Cotton LIM domain-containing protein GhPLIM1 is specifically expressed in anthers and participates in modulating F-actin
Abstract
As one form of actin binding protein (ABP), LIM domain protein can trigger the formation of actin bundles during plant growth and development. In this study, a cDNA (designated GhPLIM1) encoding a LIM domain protein with 216 amino acid residues was identified from a cotton flower cDNA library. Quantitative RT-PCR indicated that GhPLIM1 is specifically expressed in cotton anthers, and its expression levels are regulated during anther development of cotton. GhPLIM1:eGFP transformed cotton cells display a distributed network of eGFP fluorescence, suggesting that GhPLIM1 protein is mainly localised to the cell cytoskeleton. In vitro high-speed co-sedimentation and low co-sedimentation assays indicate that GhPLIM1 protein not only directly binds actin filaments but also bundles F-actin. Further biochemical experiments verified that GhPLIM1 protein can protect F-actin against depolymerisation by Lat B. Thus, our data demonstrate that GhPLIM1 functions as an actin binding protein (ABP) in modulating actin filaments in vitro, suggesting that GhPLIM1 may be involved in regulating the actin cytoskeleton required for pollen development in cotton.
Introduction
As the male gametophyte, pollen plays an important role in successful fertilisation during plant sexual reproduction. Pollen tube growth is an extremely rapid and polarised process that occurs exclusively at the tube tip, and the growth rate can reach up to 1 cm·h−1 (Bedinger et al. 1994). Double fertilisation is facilitated by the structure of the pollen tube, which provides a passage for delivery of two non-motile sperm cells to the ovule. Actin dynamics play an important role during this long-distance migration, by supporting organelle movement and cytoplasmic streaming and regulating vesicle trafficking in the tip zone (Cheung et al. 2008, 2010; Dhonukshe et al. 2008; Staiger et al. 2010). During pollen tube elongation, the actin cytoskeleton is continuously remodelled (Kost et al. 1999; Fu et al. 2001; Gu et al. 2005). A variety of actin binding proteins (ABPs) are created to maintain actin dynamics and their functional diversity in cells (McCurdy et al. 2001). They have the ability to bind to monomeric actin (G-actin), filamentous actin (F-actin) or to both forms, and their activities are tightly regulated by many cellular parameters, including calcium, pH, phosphoinositides, phosphorylation and protein–protein interactions. The coordinated regulation of ABP activities ultimately defines actin filament positioning, turnover and supra-organisation in orthogonal networks or parallel bundles (Papuga et al. 2010), so the ABPs are believed to be responsible for the formation and maintenance of higher-order F-actin structures.
Several ABPs have been identified and characterised in pollen tubes, such as formins, villins and actin-depolymerising factors (ADFs). All of these are important for maintaining organisation of the actin cytoskeleton to support pollen tube growth (Chen et al. 2003; Cheung & Wu 2004). It was shown that two lily pollen villins, P-115-ABP and P-135-ABP, assemble F-actin into bundles of uniform polarity (Yokota et al. 2003, 2005). Papaver rhoeas pollen gelsolin-like protein PrABP80 functions as an effective actin nucleator in basal calcium conditions, but severs and depolymerises actin filaments under high calcium conditions (Huang et al. 2004). These results suggest that ABPs can organise the actin cytoskeleton through bundling or severing activity. In addition, Arabidopsis formin homology5 (FH5), which is located in the tip of the pollen tube and anchored in the membrane, is essential for tip-focused growth (Cheung et al. 2010). ACTIN BINDING PROTEIN29 (ABP29) also contributes to actin cytoskeletal rearrangement during pollen germination and tube growth (Xiang et al. 2007). Recently, several proteins have been reported to play fatal functions in regulating F-actin dynamics during pollen tube growth. Down-regulation of Arabidopsis VILLIN2 and VILLIN5 led to accumulation of actin filaments at the pollen tube apex (Qu et al. 2013). A microtubule-associated protein, MAP18, regulates the direction of pollen tube growth in Arabidopsis by modulating F-actin organisation (Zhu et al. 2013). All of these results suggest that actin dynamics modulate pollen tube growth through the action of ABPs, but the underlying molecular mechanisms are still unclear.
