Adrenal insufficiency in pediatric kidney transplantation recipients
Abstract
Background
Immunosuppression of pediatric kidney transplant (PKT) recipients often includes corticosteroids. Prolonged corticosteroid exposure has been associated with secondary adrenal insufficiency (AI); however, little is known about its impact on PKT recipients.
Methods
This was a retrospective cohort review of PKT recipients to evaluate AI prevalence, risk factors, and adverse effects. AI risk was assessed using morning cortisol (MC) and diagnosis confirmed by an ACTH stimulation test. Potential risk factors and adverse effects were tested for associations with MC levels and AI diagnosis.
Results
Fifty-one patients (60.8% male, age 7.4 (IQR 3.8, 13.1) years; 1 patient counted twice for repeat transplant) were included. Patients at risk for AI (MC < 240 nmol/L) underwent definitive ACTH stimulation testing, confirming AI in 13/51 (25.5%) patients. Identified risk factors for AI included current prednisone dosage (p = .001), 6-month prednisone exposure (p = .02), daily prednisone administration (p = .002), and rejection episodes since transplant (p = .001). MC level (2.5 years (IQR 1.1, 5.1) post-transplant) was associated with current prednisone dosage (p < .001), 6-month prednisone exposure (p = .001), daily prednisone administration (p = .006), rejection episodes since transplant (p = .003), greater number of medications (β = −16.3, p < .001), 6-month hospitalization days (β = −3.3, p = .013), creatinine variability (β = −2.4, p = .025), and occurrence of acute kidney injury (β = −70.6, p = .01).
Conclusion
Greater corticosteroid exposure was associated with a lower MC level and confirmatory diagnosis of AI noted with an ACTH stimulation test. Adverse clinical findings with AI included greater medical complexity and kidney function lability. These data support systematic clinical surveillance for AI in PKT recipients treated with corticosteroids.
Abbreviations
-
- ACTH
-
- Adrenocorticotropic hormone
-
- AI
-
- Adrenal insufficiency
-
- AKI
-
- Acute kidney injury
-
- BMI
-
- Body mass index
-
- BP
-
- Blood pressure
-
- BSA
-
- Body surface area
-
- CKD
-
- Chronic kidney disease
-
- CV
-
- Coefficient of variation
-
- eGFR
-
- Estimated glomerular filtration rate
-
- MC
-
- Morning cortisol
-
- PICU
-
- Pediatric intensive care unit
1 INTRODUCTION
Kidney transplantation is the most effective treatment of end-stage kidney failure in children,1 but in many cases prevention and treatment of rejection still rely on corticosteroid therapy.2-5 Prolonged corticosteroid exposure in other chronic disease settings has been associated with the development of secondary adrenal insufficiency (AI),6-9 especially as corticosteroids are weaned or withdrawn.7, 10-12 Important clinical findings of AI include fatigue, hypotension, vomiting, lethargy, hypoglycemia, nausea, weight loss, and anorexia. Symptoms of AI are generally nonspecific, making it difficult to diagnose.6, 12 Furthermore, pediatric kidney transplant (PKT) recipients with AI may be at risk for adrenal crisis, resulting from inadequate cortisol stress response during acute stress events such as infection, surgery, and physical trauma, and can present with hemodynamic instability or shock. Precipitation of adrenal crisis is prevented by administering glucocorticoid stress doses during stressful events.9, 13, 14 The incidence of adrenal crisis in children with AI has been estimated to be 5–10 episodes/100 patient-years.15 AI is a potentially underrecognized issue in pediatric transplant centers, with few routinely screening for AI or no transplant-specific clinical guidelines that recommend it. This may leave undiagnosed patients at risk for adrenal crisis.
There are few studies that comprehensively characterize the prevalence and risk factors associated with AI specifically in PKT recipients.16-18 In two separate reports of pediatric transplant recipients on maintenance corticosteroids, the prevalence of AI varied between 31% (10/32)16 and 85% (11/13),17 with the cohort of kidney and liver transplant recipients having a lower prevalence (31%) of AI compared to the kidney-only transplant cohort (85%). In both cohorts, the risk of AI was associated with the duration of corticosteroid treatment prior to diagnosis.16, 17 A similar association was found in a third cohort of pediatric kidney and liver transplant recipients (n = 26), which found an inverse correlation between methylprednisolone exposure over time and morning cortisol levels.18 However, the relatively small size of these cohorts leaves them underpowered to effectively estimate the prevalence, additional risk factors and adverse outcomes associated with AI.
