Nutrient availability and nutrient use efficiency in plants growing in the transition zone between land and water
Abstract
The transition zone between terrestrial and freshwater habitats is highly dynamic, with large variability in environmental characteristics. Here, we investigate how these characteristics influence the nutritional status and performance of plant life forms inhabiting this zone. Specifically, we hypothesised that: (i) tissue nutrient content differs among submerged, amphibious and terrestrial species, with higher content in submerged species; and (ii) PNUE gradually increases from submerged over amphibious to terrestrial species, reflecting differences in the availability of N and P relative to inorganic C across the land–water ecotone. We found that tissue nutrient content was generally higher in submerged species and C:N and C:P ratios indicated that content was limiting for growth for ca. 20% of plant individuals, particularly those belonging to amphibious and terrestrial species groups. As predicted, the PNUE increased from submerged over amphibious to terrestrial species. We suggest that this pattern reflects that amphibious and terrestrial species allocate proportionally more nutrients into processes of importance for photosynthesis at saturating CO2 availability, i.e. enzymes involved in substrate regeneration, compared to submerged species that are acclimated to lower availability of CO2 in the aquatic environment. Our results indicate that enhanced nutrient loading may affect relative abundance of the three species groups in the land–water ecotone of stream ecosystems. Thus, species of amphibious and terrestrial species groups are likely to benefit more from enhanced nutrient availability in terms of faster growth compared to aquatic species, and that this can be detrimental to aquatic species growing in the land–water ecotone, e.g. Ranunculus and Callitriche.
Introduction
The transition zone between terrestrial and freshwater habitats is highly dynamic, with large spatial and temporal fluctuations in environmental characteristics (Naiman & Decamps 1997; Ward et al. 2002; Hoffmann et al. 2006). These characteristics are closely coupled to the dynamics of the flow regime that mediates lateral instability in the plan form of the stream channel and to periodic floods with sediment deposition within the riparian areas (Kronvang et al. 2009). Over time, these processes have created a mosaic of habitats from lotic in-stream habitats, over a variety of water bodies or backwater habitats that are almost lentic in character, to a wide range of terrestrial habitats. The environmental characteristics of the various habitats in the transition zone differ, as do the constraints that shape the plant communities. Among these constraints is mechanical stress from wind or water movement, light and nutrient availability, and substrate characteristics, which are all considered very important for species composition across the land–water ecotone (Bornette & Puijalon 2011).
Different life forms are associated with habitats in the transition zone, including submerged species living permanently under water, amphibious species able to live both submerged and emerged, and terrestrial plants living solely emerged (Sand-Jensen & Frost-Christensen 1998). The availability of nutrients for these different life forms is likely to vary across and within the transition zone, reflecting processes taking place in the catchment and in the soil matrix that moderate the availability of nutrients (Cey et al. 1999; Smith et al. 2008). Nevertheless, we expect that the availability of nitrogen (N) and phosphorus (P) is generally higher for species living submerged within the stream channel than for species living emerged in the land–water ecotone, both because concentrations are expected to be higher due to continued supply with the flowing water and because nutrients can be taken up through the entire surface area of the plants (Madsen et al. 1998).
The photosynthetic nutrient use efficiency (PNUE), defined as the ratio of CO2 assimilation rate to leaf organic N content (Poorter & Evans 1998), reflects the ability of the plants to utilise nutrients in the photosynthetic process, and can therefore be used to obtain insight into resource allocation in plants. The PNUE is independent of morphological plasticity and directly related to resource availability (Field & Mooney 1986). Low nutrient use efficiency is found in species that are stress-tolerant (Chazdon & Field 1987; Reich et al. 1994), whereas high nutrient use efficiency is found in competitive species that are normally fast growing and thus able to allocate high amounts of nutrients into the photosynthetic apparatus. We use the PNUE and the photosynthetic phosphorus use efficiency (PPUE) as measures of the nutrient use efficiency of the plants.