The LIM proteins function as actin binding and bundling proteins (ABPs) and/or as transcriptional activators of lignin biosynthesis. These proteins contain two LIM domains, characterised by the consensus sequence [C-X2-C-X17-H- X2-C]-X2-[C- X2-C- X17-C- X2-H], in which the conservative cysteine and histidine residues form the Zn2 + -binding pockets (55 amino acids that basically function in protein–protein interaction) and contribute to the stable tertiary structure (Schmeichel & Beckerle 1997; Kadrmas & Beckerle 2004). The abbreviation ‘LIM’ stems from the first letter of the proteins LIN-11 (Freyd et al. 1990), Isl-1 (Karlsson et al. 1990) and MEC-3 (Way & Chalfie 1988), from which the LIM domain was historically identified. The LIM domain is conserved over a wide variety of species. In eukaryotes, it is often associated with a protein kinase domain or a homeodomain. Whereas animals possess numerous LIM proteins of diverse structures and functions, plants only contain a limited number of LIM proteins (Arnaud et al. 2007). The LIM protein family in has CRP (vertebrate cysteine-rich proteins)-related proteins containing two LIM domains, separated by a long inter-LIM domain. So far, a number of LIM genes have been identified in a wide range of plant species, e.g. Arabidopsis, tobacco and poplar (Thomas et al. 2007; Papuga et al. 2010; Moes et al. 2013). The plant LIM protein family is divided into four groups: PLIM1, PLIM2, WLIM1 and WLIM2, based on their taxonomic class or specific expression. PLIM1 and PLIM2 are specifically expressed in pollen, while WLIM1 and WLIM2 are expressed in the whole plant. For example, LIM genes belonged to WLIM1 and WLIM2 subgroups are expressed in all tissues of sunflower, tobacco and Arabidopsis, whereas HaPLIM1 is expressed specifically in sunflower pollen (Baltz et al. 1992). Like animal CRP, most plant LIM proteins are present in the cytoplasm and/or in the nucleus. This is the case for the sunflower protein HaWLIM1, which localises in the cytoplasm, in the nucleus or in both areas (Brière et al. 2003). Many plant LIM proteins (e.g. lily LlLIM1, tobacco WLIM1 and Arabidopsis LIM proteins) such as ABPs regulate the assembly of F-actin bundles, control actin dynamics and protect F-actin from depolymerisation (Thomas et al. 2006; Wang et al. 2008; Papuga et al. 2010).
Cotton (Gossypium hirsutum), which produces textile fibres and seed oils, is an important crop. Cotton development is a complicated and ordered process regulated by many genes. Some studies reported that LIM domain proteins play an important role in cotton fibre development. GhWLIM5 is expressed preferentially in elongating fibres and involved in bundling actin filaments during fibre development of cotton (Li et al. 2013). Cotton WLIM1a performs dual functions in fibre elongation and secondary wall formation (Han et al. 2013). Although several cotton LIM genes are well characterised for fibre development, little is known about the role of cotton LIM genes in flower development, especially in pollen development. In this study, we isolated and characterised the GhPLIM1 gene from cotton, which encodes a protein containing two LIM DNA-binding doma-ins. Our experimental results indicate that GhPLIM1 is preferentially expressed in cotton anthers. GhPLIM1 protein is coupled to the actin filamentous network in cells, suggesting that it acts as an actin binding protein (ABP) in cotton. Furthermore, co-sedimentation assays confirmed that GhPLIM1 proteins directly bundle actin filaments and protect F-actin from depolymerisation.
