruster et al. 1999; Brock & Weinig 2007; Oguro & Sakai 2015). However, phylogenetic relationships in the interspecific patterns of resource allocation to reproductive structures remain unknown. A phylogenetic approach using multiple species allows us to determine whether those patterns are conserved between small- and large-flowered groups and could promote our understanding of the evolution of floral traits.
In this study, we evaluated gender bias in resource allocation to floral structures (corolla, calyx, sexual structures) among species of Cistaceae, a hermaphroditic and entomophilous family. Cistaceae represents a good model system to examine such differences as representatives show large variation in flower size (Herrera 1992). Phylogeny of the family has also been reconstructed during recently using plastid and nuclear DNA sequence data, distinguishing lineages between earlier-diverging small-flowered clades (Fumana, Lechea and Helianthemum clades) and a cohesive natural group containing large-flowered species (Cistus-Halimium complex; Guzmán & Vargas 2005, 2009a; Civeyrel et al. 2011). We specifically expect (i) flower size and relative allocation of biomass and nutrients to floral structures and gender would be adaptive and well-conserved traits across the family, showing a phylogenetic signal; and (ii) larger-flowered species would allocate more resources to male function because the fitness curve will accelerate more steeply with flower size for male relative to female function.
Material and Methods
Species and study area
Cistaceae is a family with eight genera and about 200 species of shrubs and herbs, mainly from temperate sites of the Northern Hemisphere but also in Southern America (Arrington & Kubitzki 2003). Its diversification centre is the Mediterranean area, especially the Iberian Peninsula (five genera and 64 species), being typical elements of schlerophyllous forests (Arrington & Kubitzki 2003). The Iberian Peninsula is generally characterised by a Mediterranean climate, with Atlantic-temperate climate in the northernmost coasts, and a SE-NW humidity gradient. Study sites included semiarid, dry, subhumid and hyperhumid climate with annual average rainfall of approx. 300–1600 mm and annual mean temperatures range of 9–18 °C.
Plants produce many single flowers, more commonly in inflorescences, between February and late June. All species have hermaphroditic and disc-shaped flowers with predominant outcrossing breeding systems, although annual species may show autogamy (see Table S1 and references therein). Flowers have three to five sclerophyllous sepals having a combination of diverse hair types, a distinctive trait of the family (Arrington & Kubitzki 2003) and five pink, white or yellow petals of 8–120 mm in diameter (Herrera 1992; Figure S1). Stamens are usually numerous and anthers, between four and approx. 200, contain large amounts of pollen grains, which is positively correlated to flower size (Herrera 1992). Exceptionally, in the genus Fumana, the outermost anthers are sterile and do not contain pollen of any kind (Herrera 1992; Nandi 1998). The gynoecium varies in shape and size, with three to 12 carpels, one style and one full stigma with an approximately circular surface. There is large variation among species in the number of ovules in the ovary, of between approx. seven and 1200, which is also positively correlated with flower size (Herrera 1992). Pollen-to-ovule ratios are also variable, being up to 2000 or more, consistent with an outcrossing breeding system. However, several herbs have extremely low pollen-to-ovule ratios, consistent with selfing reproduction (Herrera 1992).
A sampling of 23 species of Cistaceae inhabiting the Iberian Peninsula was selected. This sample is representative in terms of the actual diversity in this area and represents most species from the Iberian Peninsula included in the phylogeny of the family constructed by Guzmán & Vargas (2009a), as well as contrasting flower size to test the hypotheses. We collected nine species of Cistus, two of Fumana, five of Halimium, six of Helianthemum and one of Tuberaria occurring across the largest part of ecological range and covering the majority of representative habitat of the family (Table S1).
Floral size and resource allocation
For this study, one population per species was selected (Table S1). In 2009 and 2010, during the flowering peak of every species, 15 plants per species were randomly selected and, in each plant, one open flower was randomly selected to measure flower size, estimated as corolla diameter (cm) and corolla area (cm2). Corolla diameter was recorded using calipers (to the nearest mm). Additionally, we excised one petal per flower. Excised petals were carefully unfolded and photographed on a black sheet to assess their surface area, using image processing in ImageJ version 1.43 (ImageJ 2010; US National Institutes of Health, Bethesda, MD, USA, http://imagej.nih.gov/ij/). Then we multiplied each value by five to estimate corolla surface area, since flowers have five petals. Flower size estimated by both corolla diameter and corolla surface area was averaged for every species.
