Plastic responses in the metabolome and functional traits of maize plants to temperature variations
Abstract
Environmentally inducible phenotypic plasticity is a major player in plant responses to climate change. However, metabolic responses and their role in determining the phenotypic plasticity of plants that are subjected to temperature variations remain poorly understood. The metabolomic profiles and metabolite levels in the leaves of three maize inbred lines grown in different temperature conditions were examined with a nuclear magnetic resonance metabolomic technique. The relationship of functional traits to metabolome profiles and the metabolic mechanism underlying temperature variations were then explored. A comparative analysis showed that during heat and cold stress, maize plants shared common plastic responses in biomass accumulation, carbon, nitrogen, sugars, some amino acids and compatible solutes. We also found that the plastic response of maize plants to heat stress was different from that under cold stress, mainly involving biomass allocation, shikimate and its aromatic amino acid derivatives, and other non-polar metabolites. The plastic responsiveness of functional traits of maize lines to temperature variations was low, while the metabolic responsiveness in plasticity was high, indicating that functional and metabolic plasticity may play different roles in maize plant adaptation to temperature variations. A linear regression analysis revealed that the maize lines could adapt to growth temperature variations through the interrelation of plastic responses in the metabolomes and functional traits, such as biomass allocation and the status of carbon and nitrogen. We provide valuable insight into the plastic response strategy of maize plants to temperature variations that will permit the optimisation of crop cultivation in an increasingly variable environment.
Introduction
Because plants possess a sessile life cycle, they are forced to experience large temporal and spatial variations in temperature. Temperature is a key factor in controlling ecosystems with regard to plant productivity, reproduction and distribution (Thomas et al. 2004; Atkin et al. 2006; Bahuguna & Jagadish 2015). Temperature is also a strong selective agent that leads to the adaptive evolution of plant traits (Davis et al. 2005; Franks et al. 2007; Roelofs et al. 2008). In agricultural ecosystems, temperatures beyond the physiological optimum, such as heat or cold, may considerably affect the growth, development and geographic distribution of many crop species (Dawson et al. 2011). Furthermore, the frequency and magnitude of temperature extremes would increase according to global climate models because of global land surface warming, and may pose a challenge to future global food security (Sànchez et al. 2014).
A strategy for plants to adapt to these ever-changing temperature conditions is through phenotypic plastic responses, in which a plant with a given genotype can express environment-dependent phenotypes (Bradshaw 1965; Sultan 1987; Matesanz et al. 2010; Nicotra et al. 2010). Information regarding the phenotypic effects of temperature continues to increase, and a number of studies have examined phenotypic plasticity induced by temperature in plant growth patterns, biomass allocation and traits related to development, physiology and life history, particularly in Arabidopsis thaliana (Sultan 2004; Atkin et al. 2006; Suter & Widmer 2013; Frei et al. 2014). The plasticity of functional traits, which involves the modulation of developmental programmes, is a major factor in plant responses to climate change (Nicotra et al. 2010; Freschet et al. 2013). The inhibition of leaf and root growth and the adaption through biomass allocation among plant tissues are well-characterised responses to stresses that are associated with temperature (Atkin et al. 2006; Freschet et al. 2013; Gratani 2014; Ribeiro et al. 2014). The effects of temperature stress on plants involve simultaneous physiological alterations in gene expression (Chinnusamy et al. 2007; Sobkowiak et al. 2014), epigenetic regulation (Goldberg et al. 2007; Suter & Widmer 2013), signalling (Chinnusamy et al. 2004), and primary and secondary metabolism (Cook et al. 2004; Kaplan et al. 2004; Guy et al. 2008; Ramakrishna & Ravishankar 2011). Most previous studies on plastic responses of plants to temperature have focused on functional traits, and relatively little attention has been given to metabolic responses, which may be another component of plant acclimation and may provide plants with a mechanism to cope with climate change.
Plants adapt to a constantly changing environment through the adjustment of metabolic fluxes and metabolite concentrations to restore homeostasis (Benning & Stitt 2004; Shulaev et al. 2008; Janmohammadi 2012; Nägele et al. 2012). Therefore, at the metabolic level, environmental stresses to plants could be defined as any changes in growth conditions within the plant's natural habitat that alter or disrupt their metabolic homeostasis. Plant morphology, physiology and yield are recognised as parts of the phenotype, but the metabolome profile could also be part of this phenotype (Nachtomy et al. 2007; Sadras et al. 2013). Therefore, metabolic profiles, rather than functional traits that are related to plant growth and performance, must be used to investigate phenotype plastic responses to temperature stress. Metabolomics is a powerful tool that is used to obtain a comprehensive perspective of the mechanisms through which metabolic networks are regulated and metabolic pathways respond to stress-induced perturbations (Fiehn 2002). Nuclear magnetic resonance (NMR) is an efficient method to quantitatively and qualitatively analyse the metabolites that are present in a plant under certain conditions and has been successfully applied in studies of plant responses to various abiotic stress conditions (Kim et al. 2010; Obata & Fernie 2012; Sun et al. 2015).