Besides being influenced by nutrient (i.e. N and P) availability, we believe that differences in the availability of inorganic carbon (C) across the land–water ecotone may affect PNUE and PPUE. Generally, the availability of inorganic C is substantially lower in the aquatic environment because of highly reduced diffusion rates in water and thick boundary layers (Raven 1984; Sand-Jensen & Frost-Christensen 1998). Therefore, the C fixation rate per unit nutrient may be low, consequently lowering the PNUE and PPUE of species living permanently submerged compared to species living partly submerged or species living emergent.
In the present study, we investigate the nutritional status of different plant types growing in the water ecotone in lowland stream areas in Denmark, and how the photosynthetic performance of these plant types is related to their nutritional status. Specifically, we hypothesised that: (i) tissue nutrient content differs among submerged, amphibious and terrestrial species, with higher content in submerged species; and (ii) the PNUE gradually increases from submerged species over amphibious to terrestrial species, reflecting differences in the availability of N and P relative to inorganic C across the land–water ecotone.
Material and Methods
Study sites and collection of plants
We studied a total of 14 plant species: eight aquatic species, three amphibious species and three terrestrial species (Table 1). In July, plants were collected in the transition zone from the streams and in the adjacent riparian area along 12 open-canopy stream reaches situated in Jutland, Denmark. Three individuals were collected of each species at each site. Depending on the water level, the submerged species were collected by hand or with a rake. Sites were chosen to cover a wide gradient in nutrient concentration (Pedersen et al. 2006). Total N in the stream water ranged from 0.095 to 5.5 mg·l−1 and total P from 0.02 to 0.22 mg·l−1 in the 12 streams.
| species | abbreviation | life form | number of sites | number of individuals | C% DW | N% DW | P% DW | photosynthetic rate (μmol·CO2·m−2·s−1) | PNUE (μmol·CO2·mol·N−1·s−1) | PPUE (μmol·CO2·mol·P−1·s−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carex nigra | Car nig | Terrestrial | 10 | 30 | 44.67 | ±1.42 | 2.15 | ±0.75 | 0.21 | ±0.07 | 8.2 | ±4.40 | 44.3 | ±21 | 1066 | ±597 |
| Cirsium palustris | Cir pal | Terrestrial | 9 | 24 | 40.3 | ±2.06 | 2.53 | ±1.13 | 0.31 | ±0.30 | 12.61 | ±8.46 | 93.5 | ±80 | 2238 | ±1897 |
| Epilobium angustifolium | Epi ang | Terrestrial | 5 | 12 | 43.3 | ±0.78 | 2.97 | ±0.45 | 0.44 | ±0.13 | 13.9 | ±4.79 | 166.9 | ±97 | 2596 | ±1505 |
| Equisetum fluviatile | Equi flu | Amphibious | 5 | 23 | 39.65 | ±1.37 | 2.64 | ±0.61 | 0.29 | ±0.09 | 23.46 | ±5.19 | 52.2 | ±33 | 1090 | ±680 |
| Glyceria maxima | Gly max | Amphibious | 10 | 23 | 43.74 | ±1.37 | 1.85 | ±0.72 | 0.2 | ±0.09 | 9.08 | ±6.60 | 90.1 | ±92 | 1855 | ±1810 |
| Menyanthes trifoliata | Men tri | Amphibious | 5 | 14 | 45.26 | ±1.19 | 2.78 | ±0.72 | 0.23 | ±0.05 | 11.24 | ±3.49 | 55.9 | ±18 | 1446 | ±473 |
| Callitriche sp. | Cal sp | Aquatic | 10 | 16 | 43.03 | ±1.74 | 4.62 | ±0.90 | 0.49 | ±0.14 | 0.29 | ±0.20 | 8.8 | ±6 | 193 | ±134 |
| Elodea canadensis | Elo can | Aquatic | 6 | 31 | 41.43 | ±0.82 | 4.77 | ±0.65 | 0.74 | ±0.27 | 0.72 | ±0.72 | 19.6 | ±18 | 369 | ±226 |
| Myriophillum spicatum | Myr spi | Aquatic | 4 | 16 | 43.68 | ±1.61 | 3.87 | ±0.93 | 0.42 | ±0.30 | 1.12 | ±2.49 | 32.5 | ±17 | 771 | ±466 |