Two flower buds per plant, including the pedicel, were randomly harvested and kept in 70% alcohol. All flower buds were collected from the same part of the plant and in 1 day to avoid differences in floral allocation as flowering progresses. Flower buds instead of open flowers were collected so as to have suitable values for resource allocation to stamens and carpels because all pollen is still contained in the flower buds. Additionally, open flowers contain similar amounts of dry mass and nutrients unlike flower buds and, although they continue growing after opening, this is due to water allocation (Galen 2005). In the lab, each bud was divided into pedicel, sepals, petals, carpels and stamens. All the portions were subsequently oven-dried for 2 days at 60 °C and weighed to the nearest 0.1 mg with a microbalance (MX5; Mettler-Toledo, Greifensee, Switzerland) to obtain dry mass (mg). Subsequently, we examine nitrogen (N) and phosphorus (P) allocation in terms of dry mass (μg) and concentration ([N] and [P], mmol·g−1 dry mass). The objective of analysing differences between dry mass and concentration of nutrients was twofold. First, concentration provides valuable information about differential allocation among floral structures, especially to sexual structures (Méndez & Traveset 2003) regardless of allocation in terms of mass. Second, it provides evidence of whether nutrient mass allocation per unit of area is or is not allometric in relation to flower size. For N and P content analysis, pedicels were not used because of their low percentage representation in relation to total floral dry mass (Table S2). Whenever necessary, each remaining floral structure was pooled per species to reach a minimum 2.5 mg weight of plant material, and then digested in sulphuric acid and analysed for total N and P with a SKALAR San++ Analyser (Skalar, Breda, The Netherlands).
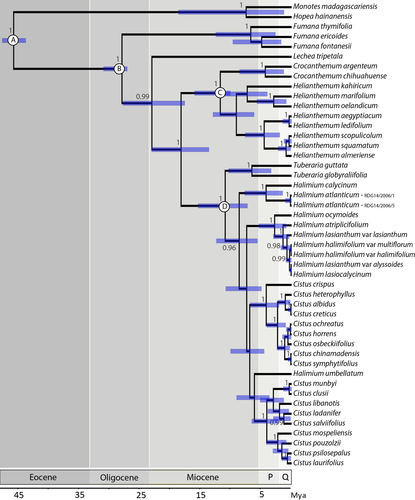
Phylogeny reconstruction and phylogenetic signal
Based on the raw aligned DNA matrix of rbcL (1367 bp) and trnL-trnF (517 bp) regions provided by Guzmán & Vargas (2009a), the most complete phylogenetic hypothesis available for Cistaceae to date, we re-built the Cistaceae phylogeny for our study with a new calibration focused on a smaller sampling. Additionally, the three fossils referenced in Guzmán & Vargas (2009b) were applied here with some modifications (Table S3). First, fossils were implemented using log-normal distribution to constrain the minimum age of the clades (instead of the maximum age as in the referenced paper) because this is a more conservative approach (detailed discussion in Forest 2009). Second, a secondary calibration point based on Bell et al. (2010) was applied in the stem node of Cistaceae representing the divergence between Cistaceae and the sister family Dipterocarpaceae (46 Ma). Third, in Guzmán & Vargas (2009b) Helianthemum fossil had been placed on the basis of Crocanthemum (New World clade) + Helianthemum (Old World clade), but we believe that the correct placement must respect the geographic origin of the fossil (France), so we changed the placement of Helianthemum fossil to correspond to the geographic origin of the clade (Old World). We included 45 taxa representing subgenera and sections of Cistaceae plus Hopea hainensis and Monotes madagascariensis (Dipterocarpaceae) as outgroup (Table S4). Methods for DNA sequence alignment were described in the original article (Guzmán & Vargas 2009a) and the same evolutionary models were applied (GTR+G for trnL-trnF and GTR+I+G for rbcL). Bayesian divergence and time estimation were performed using the package BEAST version 1.8.0 (Drummond et al. 2012). A Yule process speciation prior and an uncorrelated log-normal model of rate variation were implemented. Two runs of four chains with 30 million generations were performed, sampling one tree every 1000th generation. Parameter convergence was confirmed by examining their posterior distributions in TRACER 1.6 (Rambaut & Drummond 2007); Monte Carlo Markov Chain (MCMC) sampling was considered sufficient when the effective sampling size of each parameter was >150. All analyses were performed on the CIPRES portal (Miller et al. 2010). A maximum clade credibility tree with median branch lengths and a 