The potential need for routine AI surveillance has been recommended by Valentin et al., after identifying an AI prevalence of 43% in an adult kidney transplant cohort.9 Since cortisol secretion follows a diurnal pattern with a morning peak at approximately 8 a.m., the morning cortisol (MC) test is commonly used for this purpose.19 Selecting an accurate threshold value for diagnosing AI in children is challenging, considering variability related to sex and pubertal status.20 One review suggests that for children, an MC level of <83 nmol/L is indicative of AI, whereas ≥500 nmol/L rules it out.21 For this reason, confirmation of AI typically requires definitive testing using an adrenocorticotropic hormone (ACTH) stimulation test.22-24
In this study, we hypothesize that AI is relatively common in PKT recipients and is associated with important clinical adverse events. We report on the prevalence of AI in a single-center PKT cohort and explore potential risk factors and adverse effects associated with AI. We also evaluate the utility of an MC threshold of 240 nmol/L, which was implemented to identify risk of AI and indicate the need for confirmatory low-dose ACTH stimulation testing.
2 METHODS
We conducted a retrospective, cross-sectional, single-center cohort study of PKT recipients from a tertiary pediatric transplant center between January 2015 and July 2021. Ethics approval was obtained from the University of British Columbia Research Ethics Board at the BC Children's Hospital (BCCH; REB #H21-00546). The requirement for additional informed consent for the current study was waived.
2.1 Study population
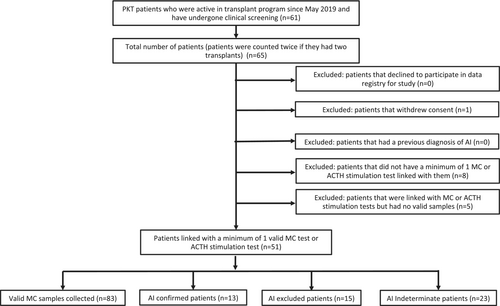
Children and adolescents younger than 21 years at the time of kidney transplantation who completed at least one valid MC test or a low-dose ACTH stimulation test during the study period were eligible for inclusion (Figure 1). Patients with a prior diagnosis of AI were excluded, as were patients who declined to participate or withdrew consent.
Standard immunosuppression for transplant recipients included IV basiliximab and methylprednisolone induction (300 mg/m2 for 2 doses), and initial maintenance immunosuppression with prednisone, tacrolimus, and mycophenolate mofetil. Prednisone was tapered from an initial daily dose of 60 mg/m2 over 4–6 months to a final dose of 3 mg/m2 on alternating days (Monday, Wednesday, and Friday). Patients on alternating day prednisone were not given hydrocortisone supplementation. Rejection episodes were treated at the discretion of the treating nephrologist with high dose IV methylprednisolone (300 mg/m2/dose) for three doses, followed by an oral prednisone taper over 2 weeks to a final daily dose of 3–5 mg/m2. The higher final dose of prednisone within this range was sometimes used for treatment of chronic inflammation.
2.2 Clinical protocol for adrenal insufficiency screening
In May 2015, AI screening was implemented as part of routine clinical monitoring at the transplant center. Due to practical limitations related to obtaining outpatient laboratory investigations before 8:00 a.m., a pragmatic approach was taken to MC testing where results taken before 9:00 a.m. were considered valid and results obtained after 9:00 a.m. were considered invalid for assessing AI risk. MC was recommended in all PKT recipients at least once with their annual review, and subsequent testing was obtained in patients considered “at-risk”: (1) treated with corticosteroids for rejection in the last 12 months or (2) a previous annual MC level was <400 nmol/L. New transplant recipients had an initial MC at 6 months and 1-year post-transplant; however, this was only implemented after June 2019.