| Potamogeton crispus | Pot cri | Aquatic | 6 | 14 | 43.36 | ±0.95 | 4.7 | ±0.83 | 0.77 | ±0.19 | 2.29 | ±2.85 | 49.1 | ±61 | 556 | ±499 |
| Potamogeton lucens | Pot luc | Aquatic | 4 | 7 | 41.93 | ±0.57 | 3.08 | ±0.45 | 0.45 | ±0.17 | 0.6 | ±0.27 | 11.5 | ±4 | 183 | ±53 |
| Potamogeton pectinatus | Pot pec | Aquatic | 9 | 34 | 41.42 | ±1.51 | 3.15 | ±0.71 | 0.37 | ±0.14 | 1.24 | ±0.75 | 22.7 | ±13 | 548 | ±464 |
| Potamogeton perfoliatus | Pot per | Aquatic | 10 | 62 | 41.98 | ±1.38 | 4.2 | ±0.90 | 0.61 | ±0.23 | 1.08 | ±0.94 | 26.9 | ±21 | 482 | ±351 |
| Ranunculus peltatus | Ran pel | Aquatic | 10 | 26 | 42.4 | ±2.07 | 4.77 | ±0.80 | 0.48 | ±0.15 | 3.12 | ±1.83 | 40.2 | ±21 | 958 | ±637 |
Measurement of photosynthetic rate and nutrient tissue content
The light-saturated rates of photosynthesis of amphibious and terrestrial plants were measured in situ using an infrared gas analyser (IRGA, LI-6400XT; Li-Cor, Lincoln, NE, USA). The instrument measures the difference in CO2 between leaf and reference sensors and thereby the CO2 uptake of the plant. The leaf chamber was supplied with atmospheric air and was air-conditioned at 15 °C. Humidity in atmospheric air in the chamber was ca. 50%; light intensity was 1800 μmol·m−2·s−1. Photosynthesis rates were measured after 3–5 min of acclimation of leaves in the chamber when the IRGA showed stable readings. Following photosynthesis measurements, the leaf area exposed in the chamber was measured.
The light-saturated rates of photosynthesis of aquatic species were measured in the laboratory on shoots incubated in glass-stoppered bottles in a standard medium (alkalinity 1.7 mEq·l−1; Smart & Barko 1985). The bottles were placed in a revolving wheel for 30 min at 15 °C and a light intensity of 250–300 μmol·m−2·s−1 PAR, ensuring light saturation for photosynthesis (Madsen & Brix 1997). The shoots were kept well stirred with the addition of three glass balls to the incubation bottles to reduce the thickness of the boundary layer. Three replicates were made for each species from each site. Moreover, we included three blanks without plants in the wheel. After 30 min, the O2 concentration in the bottles was measured using an oxygen electrode (OX500; Unisense; Aarhus, Denmark).
All samples of terrestrial, amphibious and submerged species were freeze-dried after the photosynthesis measurements and analysed for total tissue content of C and N (dry combustion and HPLC analyses) and P (acid digestion and analysis with ICP-MS).
Data analyses
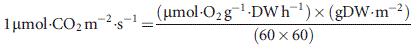


Differences among life forms in C, N, P, photosynthesis rates, PNUE and PPUE were compared using anova analysis, and to test homogeneity we used Levene′s test. All statistical analyses were performed in R (R Foundation for Statistical Computing, Vienna, Austria).
Results
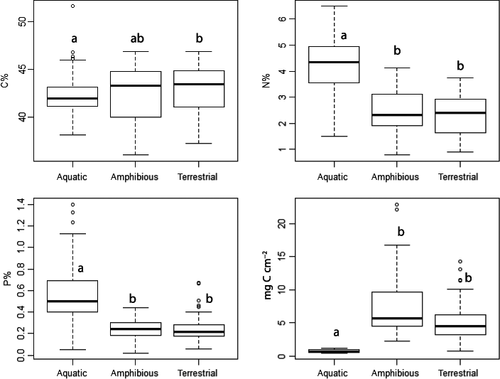
Carbon, N and P tissue content of plants of the submerged, amphibious and terrestrial groups differed significantly (Fig. 1). The C content of plants increased from submerged over amphibious to the terrestrial species group, both expressed on a shoot/leaf dry weight basis (anova P < 0.05) and on a shoot/leaf area basis (anova, P < 0.001; Fig. 1, Table 1). In contrast to the C content, N and P tissue contents were higher in submerged species compared to amphibious and terrestrial species (anova P < 0.001; Fig. 1, Table 1). The highest N and P tissue contents were found in Ranunculus peltatus and Potamogeton crispus, respectively, whereas the lowest N and P contents were in Glyceria maxima, an amphibious species (Table 1).