95% highest posterior density interval on nodes was built combining the trees with TreeAnnotator 1.8.1 (Drummond et al. 2012) based on the remaining set of trees after burn-in (for each run, a burn-in period of 3 million generations was applied). Phylogenetic relationships found here (Fig. 1) were compared with Guzmán & Vargas (2009a) and, since they were congruent based on the branches with high support (P > 0.95). The temporal divergence between clades is older in this new dating, but as the relationships among clades (younger versus older clades) are the same, we subsequently pruned this tree only for 23 species for which we have morphological data in order to examine flower size and the patterns of resource allocation. This sample is sufficiently large to detect phylogenetic patterns in floral traits, as several other studies across multiple species using phylogenetic comparative approaches have shown (e.g. Guzmán & Vargas 2005; Smith et al. 2008; Oguro & Sakai 2015).
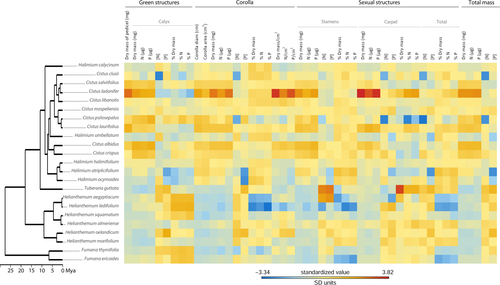
We estimated phylogenetic signal for 48 traits related to flower size and resource allocation to floral structures (Fig. 2) using Blomberg's K-statistics (Blomberg et al. 2003) implemented in the PICANTE R-library (Kembel et al. 2010), the most commonly applied model-based approach in comparative studies. As flower size showed strong phylogenetic conservatism (see Results), we considered the non-independence among species in the subsequent analyses.
Statistical analyses
To determine correlation of the allocation currencies among all floral structures across the family we used Pearson's coefficient on the corrected trait values. Traits were corrected in accordance with the phylogenetic relationships based on Phylogenetically Independent Contrasts (PIC; Felsenstein 1985). We correlated the PIC values of the percentages of all allocation currencies allocated to sepals, petals, stamens and carpels to accurately control the phylogenetic relationships. Assumptions of normality and homogeneity of variance were tested using Shapiro–Wilk's test and Levene's test, respectively.
To examine allometric patterns in resource allocation to floral structures in relation to flower size we used reduced major axis (RMA) regression analysis (Green 1999). As flower size shows phylogenetic signal (see Results), we regressed the PIC values of the percentage of all allocation currencies as well as nutrient concentration allocated to sepals, petals, stamens and carpels against the PIC values of corolla area to accurately control the phylogenetic relationships. Dry mass, N and P allocation to the above-mentioned structures were log10 (x + 1)-transformed to linearise allometric relationships and then regressed against each other (Ushimaru & Nakata 2001).
In addition, to test allometric variation between pairs of floral structures directly or indirectly related to sex (i.e. stamens versus carpels and petals versus sepals, respectively) in relation to flower size, comparisons of the slopes of the relationships between the PIC values of those floral structures across the family for the three kinds of allocation currencies (dry mass, N and P) were studied. All allocation currencies were log10 (x + 1)-transformed and, subsequently, reduced major axis (RMA) regression slopes were calculated and compared to slope of 1 (Green 1999). Departure from a slope of 1 indicates a disproportionate increase in one of the floral structures with an increase in the other floral structure's allocation when increasing flower size (Méndez & Traveset 2003). For example, if comparing stamens versus carpels, a slope significantly >1 would indicate a disproportionate increase in stamen allocation versus carpel allocation with increasing flower size, whereas a slope <1 would indicate a disproportionate increase in carpel allocation versus stamen allocation. Comparisons between slopes were studied by means of t-tests. Lastly, as allometric scaling in water and carbon allocation with corolla size has been previously reported in Cistaceae (Teixido & Valladares 2014), we tested allometry in dry mass, N and P allocation per unit of corolla area. We conducted regression analyses between the PIC values of dry mass, N and P per unit of corolla area and corolla area.
