The effects of smoke derivatives on in vitro seed germination and development of the leopard orchid Ansellia africana
Abstract
Plant-derived smoke and smoke-isolated compounds stimulate germination in seeds from over 80 genera. It has also been reported that smoke affects overall plant vigour and has a stimulatory effect on pollen growth. The effect of smoke on orchid seeds, however, has not been assessed. In South Africa, orchid seeds from several genera may be exposed to smoke when they are released from their seedpods. It is therefore possible that smoke may affect their germination and growth. Therefore, the effects of smoke [applied as smoke-water (SW)] and two smoke-derived compounds, karrikinolide (KAR1) and trimethylbutenolide (TMB), were investigated on the germination and growth of orchid seeds in vitro. The effect of SW, KAR1 and TMB were investigated on the endangered epiphytic orchid, Ansellia africana, which is indigenous to tropical areas of Africa. Smoke-water, KAR1 and TMB were infused in half-strength MS medium. The number of germinated seeds and number of seeds and protocorm bodies to reach predetermined developmental stages were recorded on a weekly basis using a dissecting microscope for a 13-week period. Infusing SW 1:250 (v:v) into half-strength MS medium significantly increased the germination rate index (GRI) and the development rate index (DRI) of the A. africana seeds. All the SW treatments significantly increased the number of large protocorm bodies at the final stage of development. Infusing KAR1 into the growing medium had no significant effect on germination or development of the seeds. The TMB treatment, however, significantly reduced the GRI and DRI of A. africana seeds.
Introduction
The germination of an orchid seed in a natural system requires not only favourable environmental conditions but also a symbiotic fungus (Arditti 1979; Chang 2006). The symbiotic fungus supplies nutrients and water to the germinating seed since the seed has virtually no reserves of its own (Arditti 1979; Baskin & Baskin 1998; Chang 2006). Of the millions of dust-like seeds that are released from an orchid seedpod, only a couple will germinate and grow into adult plants (Knudson 1922). For commercial growers and hybridisers of orchids, this process is far too lengthy and unproductive. The ability to germinate and grow orchid seeds using tissue culture techniques made it possible to create large numbers of orchids from a single orchid pod (Knudson 1922; Chugh et al. 2009) and thereby revolutionised the orchid industry.
Ansellia africana, the leopard orchid, is an African orchid and the only species in the genus Ansellia (Fig. 1). This orchid is often harvested by traditional healers for its medicinal value, resulting in a low number in the wild (Pooley 1998; Golding & Bandeira 2002). Some studies reported on cultivating this species using tissue culture techniques in order to produce large numbers of plants that can be introduced back into the wild (Bytebier et al. 1996; Zobolo 2010). In an attempt to increase the number of plants produced using tissue culture techniques, Vasudevan & Van Staden (2010, 2011) reported that plantlet generation from A. africana seeds could be enhanced by treating (sterilising) the seeds with a bleach solution and by introducing cytokinins into the growing media.

Smoke and the karrikins isolated from smoke have significant effects on the germination and growth of many plants. Several studies have reported that smoke-water (SW) and KAR1 can be used to stimulate the germination of seeds from different plant families and also increase the growth of plants (reviewed in Light et al. 2009; Kulkarni et al. 2011). Trimethylbutenolide (TMB) was shown to inhibit the germination of lettuce seeds (Light et al. 2010). A molecular study by Soós et al. (2012) indicated that karrikinolide (KAR1) down-regulates and TMB up-regulates abscisic acid (ABA), seed maturation and dormancy-related transcripts.
The orchid A. africana grows epiphytically on trees in the tropical areas of Africa, and could be exposed to smoke during the winter months when wild fires are common. The effects of smoke and the smoke-isolated compounds on orchid seed germination have never been documented, and it is unclear how the smoke treatments might affect orchid seed germination and growth, since these seeds have limited to no seed reserves. Therefore the effects of SW, KAR1 and TMB on orchid seed germination and development were investigated.