Although abundant information is available regarding metabolic responses to environmental changes, the role of these responses in determining the phenotypic plasticity of plants remains unclear. In addition, how and to what extent metabolomic profiling is correlated with the growth performance of plants has received less attention. The plasticity in functional traits is relevant to plant distribution, and the underlying mechanism is also of particular interest from an ecological or evolutionary perspective (Nicotra et al. 2010). Recent studies have shown that metabolism plays a major role in the regulatory mechanisms underlying phenotypic diversity because plants are extremely rich and variable in metabolic profiles (Keurentjes 2009; Carreno-Quintero et al. 2013). For example, the harvest index of tomato presented the highest number of associations to metabolic traits (Schauer et al. 2006). Two studies that explored the relationship between primary metabolism and phenotypic traits in A. thaliana also showed that plant biomass was related to the metabolic signature of the plants (Meyer et al. 2007; Lisec et al. 2008). A better understanding of the metabolic mechanisms underlying phenotypic plasticity will permit the optimisation of crop breeding and cultivation to obtain high yields or homeostasis in an increasingly variable environment.
Maize (Zea mays L.), which is one of the most important crops worldwide, is used as a food, feed and energy source. Because maize can be planted in diverse ecosystems from tropical to temperate regions and its highly inbred lines can also be readily produced, it is considered an ideal candidate for plasticity studies (Sultan 2000). Maize originates from subtropical regions and is very sensitive to low growth temperatures. In cool temperate climates, maize can experience cold temperatures at night or early/late in the growing season (Hou et al. 2014). Early maize hybrids are also considered the second main season crop, and young isolated maize seedlings surrounded by bare soil can be exposed to very high temperatures (Reimer et al. 2013). High soil temperatures are likely to occur in row crops, such as maize, before canopy closure, and temperatures above the optimum can negatively affect plant growth and development (Trachsel et al. 2011). To elucidate complex plastic response strategies under climate change, we analysed the functional traits and metabolic profiles of maize plants in this study. Our objectives were to determine whether: (i) the three maize lines vary in terms of their functional traits and metabolic profiles throughout the whole range of temperatures, including low, optimal and high, to which maize seedlings may be exposed, depending on cultivation and climate conditions; (ii) the three maize lines differ in the plasticity of functional traits and the metabolome, the latter which may represent the mechanism involved in adaptation to temperature stress; and (iii) the speculated phenotypic plasticity in functional traits is related to metabolomic plasticity.
Material and Methods
Plant material and growth conditions
In this experiment, we used three maize inbred lines: PH4CV (with the Lancaster background, introduced from the USA; line L), PH6WC (with the Reid background, introduced from the USA; line R) and Chang 7–2 (derivative line from Sipingtou, a Chinese landrace; line S). These lines are parents of popularly planted hybrids in China and are divided into three major germplasm groups, which represent the majority of the genetic diversity that is available for breeding and research programmes in China. The seeds were sown in pots containing 4 kg soil and then grown side-by-side in a greenhouse at the Experimental Station of the Northeastern University, Shenyang, Liaoning (123°4′ E, 41°8′ N). Plant culture was performed according to standard commercial practices. At the fourth leaf stage, the seedlings were subjected to temperature treatments.
For temperature treatments, three sets of plants were transferred to different diurnal temperature regimes of day/night: 18/10 °C (low temperature, CS), 25/15 °C (optimal temperature, CK) and 35/25 °C (high temperature, HS). The other growth conditions included a photosynthetic photon flux density of 350 μmol m−2 s−1 under a 14-h photoperiod and relative humidity of 60%. Each treatment was prepared with 12 replicates. All of the treatments were performed in parallel. After 7 days of treatment, the plants were harvested. All of the tissues were collected, divided into groups, and used for functional trait and metabolomic analyses. In NMR analysis, fresh maize leaves were obtained from different treatments, immediately frozen, and stored at −80 °C. Finally, the leaves were ground in liquid nitrogen for metabolomic analysis.
Functional trait determination
The plant height (PH) and total leaf area per plant (LA) were measured at the end of each treatment. All of the harvested plants were divided into leaves, stems (including sheaths) and roots. Biomass samples were dried (80 °C for 48 h) to a constant weight. The specific leaf area (SLA, leaf area per unit leaf mass) and root-to-shoot ratio (R/T, ratio of root to aboveground plant parts) were calculated based on plant biomass and leaf area measurements. The biomass allocation was also calculated as the ratio of the dry mass of the respective plant part to the final dry mass, with the following traits: BAL (ratio of leaf dry mass to total dry mass), BAS (ratio of stem dry mass to total dry mass) and BAR (ratio of root dry mass to total dry mass). The total C and N content in the dried leaf samples was determined by elemental analysis with an elemental analyser (Elementar Analysensysteme, Germany) in C–N operation mode.
Nuclear magnetic resonance (NMR) and metabolite analysis
The sample preparation and NMR detection used the method of Sun et al. (2015). A plant sample of 500 mg was ground in liquid nitrogen. Then, 2 ml pre-cooled water-methanol (1:1) mixture and 2 ml chloroform were added to the tube, vortexed for 30 s and sonicated in an ice bath for 1 min. The sample was then centrifuged at 4 °C for 20 min. This procedure was performed three times; the aqueous (polar phase) and organic (non-polar phase) fractions were combined and collected separately. For aqueous samples, methanol was removed under a vacuum; then the supernatants were frozen at −80 °C and lyophilised in a freeze drier for at least 24 h. Organic samples were dried under reduced pressure in a rotary vacuum evaporator. Finally, 800 μl 100% D2O and 160 μl phosphate-buffered saline (pH 7) containing 10% D2O and 0.02 mM sodium 3-trimethylsilyl [2,2,3,3-D4] propionate (TSP) were added to the dried aqueous fractions; after which 1 ml chloroform-D containing 0.03% tetramethylsilyl (TMS) was added to the dried organic fractions. TSP and TMS were used as internal standards. All contents were transferred to Eppendorf tubes and then centrifuged at 10,000 rpm for 5 min. For each sample, 0.65 ml supernatant was transferred to 5-mm NMR sample tubes. Twelve biological replicates of each sample were used for NMR analysis.