Results
Floral size and resource allocation
Flower size was highly variable among species, from approx. 1–7 cm in diameter and 0.7–36 cm2 in area (Table S1). Corolla area of Cistus ladanifer was, at least, 30% larger than that of other large-flowered species, which translated into the highest dry and nutrient mass allocation values to floral structures (Table S2). Within a flower, about a half of averaged floral mass was proportionally invested to pedicels and sepals, whereas petals comprised 28% and sexual structures 26% of floral dry mass (Table 1; see also Table S2 for percentage dry mass allocation per species). Stamens allocated about a four-fold dry mass more than carpels, which were the least costly structures in terms of floral dry mass. However, this was opposite to the pattern in the annuals Helianthemum aegyptiacum and Tuberaria guttata (Table S2). Relative to nutrients, sexual structures received the highest allocation, whereas calyx and corolla markedly decreased allocation compared to dry mass (Table 1; see also Table S2). N mass allocation to stamens was about twofold higher than that to carpels, but this difference was around 1.3-fold in terms of P (Table 1). Otherwise, nutrient concentration was higher in carpels than in stamens (Table 1).
| dry mass (%) | nitrogen (%) | phosphorus (%) | [nitrogen] | [phosphorus] | |
|---|---|---|---|---|---|
| Green structures | 10.7 ± 12.9 (46.1 ± 14.5) | 137.8 ± 180.6 (33.4 ± 14.6) | 7.5 ± 9.9 (31.0 ± 13.1) | 133.3 ± 37.1 | 3.2 ± 1.2 |
| Pedicel | 1.3 ± 2.3 | – | – | – | – |
| Sepals | 9.4 ± 14.4 | 137.8 ± 180.6 | 7.5 ± 9.9 | 133.3 ± 37.1 | 3.2 ± 1.2 |
| Petals | 6.5 ± 13.4 (28.0 ± 10.8) | 91.8 ± 186.7 (22.2 ± 7.9) | 5.7 ± 11.9 (23.6 ± 8.3) | 113.0 ± 27.6 | 3.0 ± 1.0 |
| Sexual structures | 6.0 ± 10.9 (25.9 ± 14.6) | 183.4 ± 359.4 (44.4 ± 18.0) | 11.0 ± 24.4 (45.4 ± 10.6) | 539.6 ± 125.4 | 16.7 ± 5.4 |
| Stamens | 4.7 ± 7.5 | 126.5 ± 198.4 | 6.2 ± 9.3 | 229.6 ± 116.4 | 5.8 ± 4.3 |
| Carpels | 1.3 ± 3.4 | 56.9 ± 161.0 | 4.8 ± 15.1 | 310.0 ± 107.4 | 10.9 ± 4.2 |
Phylogeny-dependent floral resource allocation
Complete phylogenetic reconstruction for 45 Cistaceae species is available in Fig. 1; here we focus results on the 23 species studied (Fig. 2). Fumana and Helianthemum are two small-flowered deep clades in Cistaceae that diverged around 25 and 20 Mya and which have the highest values of allocation to sepals and carpels within the family. In contrast, Cistus and Halimium are large-flowered and late-diverging (in the last 10 Mya) genera that emerged in the same clade and that proportionally allocate more energy to corollas and stamens.