Material and Methods
Collection of Ansellia africana pods
Mature seed pods of A. africana, were harvested from plants grown in the Botanical Garden of the University of KwaZulu-Natal (29°37.50′S, 30°24.23′E), Pietermaritzburg, South Africa, during May 2014, 9 months after flowering had occurred. The pods were surface sterilised by immersing pods in full strength commercial bleach (NaOCl) for 20 min. The pods were then blotted dry and stored at 4 °C in the presence of a desiccant in sealed plastic containers.
Preparation of growth media
Before removing the seeds, one A. africana seedpod was placed in a desiccator containing silica gel until the pod changed from a green colour to a light yellow colour (before dehiscence). The intact pod was surface-sterilised by immersing it in 3.5% NaOCl for 20 min. The pod was then immersed in absolute ethanol and set alight. After the fire was quenched, the pod was again immersed in absolute ethanol, set alight a second time and placed on a sterile laminar flow bench. The pod was cut open with a sterile scalpel and the seeds removed.
Half-strength Murashige and Skoog (Murashige & Skoog 1962) medium containing 3% sucrose was used as the growing medium. In order to test the effects of the various treatments, appropriate amounts of concentrated SW, KAR1 and TMB solutions were added to individual conical flasks, which were subsequently made up to a volume of 100 ml with half-strength MS medium. The treatments were as follows: (1) control; (2) SW 1:250 (v:v); (3) SW 1:500 (v:v); (4) SW 1:750 (v:v); (5) SW 1:1000 (v:v); (6) KAR1 (10−7 m); (7) KAR1 (10−8 m); (8) KAR1 (10−9 m); (9) TMB (10−3 m); (10) TMB (10−4 m); (11) TMB (10−5 m); (12) KAR1 (10−7 m) + TMB (10−3 m); (13) KAR1 (10−7 m) + TMB (10−4 m); (14) KAR1 (10−7 m) + TMB (10−5 m); (15) KAR1 (10−8 m) + TMB (10−3 m); (16) KAR1 (10−8 m) + TMB (10−4 m); (17) KAR1 (10−8 m) + TMB (10−5 m); (18) KAR1 (10−9 m) + TMB (10−3 m); (19) KAR1 (10−9 m) + TMB (10−4 m) and (20) KAR1 (10−9 m) + TMB (10−5 m). The pH of media in the individual conical flasks was adjusted to 5.8. The medium was solidified using 0.6% (w/v) agar (Bacteriological, Agar No. 1; Oxoid, Bastingstoke, UK) and sterilised by autoclaving at 121 °C for 20 min. The medium of each conical flask was dispersed in five Petri dishes (90 mm). After the medium cooled and solidified, 200 to 400 seeds were sown per Petri dish, and then sealed with Parafilm. The Petri dishes were incubated at 25 °C and were subjected to a 16-h light/8-h dark regime with a photosynthetic photon flux (area) density of 30 μmol·m−2·s−1.
Determining germination rate and protocorm development rate
After 6 weeks the seeds started to germinate and five different growth stages were recorded using a dissecting microscope (Kyowa Optical, Japan). Seeds were considered germinated and were differentiated from the ungerminated seeds (Fig. 2a) with the formation of rhizoids (Stage 1; Fig. 2b). The next stage of development was depicted by seeds that broke free from their testa completely (Stage 2; Fig. 2c). A seed that was enlarged and started turning green was considered a protocorm body (Stage 3; Fig. 2d). Enlarged protocorms were characterised by an increase in size, a pronounced green colour and numerous rhizoids (Stage 4; Fig. 2e). The last developmental stage considered for this study was characterised by the formation of large protocorms that could be discerned without the use of a dissecting microscope (Stage 5; Fig. 2f). The germination of A. africana seeds was recorded for an 8-week period (13 weeks after sowing of seeds). Germination rate index and developmental rate index was calculated on the data of two separate experiments (n =10).