Material and Methods
Cotton growth conditions
Cotton (Gossypium hirsutum cv. Coker 312) seeds were surface-sterilised with 70% (v/v) ethanol for 1 min and 10% (v/v) H2O2 for 60 min, followed by washing with sterile water twice. The sterilised seeds were germinated on half-strength MS medium (PH 5.8) under a 16-h light/8-h dark cycle at 28 °C for 6 days. Roots, cotyledons and hypocotyls were cut from these sterile seedlings, and other tissues were collected from field-grown cotton plants for RNA isolation.
Isolation of GhPLIM1 cDNA
More than 4,000 cDNA clones were randomly selected from a previously constructed cotton flower cDNA library (Wang & Li 2009) and sequenced. Among them, several cDNA clones encoding LIM domain proteins (including GhPLIM1) were identified for further characterisation.
DNA and protein sequence analysis
Cotton nucleotide and amino acid sequences were analysed using the comprehensive sequence analysis software DNAStar Lasergene 7.1 (DNAStar, Madison, WI, USA). The complete sequences of cotton LIM genes were confirmed using NCBI databases (http://www.ncbi.nLm.nih.gov/BLAST). Analysis of the protein structure information was obtained from the structure domain database (http://www.expasy.org/prosite/). GhPLIM1 and other plant LIM proteins were analysed using ClustalW2 (http://www.ebi.ac.uk/Tools/clustaLW2/INDEX.HTML) to find differences or similarities in structural domains and active functional sites. A neighbour-joining tree was generated with the MEGA5.0 program. A bootstrap analysis with 1000 replicates was performed to assess the statistical reliability of the tree topology.
Quantitative RT-PCR analysis
Total RNA was collected from cotton roots, leaves, stems, flowers, cotyledons, ovules, anthers, petals and fibres. To synthesise cDNA, the isolated total RNA was used for reverse transcription with MMLV reverse transcriptase (Promega, Madison, WI, USA) according to the manufacturer's instructions. Real-time quantitative RT-PCR analysis was performed to evaluate expression levels of GhPLIM1 in cotton tissues, using SYBR Green real-time PCR master mix (Toyobo, Osaka, Japan) in a DNA Engine Opticon 2 detection system (MJ Research, St. Bruno, Canada) with the method described earlier (Li et al. 2005). A cotton polyubiquitin gene (GhUBI1) was used as an internal control in RT-PCR reactions. The gene-specific primer pairs used in RT-PCR analysis were as follows: GhPLIM1-P1, ATTTGCATCATTCTTCTCTGGT and GhPLIM1-P2, CCTCCCTACGTCTCATTACACTTA; GhUBI1-P1, 5′-GGGATGCAAATCTTCGTGAAAAC-3′ and GhUBI1-P2, 5′-CTGAATCTTCGCTTTCACGTTATC-3′. All reactions were performed in triplicate, along with three independent repetitions of the biological experiments. Mean of three biological experiments was calculated for estimating gene expression levels.
Visualisation of GhPLIM1-GFP protein
To examine the subcellular localisation of GhPLIM1, the coding sequence of GhPLIM1 was cloned into the pBluescript SK-eGFP vector at BamH I and Xba I sites. Subsequently, the fragment of the GhPLIM1-eGFP fusion gene was digested from the pBluescript SK-GhPLIM1-eGFP construct with BamH I and Sac I enzymes, and cloned into the PBI121 vector, replacing the GUS gene. The construct was then transformed into Agrobacterium tumefaciens strain LBA4404 and introduced into cotton as described previously (Li et al. 2002). Stable transformed cotton cells expressing the GhPLIM1-eGFP fusion gene were selected for detecting GFP fluorescence on a SP5 Meta confocal laser microscope (Leica, Wetzlar, Germany) with a filter set of 488 nm for excitation and 506–538 nm for emission. SP5 software (Leica) was employed to record and process the digital images (Wang & Li 2009).