We found that flower size showed a phylogenetic signal (Table 2). We also detected phylogenetic signal in dry mass allocation, percentage dry mass and nutrient mass to calyx and corolla, and dry mass, percentage dry mass and N, and nutrient mass to stamens (dry mass allocation to pedicels also showed a marginal phylogenetic signal; Table 2). Therefore, congeneric species tend to share similar flower size, dry mass and nutrient allocation to calyx, corollas and stamens more often than expected by chance. Otherwise, we did not detect any phylogenetic signal in nutrient concentration, resource allocation to carpels or per unit corolla area (Table 2). Altogether, total resource allocation to flowers showed a phylogenetic signal in terms of dry mass and nutrient mass, whereas resource allocation to sexual structures (i.e. stamens + carpels) showed a phylogenetic signal in terms of percentage dry mass (Table 2).
| trait | K | P |
|---|---|---|
| Green structures | ||
| Pedicel | ||
| Dry mass (mg) | 0.31 | 0.053 |
| Calix | ||
| Dry mass (mg) | 0.47 | 0.005 |
| N (μg) | 0.45 | 0.002 |
| P (μg) | 0.41 | 0.010 |
| [N] | 0.24 | 0.087 |
| [P] | 0.21 | 0.144 |
| Dry mass (%) | 0.35 | 0.005 |
| N (%) | 0.20 | 0.155 |
| P (%) | 0.18 | 0.244 |
| Corolla | ||
| Diameter (cm) | 0.68 | 0.002 |
| Area (cm 2 ) | 0.96 | 0.001 |
| Dry mass (mg) | 0.48 | 0.002 |
| N (μg) | 0.48 | 0.003 |
| P (μg) | 0.47 | 0.002 |
| [N] | 0.16 | 0.346 |
| [P] | 0.24 | 0.137 |
| Dry mass (%) | 0.37 | 0.016 |
| N (%) | 0.29 | 0.070 |
| P (%) | 0.28 | 0.097 |
| Dry mass (mg·cm−2) | 0.18 | 0.349 |
| N (μg·cm−2) | 0.12 | 0.677 |
| P (μg·cm−2) | 0.19 | 0.259 |
| Sexual structures | ||
| Stamens | ||
| Dry mass (mg) | 0.56 | 0.001 |
| N (μg) | 0.60 | 0.002 |
| P (μg) | 0.58 | 0.001 |
| [N] | 0.05 | 0.992 |
| [P] | 0.08 | 0.875 |
| Dry mass (%) | 0.61 | 0.002 |
| N (%) | 0.37 | 0.035 |
| P (%) | 0.24 | 0.108 |
| Carpels | ||
| Dry mass (mg) | 0.17 | 0.429 |
| N (μg) | 0.18 | 0.336 |
| P (μg) | 0.17 | 0.411 |
| [N] | 0.07 | 0.933 |
| [P] | 0.05 | 0.988 |
| Dry mass (%) | 0.13 | 0.573 |
| N (%) | 0.07 | 0.925 |
| P (%) | 0.09 | 0.872 |
| Total sex | ||
| Dry mass (%) | 0.54 | 0.003 |
| N (%) | 0.28 | 0.058 |
| P (%) | 0.20 | 0.213 |
| [N] | 0.10 | 0.751 |
| [P] | 0.20 | 0.196 |
| All Structures | ||
| Dry mass (mg) | 0.59 | 0.002 |
| N (μg) | 0.51 | 0.003 |
| P (μg) | 0.49 | 0.004 |
| [N] | 0.13 | 0.588 |
| [P] | 0.24 | 0.103 |
- Traits with phylogenetic signal and their Blomberg's K-statistic and significant P-values are marked in bold.
Resource allocation to floral structures co-varied across the family (Table S5). Species with higher percentage allocation to sepals show lower percentage allocation to petals and stamens, both in terms of dry mass and nutrients, and higher percentage allocation to carpels in terms of nutrients. Accordingly, higher percentage resource allocation to petals positively correlates to percentage allocation to stamens in terms of dry mass and N and negatively to carpels in terms of nutrients (Table S5).