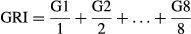
Where G1 is equal to the germination percentage x 100 at the first count after sowing; G2 is equal to the germination percentage × 100 at the second count after sowing, etc.
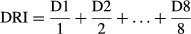
where D1 is equal to the percentage of developed seeds/protocorms of a particular developmental stage (Stage 2–5) × 100 at the first count after sowing; D2 is equal to the percentage of developed seeds/protocorms of a particular developmental stage (Stage 2–5) × 100 at the second count after sowing, etc.
Statistical analysis
Values that were significantly different to the respective controls were determined according to a two-sample t-test (P < 0.05; GenStat, version 14).
Results
The GRI and DRI for the different SW, KAR1 and TMB treatments are summarised in Table 1. The GRI of A. africana seeds sown on MS medium was significantly higher when the medium was supplemented with SW 1:250 compared to the control. However, supplementing the media with TMB (10−5 m) significantly reduced the GRI compared to the control. The GRI was also significantly reduced with KAR1 10−7 m + TMB (10−3 and 10−4 m), KAR1 10−8 m + TMB (10−3–10−5 m) and KAR1 10−9 m + TMB 10−4 m compared to the control. Smoke-water 1:250 significantly increased the number of seeds that broke free of the testa and also significantly increased the numbers of protocorms at developmental stages 3, 4 and 5 compared to their respective controls. Compared to the control, supplementing the media with SW 1:1000 significantly increased the DRI of protocorms that started turning green (Stage 3). The DRI of large protocorms (Stage 5) was significantly increased by all the SW treatments compared to the respective controls. Supplementing the media with TMB (10−3 and 10−5 m), KAR1 10−7 m + TMB (10−3 and 10−4 m), KAR1 10−8 m + TMB (10−4 and 10−5 m) and KAR1 10−9 m + TMB (10−4 m) significantly reduced the DRI of seeds at Stage 2 and protocorms at Stages 3 and 4 compared to the controls. The same set of treatments with the addition of TMB 10−4 m, KAR1 10−8 m + TMB 10−3 m and KAR1 10−9 m + TMB 10−5 m significantly reduced the DRI of large protocorms (Stage 5) compared to the control. Supplementing the medium with KAR1 had no significant effect on the GRI or the DRI at any developmental stage. The promotory effects of SW 1:250 and the inhibitory effects of TMB 10−3 m on the development of A. africana seeds 10 weeks after being sown can clearly be seen in Fig. 3.
| developmental phases: | stage 1 | stage 2 | stage 3 | stage 4 | stage 5 |
|---|---|---|---|---|---|