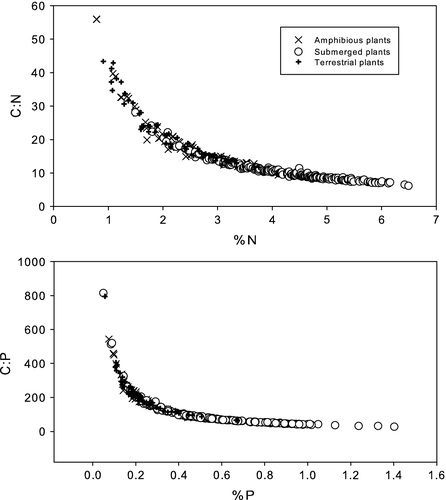
Nutrient limitation was assessed by analysing C:N as a function of percentage tissue N of the DW, and C:P ratios as a function of percentage tissue P of the DW (Duarte 1990). The C:N ratio declined markedly with increasing N tissue content, from a minimum of 0.78% DW to about 2% DW tissue N (x-axis), followed by a more gradual decline in the C:N ratio in the range of 2% DW to about 4% DW tissue N (Fig. 2), indicating that individuals with tissue N < 2% were N-limited, whereas individuals with tissue N from 2 to 4% had intermediate N availability. The C:P ratio also declined rapidly as P tissue content increased from a minimum of 0·018% DW to about 0.2% DW, followed by a more gradual decline to about 0.6% DW (Fig. 2), indicating that individuals with tissue P < 0.2% were P-limited, whereas individuals with tissue P content between 0.2 and 0.6% had intermediate P availability. On an individual basis, we found that 4% of the individuals had N content <2%, indicating N limitation, 8% had P content <0.2%, indicating P limitation, and 7% had lower content of both N (<2%) and P (<0.2%), suggesting co-limitation. There were no systematic differences in C:N and C:P ratios among the three plant groups as a whole. However, only a few aquatic specimens had tissue N and tissue P contents that could indicate limitation (<2% and <0.2%, respectively; data not shown), whereas about one-third of the terrestrial and amphibious specimens had tissue N and tissue P contents that could indicate limitation. Therefore, nutrient availability seems to be saturating for growth of most aquatic plant individuals but to a lesser extent for amphibious and terrestrial plant individuals.
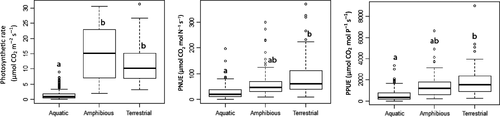
Photosynthesis rates were lower in submerged species compared to amphibious and terrestrial species (anova, P < 0.001; Fig. 3, Table 1). Callitriche sp. had the lowest photosynthesis rate of all submerged species (average 0.29 μmol·CO2 m−2·s−1) and R. peltatus had the highest rate (average 3.12 μmol·CO2 m−2·s−1), although still lower than the rates found in terrestrial and amphibious species. The photosynthesis rate of amphibious species ranged from 9.08 μmol·CO2 m−2·s−1 (average for G. maxima) to 23.46 μmol·CO2 m−2·s−1 (average for Equisetum fluviatile), while the rate of terrestrial species ranged from 8.20 μmol·CO2 m−2·s−1 (average for Carex nigra) to 13.9 μmol·CO2 m−2·s−1 (average for Epilobium angustifolium; Table 1). PNUE and PPUE were also lower in submerged species than in amphibious and terrestrial species (anova P < 0.001; Fig. 3). Callitriche sp. had the lowest PNUE among all species (8.87 μmol·CO2 mol·N−1 s−1) and P. lucens the lowest PPUE (183 μmol·CO2 mol·P−1 s−1), while E. angustifolium, a terrestrial species, had both the highest PNUE (166.97 μmol·CO2 mol·N−1 s−1) and the highest PPUE (2596 μmol·CO2 mol·P−1 s−1; Table 1) measured.