Flower size-dependent floral resource allocation
In agreement with floral resource allocation based on phylogeny, resource allocation significantly varied with flower size. Thus, larger-flowered individuals disproportionally increased the percentage dry mass and N allocation to stamens and percentage dry mass allocation to petals, but significantly decreased dry mass allocation to sepals (Table 3). This indicates an allometric pattern in the percentage resource allocation to maleness with flower size across the family. Otherwise, we did not find any significant pattern in the percentage resource allocation to carpels (Table 3). Likewise, the percentage P and nutrient concentration to floral structures did not show any flower size-dependent disproportional change (Table 3). Overall, these patterns concur with the phylogenetic relationships found across the family (Table 2, Fig. 2).
| floral structures | dry mass (%) | N (%) | P (%) | [N] | [P] | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| R 2 | b ± SE | R 2 | b ± SE | R 2 | b ± SE | R 2 | b ± SE | R 2 | b ± SE | |
| Sepals | 0.26 * | −0.12 ± 0.03 | 0.02 | −0.03 ± 0.32 | 0.03 | −0.04 ± 0.21 | 0.09 | −0.27 ± 0.22 | 0.14 | −0.26 ± 0.15 |
| Petals | 0.29 * | 0.24 ± 0.06 | 0.10 | 0.19 ± 0.20 | 0.05 | 0.12 ± 0.14 | 0.05 | 0.12 ± 0.16 | 0.09 | 0.19 ± 0.14 |
| Stamens | 0.33** | 0.49 ± 0.04 | 0.23* | 0.58 ± 0.22 | 0.13 | 0.41 ± 0.20 | 0.04 | 0.11 ± 0.14 | 0.03 | 0.13 ± 0.17 |
| Carpels | 0.09 | −0.03 ± 0.02 | 0.01 | 0.08 ± 0.26 | 0.02 | 0.16 ± 0.27 | 0.00 | −0.06 ± 0.37 | 0.02 | −0.31 ± 0.48 |
- R2 and RMA slope values with significant P-values are marked in bold.
- *P < 0.05, **P < 0.01.
Differential dry mass and nutrient allocation among pairs of floral structures is also related to flower size (Table 4). Analysis between petals and sepals revealed a slope significantly >1, indicating an allometric increase in petal dry mass in relation to sepal dry mass with increasing flower size (Table 4). We detected a similar pattern when comparing stamens versus carpels. Therefore, there was a flower size-dependent allometric increase in petals in relation to sepals and stamens in relation to carpels (Table 4).
| comparison | R 2 | b ± SE | t 21 | P |
|---|---|---|---|---|
| Petals versus sepals | ||||
| Dry mass | 0.836 | 1.136 ± 0.102 | 5.684 | <0.001 |
| N | 0.667 | 1.270 ± 0.169 | 5.895 | <0.001 |
| P | 0.407 | 1.478 ± 0.264 | 9.324 | <0.001 |
| Stamens versus carpels | ||||
| Dry mass | 0.951 | 1.900 ± 0.090 | 39.492 | <0.001 |
| N | 0.588 | 1.119 ± 0.139 | 5.445 | <0.001 |
| P | 0.596 | 1.424 ± 0.193 | 9.148 | <0.001 |
- Significant P-values are marked in bold.
Similarly to phylogenetic analysis, detailed analysis for corolla area did not show any resource allocation allometric pattern within the family. Increases in corolla area did not translate into significantly higher amounts of dry mass, N or P per unit area (R2 = 0.13, bRMA ± SE = 0.417 ± 0.083, P = 0.101 for dry mass; R2 = 0.01, bRMA ± SE = 1.434 ± 0.304, P = 0.670 for N; and R2 = 0.09, bRMA ± SE = 0.403 ± 0.082, P = 0.153 for P). Thus, larger corollas only entail disproportionate resource allocation costs for being larger but do not comprise more resources per unit area.
Discussion
We demonstrate that resource allocation to floral structures disproportionally varies across Cistaceae species in relation to flower size and the history of divergence in this animal-pollinated hermaphroditic family. In this regard, flower size and subsequent gender-biased allocation patterns are phylogenetically conserved, as large- and small-flowered species are comprised of at least two different clades that share similar dry mass and nutrient investment within flowers. Therefore, we confirmed that larger-flowered species show a male-biased sex allocation at the flowering stage by increasing resource investment to corollas and stamens. However, nutrient concentration and resource allocation to carpels seem instead to be labile, suggesting trait convergences among distantly related species during the adaptation to similar environmental conditions. Previous studies have shown that dry mass allocation to maleness increases in larger-flowered xenogamous species as compared to smaller-flowered autogamous ones (Cruden & Lyon 1985), but phylogenetic relationships in resource allocation patterns to floral structures and sexual function have so far been overlooked. We discuss our findings from an ecological and evolutionary perspective with special reference to the influence of flower size in a phylogenetic context on floral resource allocation in this Mediterranean family.