The samples were scanned through high-resolution 1-D 1H NMR spectroscopy (1H frequency, 600.13 MHz) generating polar and non-polar metabolic profiles using a Bruker Avance 600 spectrometer (Bruker Biospin, Germany). Sample handling, automation and acquisition were controlled using TopSpin 2.1 software (Bruker Biospin). For both kinds of sample, a standard 1H 90° pulse sequence was used, and residual water resonance was suppressed in the aqueous samples. After the probe was introduced, the samples were allowed to equilibrate for 1 min. Each spectrum was obtained as 32 k data points at a spectral width of 16 ppm, and as the sum of 128 transients with a relaxation delay of 2 s. For D2O samples, frequency-domain spectra were phase and baseline corrected automatically and manually referenced to the TSP residual resonance at δH 0.00 ppm. For the CDCl3 samples, the spectra were phase- and baseline-corrected automatically and referenced manually to the TMS residual resonance at δH 0.00 ppm. Metabolite resonances were assigned based on previous studies (Fan 1996; Tate et al. 2001) and publicly available databases (e.g. Spectral Database for Organic Compounds, Biological Magnetic Resonance Data Bank, Madison Metabolomics Consortium Database). These assignments were further confirmed with extensive 2-D NMR data from COSY and TOCSY spectra (Table S1). In the COSY and TOCSY experiments, 48 transients were collected in 2048 data points for each of the 256 increments, with a spectral width of 10 ppm for both dimensions. A phase-insensitive mode was used with gradient selection in the COSY experiments, whereas common MLEV-17 was used as a spin-lock scheme in the phase-sensitive TOCSY experiment, with a mixing time of 100 ms.
Data and statistical analysis
Two separate data and statistical analyses were performed for two sets of response variables: functional traits and metabolites. For functional traits, a two-way ANOVA was first performed on the effects of temperature and genetic differentiation on each trait variation. The model consists of two fixed factors, ‘inbred lines’ and ‘temperature treatments’ and their interaction ‘temperature treatments × inbred lines’. Significant differences among the treatment means were then analysed using the Student–Newman–Keuls post-hoc tests. The effects were considered significant if P < 0.05. Prior to the analysis, data were checked for normality and homogeneity of variances, and log-transformation was used to correct deviations from these assumptions if necessary.
For the metabolome, two principal components analyses (PCA) were separately performed on the polar and non-polar metabolic profiles using the SIMCA-P+ (version 11.5; Umetrics, Umea, Sweden) software package to assess the effects of temperature and genetic differentiation on metabolic profiles. Before PCA analysis, spectral intensities were scaled to TSP for the polar extract and to TMS for the non-polar extract. The spectral intensities were then reduced to integrated regions with an equal width (0.04 ppm) corresponding to the region of δ 9.00 to δ −0.04 (polar phase) or δ 10.00 to δ −0.04 (non-polar phase). For the polar phase, the regions of δ 5.00–δ 4.70 and δ 3.38–δ 3.30 were excluded from analysis because of the residual signals of water and methanol. For the non-polar phase, the region of δ 7.40–δ 7.20 was discarded from analysis because of the residual signal of chloroform. The results were visualised with score plots, in which each point represents the metabolome of a sample. The relative levels of metabolites were calculated from the least overlapping NMR signals of metabolites with known concentrations of TSP and TMS under the assumption of the minimal inter-sample variations in the spin–lattice relaxation time of the same protons. Heat map and hierarchical cluster analyses were performed with MeV version 4.2 on semi-quantitative data for polar and non-polar metabolites. Differences in metabolite levels between CK and each treatment were considered significant at P < 0.05.
To compare the degree of plasticity among the lines as a response to temperature, we calculated the plasticity indices (PIs) for the measured parameters as the difference between the maximum mean value and the minimum mean value divided by the maximum mean value across the levels of a treatment, according to Valladares et al. (2002). PIs were calculated independently for each level of two treatment factors (PIL, PIR, PIS, PICS and PIHS) and among each treatment factor (PIG for lines and PIE for temperatures). The mean for each PI was also calculated by averaging 11 functional traits, 31 polar metabolites and 14 non-polar metabolites.
A linear regression analysis was performed to investigate the relationship between functional traits and metabolomic profiles under different temperature stress conditions. The ANOVA and linear regression were performed using SPSS 17.0 for Windows (PSS, Chicago, IL, USA).
Results
Responses of the metabolome to different temperature changes
Non-supervised PCA was performed to elucidate responses in the maize plant metabolome to temperature variations. The 1H NMR results on the extracted polar phase showed that the samples that experienced HS had distinct clusters far from those of plants that experienced CS for all three lines (Fig. 1A). A separate PCA for the three lines showed that PC1 discriminated samples that were grown under HS from those that were grown under CK and CS, whereas PC2 discriminated samples grown under CS from those grown under CK (Fig. 1B–D). In a separate observation of temperature treatments, differences in the responses among the lines became evident (Fig. 1E–G). However, the clusters that were based on non-polar metabolic profiles were less distinct than those that were based on polar metabolic profiles (Figure S1).