Discussion
As predicted, tissue nutrient content was generally higher in submerged species than in amphibious and terrestrial species. The tissue content measured was within ranges previously reported for submerged species (1.2–6.3 N w/w; Baattrup-Pedersen et al. 2013a; 0.05–0.6% P; Demars & Edwards 2008). The higher content in submerged species likely reflects that these species can take up nutrients from both the continually renewed interstitial water in the sediment and from open water (Cedergreen & Madsen 2002), and therefore have access to higher amounts of nutrients than amphibious and terrestrial species, despite the fact that these species groups live side-by-side in the land–water ecotone.
To investigate potential nutrient limitation in the three species groups, we assessed threshold values derived from C:N to tissue N relationships and C:P to tissue P relationships, as suggested by Duarte (1990). We found that the three species groups overlapped in C:N to tissue N relationships and in C:P to tissue P relationships and, additionally, that threshold values indicating limitation was <2% for tissue N and <0.2% for tissue P. These threshold values were very close to previously reported thresholds for nutrient limitation of 1.8% for N and 0.2% for P in seagrasses using the same approach (Duarte 1990). They were also very close to values reported by Gerloff & Krumbholz (1966) for angiosperm aquatic plants: 1.3% for tissue N and 0.13% for tissue P. Using the derived threshold values, the number of individuals observed that could possibly suffer from nutrient limitation was comparable among the three plant groups. Taken together, we only found indications of potential N limitation in 4% of individuals, potential P limitation in 8% of individuals, and potential co-limitation by N and P in 7% of individuals. Submerged species had the lowest number of individuals experiencing potential nutrient limitation, as expected from their high nutrient tissue content compared to the contents measured in amphibious and terrestrial species.
We also considered the possibility of assessing nutrient limitation by applying tissue N:P ratios, as suggested by Koerselman & Meuleman (1996). The N:P ratios of leaves can, however, be higher compared to that of whole plant tissues (T. Riis & A. Baattrup-Pedersen, unpublished data), reflecting the particular high content in photosynthetically most active tissues (Feller et al. 2008). This is primarily due to Rubisco, which is the predominant protein in leaves of C3 plants and may contribute up to 50% of the soluble leaf protein (Spreitzer & Salvucci 2002). Consequently, ratios obtained here based on leaf N and P are not directly comparable with the suggested ratios of Koerselman & Meuleman (1996) that are based on whole plant content. Furthermore, it has also been observed that optimal N:P ratios for plant growth may change over time, depending on plant growth rate and age of the tissues, and also may vary over seasons and with the availability of light (Gusewell & Koerselman 2002), which all add some uncertainty to the use of N:P ratios in assessing nutrient limitation. Finally, C:N and C:P ratios are likely to better reflect the relative resource availability than N:P ratios in general, since C:N and C:P threshold values take into account the amount of C in the plant tissues, which is normally less variable than the N and P content.