In Cistaceae, sepals comprised, on average, the higher percentage of dry mass, whereas sexual structures markedly increased the investment in nutrients. The prevailing dry mass allocation to sepals found here concurs with results reported for the xenogamous and hermaphroditic Mediterranean plant Paeonia cambessedesii (Méndez & Traveset 2003). Although sepals generally protect the flower buds and developing fruits from floral enemies, differential allocation to the calyx may be particularly important in Mediterranean environments, wherein heavy, sclerophyllous and photosynthetic sepals might increase water storage, a key conservative resource use strategy for evergreen woody plants in these ecosystems (Thompson 2005). In relation to nutrients, previous studies have also found that stamens and especially carpels contained the highest N and P concentrations (Ashman & Baker 1992; Méndez & Traveset 2003). Our results reinforce the nutritive importance of pollen as reward to pollinators in Cistaceae, which usually lacks nectar (Herrera 1992; Arrington & Kubitzki 2003), and emphasise the general relevance of the nutrients allocated from the maternal component for fruit and seed development (Case & Ashman 2005).
We found evidence that flower size-dependent allometric scaling in floral resource allocation and male–female functional balance show a significant phylogenetic structure. Although investment to carpels is not conserved across the phylogeny, we still detected that resource allocation to stamens disproportionally increases in relation to female function in large-flowered species. Our results might reflect different optima related to the mating systems, as larger-flowered and xenogamous species allocate a higher percentage of dry mass to corollas and stamens in relation to the percentages dry mass allocation to sepals and carpels in autogamous, smaller-flowered species (Cruden & Lyon 1985; Goodwillie et al. 2010). In Cistaceae, larger-flowered species tend to follow the patterns of maleness-biased xenogamous flowers, showing predominant self-incompatibility (Table S1). Otherwise, resource allocation patterns in small-flowered species are more related to self-fertilisation, especially common among Fumana and Tuberaria (Herrera 1992; Carrió & Güemes 2013; Table S1). Although Helianthemum forms a heterogeneous and highly diverse group with a large variation in life form, flower size, number of anthers and ovules and mating systems (Arrington & Kubitzki 2003; Guzmán & Vargas 2009a), largest-flowered species share a mating system that is mainly xenogamous with high pollen production (Tébar et al. 1997; Rodríguez-Pérez 2005). However, solid conclusions associated with mating systems in Cistaceae should be taken with caution since we lack complete data for pollinator requirements and compatibility systems in the present study. Nevertheless, our findings support the classical assumptions for models and theory of sexual allocation in plants (e.g. Lloyd 1980; Charlesworth & Charlesworth 1981; Charnov 1982; Bell 1985). Moreover, our results show that phylogeny strongly drives flower size-based relative investment into sexes and that larger flowers disproportionally increase maleness, which is in agreement with the male function hypothesis (Bell 1985; see also Teixido et al. 2016 for Cistaceae).
Pollinator-mediated selection might have modulated the patterns reported here, as selective pressures on floral phenotypes via pollination agents primarily exhibit a phylogenetic signal (Smith et al. 2008; Gómez et al. 2015). In our study system, Cistus-Halimium forms a cohesive natural clade with parallel evolution and related characters (Guzmán & Vargas 2005, 2009a). These large-flowered genera receive a high number and diversity of pollinators, which translates into direct returns in terms of high fruit and seed production and pollen dispersal rates (Bosch 1992; Talavera et al. 2001; Barrio & Teixido 2015). This is specifically relevant in the largest-flowered species, Cistus ladanifer, in which pollinator visitation rates show about a five-fold increase and seed production (ca. 1000 seeds per fruit) is extraordinarily high in relation to other Cistus (Teixido et al. 2016). These processes concur with the elevated resource allocation patterns in this species and its differences from closely related taxa (Fig. 2). Otherwise, pollen limitation is not uncommon in large-flowered Cistaceae and positive selection on flower size through female function components is feasible, also being permanent and especially strong through male fitness (reviewed in Teixido et al. 2016). Conversely, when pollinator frequency is low and female fitness is strongly pollen-limited, the selective ability to self-fertilise may assure reproduction independent of pollinator visitation, subsequently promoting smaller corollas and lower pollen production (Lloyd 1992; Goodwillie et al. 2010; Tedder et al. 2015). Following these assumptions, pollinator environment may have operated as an important selective pressure on evolution of flower size and resulting gender-biased allocation across lineages in this hermaphroditic family.