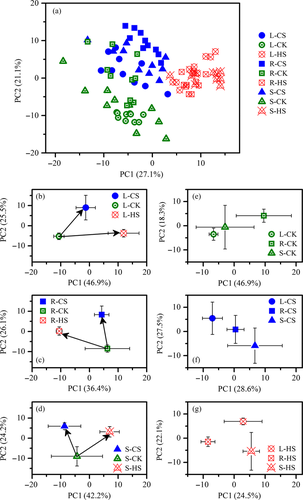
To investigate the metabolic states of maize plants under different temperatures, we assigned the metabolites that were identified through NMR and listed in additional files (Figure S2, Table S1). The heatmap and hierarchical cluster showed that the amplitude of metabolic changes in maize leaves caused by HS was larger than that attributed to CS (Fig. 2A). Correspondingly, a comparative analysis revealed that the pool sizes of amino acids derived from pyruvate (leucine, valine and alanine), α-ketoglutarate (γ-amino-butyrate, proline and glutamine) and oxaloacetate (alanine and isoleucine) significantly increased under CS and HS (Figs 2B and 3). Accumulation of compatible solutes (choline, malate) was another adaptive response. In addition, sugars (sucrose and glucose) and other metabolites (inositol, pyruvate, fatty alcohols and diacylglyceride) significantly decreased in response to CS and HS and were likely responsible for the common responses of maize to these temperature treatments. The higher sensitivity in the metabolome of maize lines to HS than to CS could be attributed to the relatively large changes in the levels of metabolites involved in the tricarboxylic acid (TCA) cycle, fatty acid metabolism and secondary metabolism related to defence responses via the shikimate pathway.
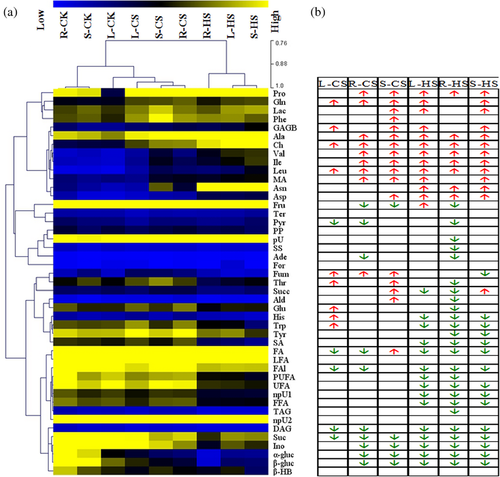
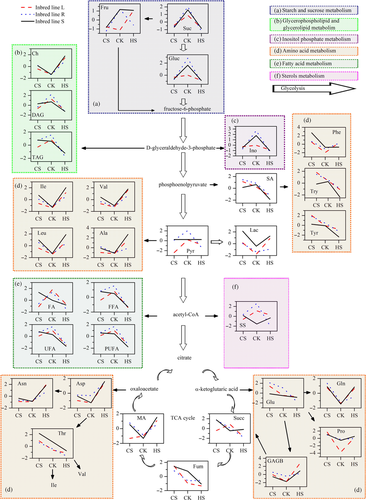
In addition, despite sharing similar response patterns to temperature stresses, the three lines showed several remarkably different metabolic responses, such as changes in fructose, fatty acids and succinate (Figs 2B and 3). Considerable differences were also observed in the strength of metabolic responsiveness among the three lines.
Responses to functional traits to different temperature changes
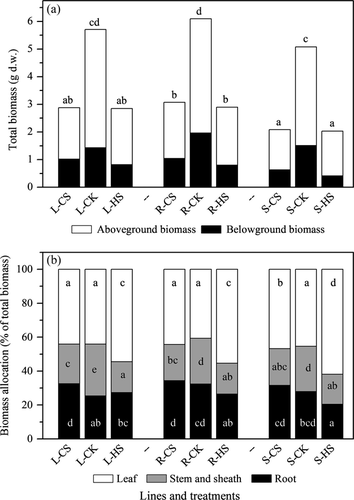
Temperature treatments significantly affected the phenotypes of maize plants for all of the evaluated traits (Table 1). Exposure to CS and HS reduced biomass production in all of the inbred lines compared to that in plants grown under control conditions (Fig. 4A). Biomass allocation differed between temperature treatments depending on the line, as shown by the significant interactions between genotype and temperature in the ANOVA results (Table 1, Fig. 4B). Generally, higher investments were observed in leaves and roots under HS and CS, respectively. In addition, maize plants exposed to HS and CS were shorter and had reduced leaf area, and both HS and CS decreased the total leaf C and increased the total leaf N for the three lines (Figure S3).
| Source | PH | LA | SLA | TDM | R/T | BAL | BAS | BAR | N | C | C/N |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | 12.1*** | 18.8*** | 19.7*** | 37.0*** | 3.3* | 12.4*** | 3.2* | 4.1* | 1.2 | 95.2*** | 3.3* |
| Temperature | 170.4*** | 41.7*** | 54.8*** | 144.7*** | 24.1*** | 90.1*** | 57.8*** | 24.6*** | 112.1*** | 185.2*** | 162.8*** |
| Genotype×Temperature | 3.6** | 3.6** | 5.0** | 3.3* | 5.7*** | 3.7** | 2.3 | 6.0*** | 3.2* | 48.0*** | 6.0** |
- PH, plant height; LA, leaf area per plant; SLA, specific leaf area (leaf area per unit leaf mass); TDM, total dry mass per plant; R/T, root/shoot ratio; BAL, ratio of leaf dry mass to total dry mass; BAS, ratio of stem dry mass to total dry mass; BAR, ratio of root dry mass to total dry mass; N, total leaf N content; C, total leaf C content; C/N, ratio of total leaf C to total leaf N.