F-actin co-sedimentation assay
The coding sequence of GhPLIM1 cDNA from the pBluescript SK-GhPLIM1 vector was cloned into the pET28a vector at BamH I and Sac I sites. The recombinant vector was introduced into Escherichia coli strain BL21 to express GhPLIM1 proteins. The His-GhPLIM1 proteins were purified using Ni-NTA agarose under native conditions according to the manufacturer's instructions, and then exchanged with buffer (100 mm NaH2PO4, 10 mm Tris-HCl, pH 7.4) using 10 K molecular weight cut-off dialysis cassettes (Merck Millipore, Darmstadt, Germany). Protein concentration was determined using the Actin Bing Protein Biochem Kit (Cytoskeleton, Denver, USA).
A high-speed co-sedimentation assay was performed to test the ability of GhPLIM1 protein to bind F-actin, using the method described earlier (Li et al. 2013). In brief, all proteins were centrifuged at 200,000 g for 1 h at 4 °C before use. Then, actin filaments (10 μm) were incubated with increasing concentrations (0.5–4.0 μm) of GhPLIM1 protein at 25 °C. After incubating for 1 h, all samples were centrifuged at 200,000 g for 1 h at 4 °C. The proteins in supernatants and pellets were analysed with SDS-PAGE.
Bundling activity of GhPLIM1 protein was analysed with a low-speed co-sedimentation assay (Huang et al. 2005). Similar to the high-speed assay, all samples after incubation were centrifuged at 14,000 g for 1 h at 4 °C, then, the proteins in supernatants and pellets were analysed using SDS-PAGE.
Latrunculin B (Lat B, an F-actin depolymeriser) was used to examine the effect of GhPLIM1 proteins on stabilising F-actin. In this high-speed co-sedimentation assay, 4 μm actin was polymerised at 25 °C for 1 h in the absence (control) or the presence of various amounts (0.5, 1, 2 and 4 μm) of GhPLIM1 protein, and then 100 μm Lat B was added to each sample, using DMSO as control. After 20 h of incubation at 25 °C, the samples were centrifuged at high speed (200,000 g) at 4 °C for 1 h, and analysed with SDS-PAGE, as described above.
Results
Characterisation of anther-expressed GhPLIM genes
To identify the function of actin-binding proteins (ABPs) regulating actin dynamics in pollen development and germination, we isolated several full-length GhPLIM cDNAs, one of which is designated as GhPLIM1 (accession number in GenBank: JX290319), from a cotton flower cDNA library by randomly sequencing over 4,000 cDNA clones. Subsequently, the genomic DNA of GhPLIM1 was amplified by PCR using gene-specific primers. GhPLIM1 cDNA is 919 bp in length, including a 651 bp open reading frame (ORF) that encodes an LIM protein of 216 amino acids. GhPLIM1 protein contains two LIM DNA-binding domains (one from C10 to K67 and the other from C108 to H155) separated by a 42 residue spacer region and a variable C-terminal domain. Sequence analysis showed that GhPLIM1 shares high similarity with other cotton LIMs (e.g. GhLIM1, GhWLIM2, GhWLIM5 and WLIM1a). However, compared with others highly expressed in cotton fibres, GhPLIM1 contains the C-terminal repeat domain specifically present in pollen protein (Fig. 1). In addition, for all plant LIMs examined, the tandem zinc finger motifs present in both LIM domains contain eight conserved Cys/His residues for zinc binding. In each zinc finger motif, the pairs of Cys/His residues are separated by 17 amino acid residues, except for the last one in the second LIM domain, which was separated by 15 amino acid residues.