As predicted, we found that the PNUE declined from terrestrial over amphibious to submerged species. Accordingly, the higher amount of nutrients likely allocated to supporting structural tissues in the terrestrial and amphibious species groups than in the aquatic species group (Poorter & Evans 1998; Onoda et al. 2004) seems to be more than offset by more optimal allocation of N to and between photosynthetic components in these species compared to submerged species. We infer that this difference in PNUE among the groups can be mediated by contrasting CO2 availability in the terrestrial and aquatic environments. Although the concentration of inorganic C in water can be high compared to that in air, the availability of inorganic C for photosynthesis will be low because of 104 times slower diffusion of gases in water and a long diffusion path through the boundary layer (100–500 μm; Madsen & Sand-Jensen 1991). Therefore, to obtain the same flux of CO2 across the boundary layer in air and water and, hence, the same rate of photosynthesis, the CO2 concentration should be more than 104 higher in water. However, the concentration of CO2 seldom exceeds 25 times equilibrium concentrations (Rebsdorf et al. 1991; Sand-Jensen & Frost-Christensen 1998). The lower PNUE of submerged species may thus reflect that these species are better acclimated to lower availability of inorganic C than terrestrial species, yielding differences in nutrient allocation patterns to and between the various photosynthetic components among the species groups. It was previously shown that high availability of CO2 can lower the amount of N allocated into Rubisco without negatively affecting carboxylation capacity of the plants, probably due to down-regulation of the photosynthetic apparatus (Ge et al. 2012). Consequently, the higher PNUE of terrestrial and amphibious species than of submerged species may reflect the fact that these species allocate proportionally more N into processes of importance at high CO2 availability, i.e. enzymes involved in substrate regeneration. Furthermore, many submerged species possess C concentrating mechanisms that serve to improve the availability of inorganic C in the aquatic environment (Raven et al. 2011), which may also lower the PNUE of species in the submerged species group. Thus, the capacity and efficiency of bicarbonate (HCO3−) uptake, which is by far the most widespread C concentrating mechanism in aquatic plants (Sand-Jensen 1983), are largely dependent on the amount of tissue N in the plants, indicating that the N cost associated with HCO3−uptake can be substantial (Madsen & Baattrup-Pedersen 1995; Baattrup-Pedersen et al. 2013b).
In addition to differences in nutrient allocation to and among photosynthetic components, it is also possible that the lower PNUE of submerged species reflects that higher amounts of nutrients are stored in this species group compared to the amphibious and terrestrial species groups because of the relative availability of N and P to inorganic C is higher in the aquatic environment. Most perennial plants can store N when uptake from the external environment exceeds immediate growth requirements. This has been interpreted as an adaptation whereby growth is better matched to N availability (Chapin 1980; Proe & Millard 1994). Nitrogen may be stored in a variety of forms in plants, but in foliage it is most often stored as inorganic N (nitrate or ammonium), free amino acids or proteins (Millard 1988), which may all lower the PNUE. Similarly, excess P can be stored in vacuoles (Bieleski 1973), which may lower the PPUE since it is not used in compounds affecting the photosynthetic performance of the plants, e.g. nucleic acids, sugar phosphates, ATP and phospholipids, which all have important roles in the photosynthesis process (Hidaka & Kitayama 2009).
Finally, lower PNUE in submerged species could also reflect that species within this group are adapted to lower light availability and therefore invest proportionally more N into light capture than terrestrial and amphibious species, translating into higher light use efficiency. The restricted availability of light in the aquatic environment compared to the terrestrial environment also implies that photosynthesis saturates at a lower irradiance in submerged species (ca. 250 μmol·m−2·s−1) compared to amphibious and terrestrial species (ca. 1800 μmol·m−2·s−1). Consequently, at saturating N the PNUE of amphibious and terrestrial species will be higher.
In conclusion, we found that ca. 20% of plant individuals growing in the transition zone between land and water were nutrient-limited and that these were primarily amphibious and terrestrial species. Additionally, we found that the nutrient use efficiency increased from submerged over amphibious to terrestrial species. This pattern likely reflects differences in nutrient allocation to and within the photosynthetic apparatus among the species groups, mediated by large differences in the relative availability of N and P to inorganic C in the aquatic and terrestrial environments. Taken together, our results indicate that enhanced nutrient loading may affect the relative abundance of the three species groups in the land–water ecotone of stream ecosystems. Thus, species belonging to the amphibious and terrestrial groups are likely to benefit more from enhanced nutrient availability in terms of faster growth, compared to aquatic species, and also that this can be detrimental to aquatic species growing in the land–water ecotone, e.g. Ranunculus and Callitriche. Enhanced growth of e.g. G. maxima may intensify competition for light in the transition zone for aquatic species and lead to a decline in their relative abundance, in particular in streams subjected to low summer flows and with minimal physical stress – a situation that is likely to be intensified in the coming years as lower summer precipitation associated with climate change becomes more common.
Acknowledgements
We thank the Carlsberg Foundation, Denmark (grant to TR #2013_01_0258), the Danish Research Council (FNU; grant to TR #272-09-0012) and the European Union 7th Framework Projects REFRESH (contract no. 244121) and MARS (contract no. 603378). The authors wish to thank Anne Mette Poulsen for editorial support.