- *P < 0.05, **P < 0.01, ***P < 0.001.
Assessment of plasticity
To assess the plasticity, we independently calculated the PI for each level of two treatment factors (PIL, PIR, PIS, PICS and PIHS) and among each treatment factor (PIG and PIE). In general, the degree of plasticity differed among the three lines for functional traits and metabolite levels in response to CS and HS, which depended on the line and trait examined and the temperature applied (Table 2). The means of PIs exhibited a similar response, which was higher for polar metabolites than for functional traits and non-polar metabolites. At the metabolic level, non-polar metabolites showed a more stable response pattern across a wider range of temperatures than did polar metabolites. Moreover, the mean of PIE was also higher than that of PIG for both metabolite levels and functional traits. This finding indicates that the three lines exhibited plasticity with respect to temperature, whereas genetic differentiation and differences in plasticity between lines were less important (Table 2). In addition, the three maize lines had the lowest mean PICS compared to PIHS, indicating that CS had less influence on phenotypic variability than did HS. Although the studied lines differed minimally in terms of the mean overall plasticity (calculated for each line by averaging PIs obtained for each of the 56 variables, achieved in different ways), the plasticity of each individual trait varied among the three maize lines (Table S2).
| Variables | PIE | PICS | PIHS | PIL | PIR | PIS | PIG |
|---|---|---|---|---|---|---|---|
| Polar metabolites | 0.55 | 0.38 | 0.48 | 0.54 | 0.56 | 0.59 | 0.26 |
| Non-polar metabolites | 0.31 | 0.12 | 0.28 | 0.31 | 0.39 | 0.32 | 0.11 |
| Functional traits | 0.29 | 0.24 | 0.24 | 0.28 | 0.30 | 0.33 | 0.23 |
Association between the metabolome and functional traits at different temperatures
To relate metabolic adjustment to functional responses to changes in temperature, we investigated the relationships between PC1 scores from the PCA analysis and functional traits through linear regression analysis. Among the studied functional traits, BAL, BAS, BAR, C, N and C/N showed a significant linear regression with PC1 scores (Fig. 5). Thus, changes in biomass allocation and the status of C and N coincided with the metabolomic separation based on the polar metabolic alterations of maize lines in response to different temperature conditions. Metabolic plasticity and functional trait plasticity were related to each other as the two components of plant adjustment to temperature change. Therefore, environmental changes could induce maize plants to adjust their metabolomes and physiological processes in an interrelated manner to maintain optimal performance under temperature stress conditions.
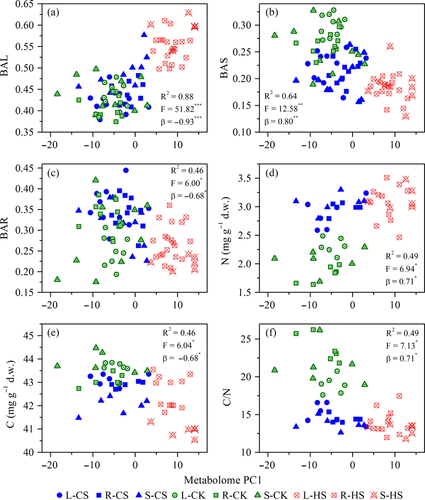
Discussion
Common metabolic responses to CS and HS
Plants can precisely sense absolute and gradual changes in diurnal and seasonal temperature using a wide array of thermosensors, and then alter thermal responsiveness associated with metabolic composition (Bahuguna & Jagadish 2015). A comparative analysis revealed that extensive cross-talk and specificity were involved in complicated pathways between responses triggered by low and high temperature variations (Figs 2B and 3). The significant changes in amino acids (alanine, leucine, isoleucine, valine, γ-amino-butyrate, proline and glutamine), sugars (sucrose and glucose) and compatible solutes (choline and malate) were responsible for the common responses of maize to temperature variation.