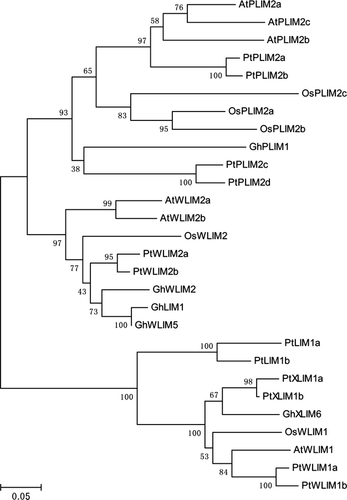
Phylogenetic relationship of GhPLIM1 with other plant LIM proteins
We analysed the phylogenetic relationship of the GhPLIM1 protein with other plant LIM proteins during evolution. A total of 26 plant LIM proteins reported previously were selected from the GenBank database for phylogenetic analysis. The 26 LIM proteins in the phylogenetic tree can be divided into three groups (Fig. 2). GhPLIM1 is classified in first group, where it forms a distinct clade basal to PtPLIM2c and PtPLIM2d. In this branch of the first group of the tree, GhPLIM1 shares relatively higher similarity (38% identity) to PtPLIM2c and PtPLIM2d, implying that GhPLIM1 has a close evolutionary relationship with the two LIMs. On the other hand, GhPLIM1 shares relatively low similarity to the previously reported GhLIM1, GhWLIM2 and GhWLIM5, which are highly expressed in cotton fibres. Together with expression patterns of the other GhLIMs, it is suggested that GhPLIM1 may have diverged independently from GhLIM1 during evolution. Furthermore, plant LIM proteins exhibit high diversity in phylogeny.
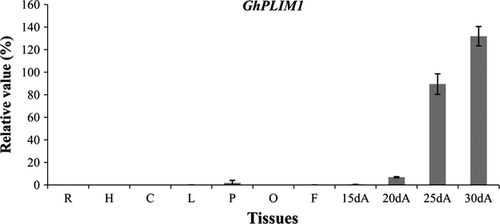
GhPLIM1 is preferentially expressed in anthers
To identify the expression profiling of GhPLIM1 in cotton tissues, quantitative RT-PCR analysis was performed. GhPLIM1 mRNA largely accumulated in anthers, but there was weak to no expression of this gene in the other tissues examined (Fig. 3). As anthers developed, GhPLIM1 expression gradually increased, and reached a peak in 30-day-old mature anthers on the day of flowering, suggesting that it may play an important role in anther development, especially in pollen development and germination.
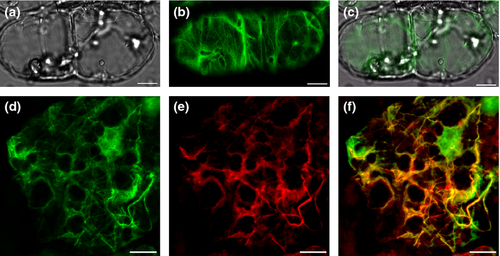
The GhPLIM1 protein is mainly localised to the cell cytoskeleton
To determine the role of GhPLIM1 in regulation of anther development, it is important to first determine where it is localised in the plant cell. eGFP (enhanced green fluorescence protein)-tagged GhPLIM1 fusion protein, driven by the cauliflower mosaic virus (CaMV) 35S promoter, was introduced into cotton via Agrobacterium-mediated transformation. Stable transformed cells expressing GhPLIM1-eGFP fusion protein were examined with a Leica confocal laser scanning microscope (TCS SP5). Strong GFP fluorescence was detected in the cytoplasm (Fig. 4). In the cytoplasm, GhPLIM1-eGFP proteins decorated a filamentous network, suggesting that GhPLIM1 is mainly localised to the cell cytoskeleton. In addition to its cytoplasmic localisation, a few GhPLIM1-GFP proteins seem to also be presented within the nucleoplasm (Fig. 4A–C). To further verify our results, GhPLIM1:eGFP transformed cotton callus cells were stained with rhodamin-phalloidin, which is an actin cytoskeleton-specific fluorochrome. The fluorescent signals of GhPLIM1:eGFP overlapped with the actin cytoskeleton stained with rhodamine-phalloidin (Fig. 4D–F), demonstrating that GhPLIM1 protein is localised on filamentous actin.