| treatments | GRI (% per week) | DRI (% per week) | DRI (% per week) | DRI (% per week) | DRI (% per week) |
| Control | 70.8 ± 6.5 | 41.7 ± 4.0 | 20.2 ± 2.2 | 7.8 ± 1.6 | 7.1 ± 1.0 |
| SW 1:250 | 91.6 ± 3.2* | 56.9 ± 3.7* | 37.9 ± 2.3* | 18.7 ± 1.1* | 31.4 ± 2.1* |
| SW 1:500 | 59.0 ± 8.4 | 34.1 ± 4.8 | 22.8 ± 3.1 | 9.1 ± 1.2 | 12.4 ± 1.8* |
| SW 1:750 | 55.4 ± 6.1 | 33.1 ± 2.6 | 21.9 ± 1.3 | 8.0 ± 1.0 | 12.2 ± 1.2* |
| SW1:1000 | 74.7 ± 4.6 | 43.9 ± 2.0 | 26.6 ± 0.6* | 9.2 ± 0.6 | 11.8 ± 0.9* |
| KAR 10−7 m | 59.0 ± 3.4 | 31.3 ± 1.9 | 18.2 ± 1.2 | 5.3 ± 0.4 | 6.4 ± 0.6 |
| KAR 10−8 m | 62.0 ± 2.5 | 34.1 ± 1.2 | 18.0 ± 1.1 | 4.8 ± 0.4 | 6.2 ± 0.6 |
| KAR 10−9 m | 55.3 ± 2.3 | 30.2 ± 4.8 | 18.4 ± 2.3 | 5.3 ± 1.1 | 4.9 ± 1.2 |
| TMB 10−3 m | 57.8 ± 7.6 | 30.6 ± 1.8† | 12.7 ± 0.9† | 3.1 ± 0.3† | 2.4 ± 0.3† |
| TMB 10−4 m | 69.5 ± 5.3 | 39.9 ± 3.4 | 16.6 ± 1.7 | 3.9 ± 0.6 | 3.5 ± 0.6† |
| TMB 10−5 m | 32.3 ± 4.8† | 16.0 ± 2.5† | 9.4 ± 1.6† | 2.9 ± 0.7† | 2.5 ± 0.8† |
| KAR1 10−7 + TMB 10−3 m | 24.0 ± 1.6† | 13.0 ± 1.4† | 7.6 ± 0.9† | 2.5 ± 0.3† | 2.5 ± 0.4† |
| KAR1 10−7 + TMB 10−4 m | 39.7 ± 5.7† | 19.6 ± 2.2† | 11.8 ± 1.6† | 3.4 ± 0.6† | 4.7 ± 1.1 |
| KAR1 10−7 + TMB 10−5 m | 79.1 ± 10.7 | 40.8 ± 4.1 | 22.3 ± 1.9 | 6.7 ± 0.9 | 7.9 ± 1.2 |
| KAR1 10−8 + TMB 10−3 m | 33.7 ± 3.8† | 27.3 ± 4.7 | 14.8 ± 1.2 | 4.6 ± 0.5 | 4.1 ± 0.6† |
| KAR1 10−8 + TMB 10−4 m | 35.7 ± 6.2† | 20.1 ± 3.2† | 13.5 ± 0.7† | 3.2 ± 0.4† | 3.2 ± 0.8† |
| KAR1 10−8 + TMB 10−5 m | 40.4 ± 1.7† | 21.1 ± 0.5† | 13.7 ± 0.8† | 3.6 ± 0.3† | 2.5 ± 0.4† |
| KAR1 10−9 + TMB 10−3 m | 80.7 ± 6.9 | 41.7 ± 4.9 | 27.4 ± 4.1 | 9.1 ± 1.5 | 4.9 ± 1.1 |
| KAR1 10−9 + TMB 10−4 m | 38.2 ± 5.2† | 20.0 ± 3.5† | 11.2 ± 2.1† | 2.3 ± 0.4† | 1.1 ± 0.3† |
| KAR1 10−9 + TMB 10−5 m | 66.4 ± 10.8 | 36.9 ± 7.2 | 23.2 ± 4.5 | 4.8 ± 0.8 | 3.3 ± 1.1† |
- Values that were significantly higher than control treatments are indicated with an asterisk symbol (*), while values that were significantly lower than the control treatments are indicated with a hash symbol (†) according to a two-sample t-test (P < 0.05).

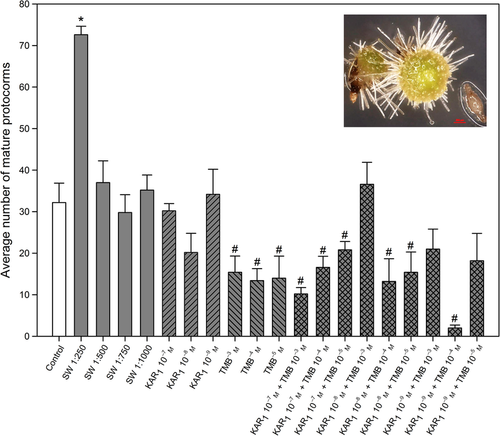
The average number of seeds that developed into large protocorms is summarised in Fig. 4. Smoke-water 1:250 produced on average significantly more large protocorms compared to the control. Trimethylbutenolide (10−3–10−5 m) significantly reduced the average number of Stage 5 protocorms compared to the control. The combined treatments KAR1 10−7 + TMB (10−3–10−5 m), KAR1 10−8 m + TMB (10−4 and 10−5 m) and KAR1 10−9 m + TMB 10−4 m significantly reduced the average number of Stage 5 protocorms produced compared to the control.