Sugars play critical roles in plant growth and adaptation to temperature stress (Gibson 2005; Hasanuzzaman et al. 2013). The increase in sugars under temperature stress was reported previously (Rizhsky et al. 2004; Usadel et al. 2008; Janmohammadi 2012). Notably, decreased sugar levels were recently observed in thermal bentgrass (Agrostis scabra) and Ricinus communis seedlings in response to increasing temperature (Xu et al. 2013; Ribeiro et al. 2014). Sugar content also declined after long-term cold shock (Kaplan et al. 2007). Similarly, our results showed that the levels of sucrose in the three maize lines decreased within 7 days of exposure to CS and HS (Fig. 2B), probably because sucrose can be quickly mobilised and stored throughout the plant and used for respiratory needs while gross morphological and biochemical changes occur during acclimation (Guy et al. 1992; Xu et al. 2013). Because dark respiration is induced by heat stress in leaves, a decrease in starch levels would have occurred at high temperature, thereby possibly influencing metabolism of soluble sugars (Nägele et al. 2012). As an extraordinarily versatile precursor, glucose can supply a vast array of metabolic intermediates for biosynthetic reactions (Ribeiro et al. 2014). Glucose and pyruvate levels also decreased in response to increasing temperature, thereby indicating that downstream reactions in the glycolytic pathway may be up-regulated at high temperature (Fig. 3). This result is supported by the findings of Minhas & Grover (1999), who reported that transcript levels of several enzymes in the glycolytic pathway exhibited sufficient flexibility to adjust to increased energy demands and supply of intermediates for acclimatisation to high temperature. Lactose is a product of anaerobic respiration, and its accumulation is potentially toxic to cellular metabolism (Xia & Saglio 1992). In this study, we detected increased levels of lactose in the leaves of maize inbred lines in response to temperature stress, suggesting shifts from normal respiratory pathways to the energetically less-efficient fermentation pathway.
Among numerous highly regulated metabolic networks in plant cells, those leading to amino acid synthesis have received considerable interest because amino acids are required for the synthesis of proteins and serve as precursors for a large array of metabolites with multiple functions in plant growth and response to various stresses (Less & Galili 2008). The findings of the present study showed that amino acids, such as valine, isoleucine, leucine, alanine, proline, glutamine and γ-amino-butyrate, differentially accumulated in the three maize lines exposed to cold or heat stress, which could contribute to maize adaptation to temperature stress (Figs 2B and 3). Branched-chain amino acids (BCAAs; valine, isoleucine, and leucine) may be produced as an alternative energy source in response to increased energy demand from plants struggling with abiotic stress, resulting in sugar starvation if photosynthesis is not sustained under severe stress (Taylor et al. 2004). The accumulated BCAAs could also serve as primary metabolites to support increased production of secondary metabolites for stress defence. For example, Kaplan et al. (2004) found the accumulation of isoleucine and valine in Arabidopsis under high temperatures was as a part of the defence response of the stress-weakened host against attack by pathogens during temperature stress. As a non-protein amino acid, the possible roles of γ-amino-butyrate include the regulation of cytosolic pH, N metabolism, signalling, osmoregulation, energy production, protection against oxidative stress and maintenance of the C/N balance (Mayer et al. 1990; Fait et al. 2008). As shown in Fig. 3, metabolic modulation occurred with changes in levels of γ-amino-butyrate, succinate, glucose and glutamine via the γ-amino-butyrate shunt.
Different metabolic responses to CS and HS
Hierarchical cluster analysis and PCA revealed that heat was the major stress factor that clearly separated heat-stressed plants and cold-stressed plants from controls (Fig. 1A). Regarding the identified metabolites, HS influenced metabolism more profoundly than did CS in a quantitative sense (Fig. 2B). As TCA cycle intermediates, fumarate and succinate significantly increased under CS but generally decreased under HS. These organic acids are involved in cation charge balance or osmotic maintenance functions (Xu et al. 2013). The higher levels of fumarate and succinate under CS could also reflect increased mitochondrial activity, which is beneficial in generating more reducing agents and ATP or provide C skeletons for amino acid biosynthesis in response to CS.
We observed that decreased shikimate levels were associated with decreasing levels of the amino acid derivatives, namely, tryptophan, tyrosine and phenlyalanine, with increasing temperature (Fig. 3). Moreover, the considerable increase in these aromatic amino acids depended on increased levels of shikimate under CS. In plants, aromatic amino acids act as precursors for the production of several important compounds, such as phytohormones, electron carriers, enzyme cofactors and antioxidants (Ribeiro et al. 2014). For example, phenylalanine and tyrosine serve as precursors for phytoalexins, alkaloids, lignins, flavonoids, isoflavonoids and hydroxycinnamic acids (Dixon 2001). Heat shock also induced the accumulation of tyrosine in cowpea and Arabidopsis (Mayer et al. 1990; Kaplan et al. 2004). Low temperatures have been reported to significantly affect the synthesis of flavonoids in plants, which could reflect the response in phenylalanine, as the starting component in the polypropanoid pathway (Janmohammadi 2012). Our results emphasise the importance of aromatic amino acid metabolism under cold and heat stress conditions.
Other differences in responses between CS and HS were the level and composition of fatty acids. HS influenced fatty acid metabolism more profoundly than did CS (Fig. 2B and 3). Cell membranes are the major targets of environmental stress factors, and the maintenance of membrane stability and fluidity through adjustments to membrane lipid composition and fatty acids saturation levels is a well-characterised adaptive response to stresses associated with high and low temperatures (Gigon et al. 2004). Membrane phospholipids constitute a dynamic system that generates a multitude of signalling molecules, such as inositol 1,4,5-triphosphate and diacylglycerides, which act as secondary messengers. When released during abiotic stress by the action of lipases on polar lipids, free fatty acids can be stored in triacylglycerides to avoid oxidation by free radicals and active oxygen forms (Gigon et al. 2004). In this study, triacylglycerides and diacylglycerides produced from the glycerolipid metabolic pathway significantly decreased when maize seedlings were exposed to low temperatures (Figs 2B and 3). Plant sterols play important roles in plant adaptation to different stress conditions and are involved in the regulation of stress-driven membrane dynamics (Dufourc 2008). Ribeiro et al. (2014) found that several phytosterols, including campesterol, squalene and β-sitosterol, in the roots and cotyledons of R. communis seedlings were affected by temperature. Our results showed that the content of sterols, fatty acids, free fatty acids, unsaturated fatty acids and polyunsaturated fatty acids in maize plants significantly decreased under HS for at least two of the three maize lines, indicating that the metabolic regulation of glycerolphospholipids, glycerolipids, fatty acids, terpenoids and sterols could be driven by temperature stress and play important roles in maize adaptation.