Alternatively, other non-pollinator agents of selection could exert relative evolutionary constraints on floral traits analysed here. Overall, such agents might explain why we did not detect any phylogenetic signal in resource allocation to carpels. For example, site-dependent environmental conditions (temperature, humidity) and specific differences in growth form and life traits strongly influence sexual allocation (e.g. Lloyd & Bawa 1984; Dawson & Geber 1999; Guo et al. 2010). In Cistaceae, annual species of Tuberaria have low anther-to-ovule ratios and a female function-biased allocation to optimise investment to fruit and seed production (Herrera 1992). This may also explain the extraordinarily high percentage of dry mass allocation to carpels in T. guttata (Fig. 2, Table S2). Likewise, a high reproductive effort at the expense of reduced survival to deal with the most stressful conditions seems to be the strategy of Helianthemum squamatum, a perennial shrub inhabiting semiarid environments (Aragón et al. 2009). Similar patterns could be plausible to understand patterns of nutrient allocation to floral structures in terms of concentration in our study. In this regard, some species-level trait and environmental convergence across unrelated evolving plant lineages likely explain the relationships that constrain the partitioning of nutrients in plants (Chapin et al. 1986; Thompson et al. 1997; Kerkhoff et al. 2006). We suggest that functional analysis beyond the flowering stage, linking resource allocation to reproductive success (e.g. number of seeds), and between different habitats and life forms would provide reliable estimates of sexual allocation. Overall, our results suggest that although flower size in Cistaceae is a phylogenetically conserved trait and its evolution is therefore rather limited, species may independently balance allocation to carpels to assure fruit and seed production. Therefore, direct resource investment to the primary female structure in flowers seems a labile trait that varies among closely related species that have diversified into different ecological niches.
In conclusion, our study demonstrates that the phylogenetic relationships among species determine flower size and consequent gender-biased patterns of resource allocation to floral structures in Cistaceae, a typical hermaphroditic Mediterranean family. Alternatively, habitat and life form may be relevant in controlling allocation to carpels. Still, we found that larger-flowered species allometrically allocate more resources to maleness, by increasing allocation to corollas and stamens. From an evolutionary context, pollinator-mediated selection may maintain larger, xenogamous and male function-biased flowers in this hot and dry ecosystem. Otherwise, persistent pollen limitation may operate on reproductive assurance by autonomous selfing, whereas most stressful climatic conditions may disproportionally increase reproductive effort, thus preserving smaller-flowered species with biased floral femaleness. Analysing the selective mechanisms underlying correlated shifts in sexual allocation to flowers and pollination systems (i.e. mating systems) will ultimately provide a more complete understanding of the nature of resource allocation and the evolution of flower size under stressful Mediterranean conditions.
Acknowledgements
We thank two anonymous reviewers and P. Vargas and F. A. O. Silveira for valuable comments during the first versions of the manuscript. We are also grateful to J. Arroyo, E. Carrió, J. P. González-Varo, J. Güemes, J. Herrera, A. L. Luzuriaga, M. Méndez, M. A. Rodríguez-Gironés, A. Sánchez, S. Santamaría, E. Triano and R. Torices for collecting flower buds from several species; and to E. Galisteo and Y. Valiñani for lab assistance. This study was supported by the Spanish-funded projects REMEDINAL3eCM (S2013/MAE-2719) and Ecometas excellence network (CGL2014-53840-REDT). ALT held a doctoral fellowship at Rey Juan Carlos University, Spain. BG received a Juan de la Cierva fellowship, and VGS a post-doctoral fellowship (grant #2014/13899-4) from São Paulo Research Foundation (FAPESP).