Discussion
It is common practice to germinate orchid seeds on a growing medium such as MS medium used in this study (Chugh et al. 2009). Using tissue culture techniques, many plantlets can be generated from the seed of one seedpod. Culturing orchids from seed is, however, very time consuming, with most species taking between 5 to 7 years to reach maturity and flower. Shortening this process at any point, from germination to growth, could be very beneficial for orchid growers and breeders.
From a visual inspection, it was evident that the seeds that were sown on media containing SW (1:250) developed much faster than those of the control treatment (Fig. 3). The SW (1:250) treatment not only significantly increased the average number of large protocorms compared to the control treatment (Fig. 4), but also increased the GRI and DRI of every stage of development (Table 1). All the SW treatments significantly increased the DRI of large protocorms (Stage 5; Table 1). These results indicate that smoke has a stimulatory role on the growth and development of A. africana seeds. It is therefore possible that the smoke from fires may stimulate A. africana protocorm development in natural systems. More importantly, smoke could increase the rate of development of orchid protocorms grown in vitro. More orchid species will need to be evaluated to determine if SW can be incorporated into the growing media of orchid seeds.
Use of KAR1 has been found to stimulate germination in seeds from many plant species. It was reported by Downes et al. (2010) that the seeds of some plant species may be stimulated to germinate by SW and not by KAR1 while in other cases, by KAR1 and not by SW. In this instance, SW stimulated the germination and growth of A. africana seeds while KAR1 had no effect. This might be due to the fact that orchid seeds do not contain any nutrient reserves that can be mobilised, since the activity of KAR1 is often associated with up-regulating the activity of hydrolytic enzymes. This result correlates well to a study conducted by Papenfus et al. (2014), which showed that SW stimulated pollen growth in three Amaryllidaceae species and that the effects of KAR1 on pollen growth were less consistent than the SW treatment.
It is possible that the other karrikins or combinations of karrikins present in plant-derived smoke (Flematti et al. 2007) may be responsible for the stimulatory effect of the SW 1:250 treatment. It was, however, reported by Flematti et al. (2007) that KAR1 is the most active and most abundant karrikin in plant-derived smoke. It is therefore possible that other compounds may be present in plant-derived smoke with biological activity, and that these compounds were responsible for the increased germination and development in pollen grains (Papenfus et al. 2014) and in A. africana seed germination and development. It may therefore be worthwhile to develop a bioassay using pollen or orchid seeds to identify other compounds with biological activity in plant-derived smoke.
Similar to species with seeds that contain nutrient reserves (Light et al. 2010; Papenfus et al. 2015), TMB reduced the rate of development of A. africana orchid protocorms (Table 1). Soós et al. (2012) reported that KAR1 and TMB do not compete for the same binding site when applied to ‘Grand Rapids’ lettuce seeds, which is consistent with our results of TMB reducing the growth of A. africana seeds, while KAR1 had no effect. The same study from Soós et al. (2012) showed that TMB up-regulated dormancy-related transcripts when applied to ‘Grand Rapids’ lettuce seeds. The effect of TMB on A. africana seed germination and growth could be due to the fact that TMB up-regulates dormancy-related transcripts. More in-depth studies are required to elucidate the role of TMB in orchid seed germination.
Acknowledgements
We thank the National Research Foundation South Africa and the University of KwaZulu-Natal (UKZN), South Africa, for financial support.