In general, the three maize lines could cope with changes in growth temperature through plastic responses in the metabolome. This investigation has provided novel insights into the mechanisms of maize plant adaptation to temperature stress at the metabolite level, and revealed the relationships between heat and cold stress responses.
Response of functional traits to different temperature changes
Plants, particularly those exposed to local abiotic conditions and environmental fluctuations, could respond to these variations by adjusting their functional traits (Hennion et al. 2012). Functional traits are well known for their high developmental plasticity, which involves the modulation of developmental programmes induced by the environment (Nicotra et al. 2010). Our results showed that the studied functional traits differed between lines and were significantly affected by temperature (Table 1, Fig. 4; Figure S3). Plant growth (PH, LA and biomass) at sub-optimal temperature can be limited through a direct temperature effect on shoot activity and an indirect effect via reduced nutrient acquisition and water uptake by the roots (Reimer et al. 2013). The observed higher investment in leaves under HS and the higher investment in roots under CS are similar to the general trend, in that allocation of root biomass increases with decreasing temperature (Fig. 4B; Frei et al. 2014). Growth regulation under high temperature was probably mediated by key phytohormones, such as auxin, gibberellic acid and salicylic acid (Scott et al. 2004; Franklin et al. 2011).
In addition to responses in growth-related traits, the total N content significantly increased, but the total C content decreased under CS and HS among the three lines (Table 1, Figure S3). The increased total leaf N under temperature stress was caused by protein turnover and degradative processes, which are typically accelerated under environmental stresses (Huffaker 1990). Under temperature stress, plants are unable to maintain whole-plant C accumulation because of a stress-induced decline in photosynthesis and increased respiration (Lyons et al. 2007; Xu et al. 2013). The shift in C-N metabolism toward the accumulation of N-containing compounds seems to be the main biochemical response to support growth under temperature stress. In general, the three maize lines could adapt to changes in growth temperature through plastic responses in the functional traits.
Phenotypic plasticity under different temperature changes
Phenotypic variation between environments can be partitioned into intraspecific genetic variation and environmentally inducible phenotypic plasticity (Valladares et al. 2002; Poorter et al. 2012). Based on a wide variety of traits, ranging from those at the cellular level to those at whole plant level, our results suggest that the influence of across environment variation (PIE) to the phenotypic variation was more important than that of intraspecific genetic variation (PIG) for the three maize inbred lines (Table 2). Plasticity is recognised as a major source of phenotypic variation in the real world because it influences natural selection and, consequently, distribution and composition (Sultan 2004). In particular, under rapid climate change, phenotypic plasticity rather than genetic diversity is likely to play a crucial role in allowing plants to persist in their environment (Vitasse et al. 2010).
Plasticity in growth determines the ability of maize to respond to sub-optimal temperature by buffering against the detrimental effects of rapid climate change and providing time for evolutionary adaptation. Both CS and HS decreased overall fitness (based on biomass parameters) in the three lines (Table 2, Fig. 4). However, this result differed from the findings of Suter & Widmer (2013), who observed increased fitness of A. thaliana (based on rosette growth) at elevated temperatures. Generally, temperature stress is expected to have negative fitness consequences, and the timing and severity of stress may be very important (Sultan 2004; Tonsor et al. 2008). At the metabolic level, a common feature that is shared by several studies on temperature response metabolite profiling is the fact that carbohydrate and amino acid metabolism seem to be the key responsive elements of plasticity and tolerance mechanisms (Kaplan et al. 2004; Guy et al. 2008; Obata & Fernie 2012; Ribeiro et al. 2014). Our results show that plasticity in the metabolism of sugars, amino acids and compatible solutes seems to be a common feature shared between CS and HS (Fig. 3, Table S2). The metabolite variables with high PIs may have more important implications for the superior maize in responses to temperature stress than those with low PIs. High trait plasticity is often assumed to be an advantage for plants experiencing large spatial and temporal habitat heterogeneity because the fitness can be maximised under different environmental conditions (Valladares et al. 2006; Frei et al. 2014). However, strong responses are also accompanied by costs and limitations to fitness, such as more resources needed to generate responses, intrinsic genetic costs due to pleiotropy, gene linkages and epistasis, or unstable plant development when environmental signals are unreliable (Freschet et al. 2013). The costs of plasticity that benefit homeostasis are also thought to be generally low because plasticity can evolve even when the response does not lead to the optimal phenotype under each condition (Vermeulen 2015).
The difference in PI between cold and heat treatment indicated that maize plants achieve plasticity in different ways in response to temperature variations (Fig. 3, Table S2). High temperature is one of the most detrimental abiotic stresses for plant growth and is expected to become more significant in future because global temperature is predicted to increase by 1–4.5 °C over the next 50 years (Sànchez et al. 2014). Therefore, our results are important for elucidating complex plastic response strategies and improving crop yield under future climate change.