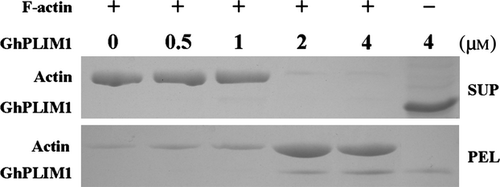
The GhPLIM1 protein binds F-actin in vitro
To investigate whether GhPLIM1 functions as an ABP to regulate dynamics of the actin cytoskeleton in anther/pollen development, the ability of GhPLIM1 to bind to filamentous actin (F-actin) was analysed with a variation of the high-speed co-sedimentation assay. Recombinant His-GhPLIM1 protein was prepared and purified. We used various concentrations of recombinant His-GhPLIM1 to incubate with preformed F-actin, following centrifugation at high speed at 4 °C, the pellet and supernatant fractions were detected with SDS-PAGE. Most GhPLIM1 was in the supernatant when GhPLIM1 was centrifuged alone under high-speed centrifugation (Fig. 5). In contrast, GhPLIM1 was enriched in the pellet, together with F-actin when GhPLIM1 with F-actin was centrifuged at same speed. With the increasing concentration of GhPLIM1 protein, the amount of GhPLIM1 in the pellet gradually increased, suggesting that GhPLIM1 protein directly binds and co-sediments with F-actin in vitro.
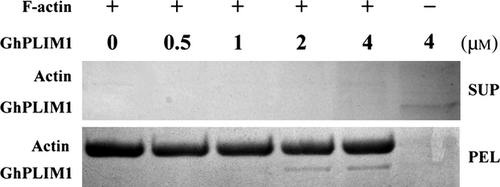
The GhPLIM1 protein bundles actin filaments
It is known that some binding proteins also bundle actin filaments, so we examined the F-actin bundling activity of GhPLIM1 using a low-speed co-sedimentation assay. Preassembled F-actin was incubated with increasing concentrations of GhPLIM1 protein, and centrifuged at low speed at 4 °C. Like the high-speed co-sedimentation assay, the pellet and supernatant fractions were also detected with SDS-PAGE. Little GhPLIM1 protein was detected in the pellet when GhPLIM1 protein was centrifuged alone (Fig. 6). Furthermore, most F-actins were observed in the supernatant without the addition of GhPLIM1. However, with increasing concentrations of GhPLIM1, the amount of F-actin in the pellet fraction increased in proportion. These data demonstrate that GhPLIM1 proteins could bundle actin filaments in vitro.
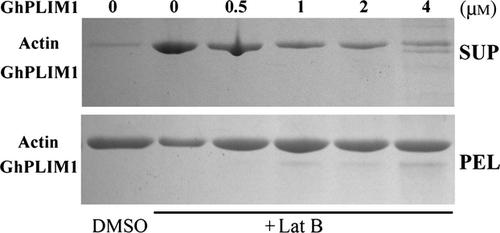
The GhPLIM1 protein stabilises F-actin in vitro
It was reported that plant LIM proteins (such as tobacco WLIM1 and lily LlLIM1) play a role in regulation of F-actin stabilisation (Thomas et al. 2006; Wang et al. 2008). To determine whether GhPLIM1 stabilises actin filaments, we used Lat B to test the effect using methods described earlier (Wang et al. 2008; Zhao et al. 2011). Preformed F-actin was polymerised alone or in the presence varying concentrations of recombinant GhPLIM1 and subsequently treated with Lat B, using DMSO as a negative control. Samples were centrifuged at 200,000 g, and then the supernatants and pellets analysed separately with SDS-PAGE. Little actin was detected in the supernatant without Lat B treatment (lane 1; Fig. 7). In contrast, the addition of Lat B increased the amount of actin in the supernatant. When increasing GhPLIM1 protein was incubated with actin, even when treated with Lat B, the amount of F-actin in the supernatant also gradually decreased (lane 3–7), indicating that GhPLIM1 protein can protect F-actin against depolymerisation by Lat B.