The slight difference in mean PI among the three inbred lines found here is probably caused by genetic variation in domesticated maize populations that can be reduced or restructured through genetic drift and selection, both natural and artificial, by early farmers (Xu et al. 2013). In addition, the three inbred lines developed for high yield and extensive adaptability were selected under natural conditions rather than for a direct temperature response. Natural selection favours the competitiveness of individual plants, and this effect is reduced by selection for yield in crops (Sadras et al. 2013). Thus, several yield-related traits do not scale from plant to crop. Among the measured metabolites, fructose, succinate and fatty acids differed considerably among the three lines (Figs 2B and 3), whereas the magnitude of plastic responses across the three lines was similar (Table 2), suggesting that the major differences between these three lines are probably in terms of traits rather than the extent of plasticity (Chown et al. 2007; Godoy et al. 2011).
However, despite minimal differences in mean PIs, evident variation was noted in the plasticity of a given trait among the three maize lines because these lines did not always achieve plasticity in the same way (Table S2). Although phenotypic plasticity may facilitate short-term adaptation to environmental changes, genetic adaptation may ultimately be necessary for the persistence of a species in extreme habitats. Our results indicate that both phenotypic plasticity and genetic diversity contributed to phenotypic differences, although the two components of the phenotypic variance were ranked as environmental > genetic. The observed genetic differences may be attributed to neutral genetic processes (Linhart & Grant 1996). Alternatively, the genetic differentiation of plastic responses between the three maize lines in some growth-related and metabolic traits might indicate that selection in the past acted differently on the three maize lines.
Association between metabolome and functional traits in response to temperature change
Although functional trait plasticity is a major component of plant adjustment to environmental stress, researchers have increasingly recognised that some functional traits are determined by the evolutionary history of the species and the present-day environment (Ackerly 2009). Here, the three maize inbred lines seemed to adapt to temperature variations through a large number of responses in the traits of plant biomass allocation that were related to fitness (Fig. 4, Table 2). Moreover, the relationship between the metabolome and biomass allocation is significant (Fig. 5A–C). Plant growth must be constantly controlled through a variety of molecular and metabolic networks to protect and repair plant cells and provide an appropriate response to ever-changing environmental conditions (Meyer et al. 2007). Therefore, changes in biomass allocation will be preceded by alterations in plant metabolic processes. Changes in C and N concentrations mirror the general status of C and N in plants under stress conditions (Sun et al. 2015). The significant relationships between the metabolome and content of C and N also showed that related changes in the leaf metabolome occurred in response to reduced C and increased N substrates under stress conditions (Fig. 5E and D). Our finding that the content of glutamine, γ-amino-butyrate, proline, valine, leucine, isoleucine, aspartate, asparagine and alanine significantly increased under CS and HS indicates that the abundance of metabolites with a storage function associated with N assimilation was highly variable and that these amino acids were involved in the process of plant adaptation to temperature stress (Fig. 2B). The significant linear relationship between C/N and PC1 indicated that maize seedlings shift their metabolic pool from carbohydrates to amino acids as a dominant biochemical response to adjust growth and developmental processes at sub-optimal temperatures and maintain cellular metabolic homeostasis. Temperature strongly influences plant metabolism and consequently determines plant growth and physiological performance (Nägele et al. 2012). Our results suggest that both functional traits and characteristics at the metabolome level could serve as estimators for phenotypic plasticity.
The low functional trait responsiveness of maize lines to temperature variations (mean PIE for functional traits was 0.28) found in this study was not paralleled by the high metabolic responsiveness (mean PIE for polar metabolites was 0.55), indicating that phenotypic plasticity was independent from that at the organisation level. This finding emphasises the importance of phenotypic plasticity at the metabolic level as a consequence of different functional traits (Valladares et al. 2002). Alternatively, a lack of functional responses was likely due to these traits being genetically fixed, and these lines probably could only partially adjust to climate change through the relatively slow process of evolutionary adaptation (Jump & Penuelas 2005). Nevertheless, morphological, physiological and metabolic plasticity might have different roles in plant adaptation to environmental change. In fact, plants grown under stress conditions tend to have a conservative leaf morphological pattern to avoid production of structures that are too expensive to sustain (Gratani 2014). Metabolic plasticity is more related to the enhanced capacity to grow and reproduce in spatially or temporally variable environments (Zunzunegui et al. 2011). Our results indicate the importance of metabolic plasticity in plant acclimatisation to sub-optimal temperature environments, whereas functional trait plasticity plays a secondary role.
Conclusions
Our research provides clear evidence that various metabolites and functional traits are involved in maize adaptation to temperature stresses through an interrelated relationship. The response pattern and degree of specific variables varied among the plant lines and applied temperatures. A comparison of heat and cold stress response patterns revealed that maize plants share common plastic responses in biomass, C, N, sugars, some amino acids and compatible solutes. We also found that the plastic response of maize to HS was different from that to CS, which involved biomass allocation, shikimate and its aromatic amino acid derivatives, amino acids derived from oxaloacetate and other non-polar metabolites. Despite the significant relationship between the metabolome and functional traits in biomass allocation and the status of C and N, functional and metabolic plasticity may play different roles in maize adaptation to temperature variations.
Acknowledgements
This study was financially supported by National Natural Science Foundation of China (No. 31300331) and Fundamental Research Funds for the Central University (No. N120405008), China.