Discussion
Members of the LIM family have been identified in wide range of plant species. LIM proteins share common elements, including two LIM domains; the first LIM domain showed a conserved sequence similar to the animal LIM sequence (Schmeichel & Beckerle 1997), but the second LIM sequence changed from C-X2-CX17-C-X2-H to C-X4-C-X15-C-X2-H (Wang et al. 2008). In this study, we cloned a gene (cDNA) encoding a LIM domain-containing protein (GhPLIM1) from cotton. Quantitative RT-PCR analysis revealed that this gene is specifically expressed in cotton anthers. Because actin dynamics are important for pollen development and germination (including pollen tube growth), and most LIM proteins previously reported contribute to actin cytoskeleton architecture (Thomas et al. 2006; Wang et al. 2008; Papuga et al. 2010), GhPLIM1 might participate in pollen development and germination via mediating actin dynamics and remodelling.
Pollen development is a highly programmed process, in which many genes are involved. Some LIM proteins have been demonstrated to play essential roles in pollen development and pollen tube growth, e.g. lily LlLIM1 is preferentially expressed in pollen and pollen tubes, likely contributes to actin bundle formation and maintenance, and protects F-actin against depolymerisation in pollen and the elongating pollen tube. Overexpression of LILIM1 significantly disturbs pollen tube growth and morphology, with multiple tubes protruding from one pollen grain (Wang et al. 2008). Tobacco WLIM1 protein, located in cell nucleus, functions as a novel ABP to promote the assembly of rigid F-actin bundles, control actin dynamics and protect F-actin bundles against depolymerisation. Further study showed it can function as a transcription factor, regulating the expression of genes involved in lignin biosynthesis (Thomas et al. 2007). In addition, NtWLIM2 contributes to cell proliferation and cell cycle progression by binding to the octameric cis-elements (Oct) and activates expression of the basal histone gene (Moes et al. 2013). It was found that Arabidopsis LIM proteins are vital to bundle actin filaments and protect the actin cytoskeleton against disassembly (Papuga et al. 2010). Sunflower HaPLIM1 is detected both in small cytoplasmic structures of the microspores and in the cortical region of mature pollen grains, where it concentrates in the actin-enriched germination cones, suggesting its interaction with the actin cytoskeleton (Baltz et al. 1999). Similarly, the data presented in the present study revealed that GhPLIM1 transcripts are predominantly/specifically accumulated in cotton anthers. It is possible that GhPLIM1 is involved in controlling pollen-specific processes, such as male gamete maturation, pollen tube formation or even fertilisation. This should be further elucidated in future studies.
The LIM domain-containing proteins retain the dual localisation and participate in cytoplasmic as well as nuclear actions in many cell types. Most LIM proteins in the cytoplasm act as developmental regulators in basic cellular processes, but their roles in the cell nucleus are unclear. Recently, biochemical analysis revealed that cotton WLIM1a has both cytoplasmic and nuclear localisation, and exerts its dual functions in the developing fibre cells (Han et al. 2013). Moreover, NtWLIM2 has dual functions in actin bundling and transcriptional activation of the histone gene, and shuttles to the nucleus in response to cytoskeletal remodelling (Moes et al. 2013). In this study, microscopic observation indicated that eGFP-tagged GhPLIM1 protein is localised to the actin cytoskeleton in the cytoplasm and the nucleus. Further experimental data demonstrated that GhPLIM1 functions in bundling and stabilising F-actin. These results suggest that GhPLIM1 may be involved in regulating actin dynamics required for pollen development of cotton.
Acknowledgement
This work was supported by the National Natural Sciences Foundation of China (Grant No. 31070281) and a project from the Ministry of Agriculture of China for transgenic research (Grant No. 2014ZX08009-027B, 2014ZX08009-003-004).