MC < 240 nmol/L was considered at risk for AI and used to indicate confirmatory testing with a low-dose ACTH stimulation test, which was typically performed within 6 months after the initial MC that indicated AI risk. The low-dose ACTH stimulation test included a baseline cortisol (i.e., MC screening repeated on the morning of the ACTH stimulation test) level followed by a single dose of IV ACTH (1 mcg Cosyntropin). This test was used clinically for our cohort, as it is the gold-standard test for secondary AI.21 Serum cortisol levels were drawn at 20 and 30 min after administering ACTH.22-24 Patients on maintenance prednisone were instructed to withhold the prednisone dose for 48 h prior to the test. A definitive diagnosis of AI was made if the stimulated 20 or 30-min cortisol level was <400 nmol/L, or if the patient had a clinical presentation consistent with adrenal crisis and contemporaneous MC level <400 nmol/L. AI was definitively excluded if either the peak stimulated cortisol level or their MC level was ≥400 nmol/L. Patients were considered indeterminate for AI risk if the MC was ≥240 and <400 nmol/L, and were followed clinically for risk of AI but did not receive a confirmatory ACTH stimulation test. Repeat MC screening (annual) was clinically indicated if considered “at-risk” with a previously indeterminate MC (240–400 nmol/L) or with an interval increase in steroid exposure (e.g., treatment for rejection). In patients with MC, ≥400 nmol/L annual surveillance was stopped after 1 year post-transplant unless risk of AI re-emerged. The AI result (i.e., confirmed, excluded, or indeterminate) was then associated with the patients' most recent transplant prior to the test. There is currently no consensus in the literature for optimal thresholds for MC testing for AI, with recommended values ranging from 34 to 138 nmol/L to include, and from 348 to 470 nmol/L to exclude AI in adults.25-28 Due to this variability in recommended thresholds, the ones used in our study (240 and 400 nmol/L) were set by consensus for our transplant program.
2.3 Baseline transplant and contemporaneous clinical data collection
Our study treated each transplant as a separate case; therefore, if a patient had two transplant instances that each fit the inclusion criteria (i.e., had valid cortisol samples linked to each transplant), they were reported as two separate patients in our cohort. Select clinical characteristics were ascertained at the time of testing or up to 6 months prior and classified as potential risk factors or adverse effects associated with AI. Multiple MC tests per patient were included if there was no diagnosis of AI prior to the transplant and were adjusted for in the analysis with mixed effect models.
Baseline transplant characteristics included age at transplant, sex, race, glomerular (vs. nonglomerular) diagnosis, preemptive transplant, years on dialysis, type of dialysis, and previous history of transplant. Preemptive transplant was defined as never having been on dialysis prior to transplant. Type of dialysis was defined as the method of dialysis used by the patient for >2 weeks prior to transplant. Contemporaneous characteristics included age, number of years since transplant, and height and body mass index (BMI) reported as Z-score adjusted for sex and age.29
2.3.1 Quantifying prior corticosteroid exposure
Corticosteroid exposure-related factors to evaluate its significance for risk prediction of AI included daily prednisone dosage at the time of event, prior 6-month prednisone exposure, prednisone administration schedule (daily versus not daily), number of acute rejection episodes since transplant, and number of months since the last rejection. Prednisone dosage was reported with adjustment for body surface area (mg/m2). Daily prednisone dosage was adjusted for mean daily exposure over 7 days when given on alternate days (Mondays, Wednesdays, and Fridays). Patients not taking prednisone or taking on alternating days were classified as “not daily.” A separate group for patients not taking prednisone was not created, as there was only one such patient within our cohort. The prior 6-month prednisone exposure was calculated using the cumulative prednisone dosage over 183 days and reported as the average daily exposure (mg/m2/day). Acute rejection episodes were defined by treatment with IV methylprednisolone and were therefore a proxy for recent high-dose corticosteroid exposure. If a patient was never treated for rejection, then the time since transplant was used to represent the time since the last episode of high-dose IV methylprednisolone.
2.3.2 Potential adverse effects associated with AI
We screened for potential adverse effects associated with AI as follows: 1) Measures of medical complexity, including current number of regular use medications, number of hospitalizations, hospitalization days, or admissions to the pediatric intensive care unit (PICU) in the previous 6 months; 2) Measures of hemodynamic stability including systolic and diastolic blood pressure; 3) Measures of metabolic control including glucose levels, insulin use, and serum sodium; and 4) Kidney function lability-related effects, including estimated glomerular filtration rate (eGFR) at time of event, serum creatinine lability (coefficient of variation), episodes of acute kidney injury (AKI) defined by >50% creatinine increase from baseline creatinine (AKI50%), and number of AKI-related hospitalizations in the prior 6 months. Hospitalization was defined as admission for >24 h. Number of medications30-34 and the rates of hospitalization35-37 are both indicators of medical complexity and comorbidity. Regular use medications were defined as any medications being taken regularly at the time of event and excluded those being taken as needed. Systolic and diastolic pressures were adjusted for sex, age, and height and reported as percentiles.38 Blood pressure measures were included as they could be indicators of hypotension. The estimated glomerular filtration rate (eGFR) was calculated using the bedside Schwartz equation.39 Coefficient of variation (CV) of creatinine levels during 6 months prior to the event was calculated with the following equation: standard deviation/mean*100. A minimum of 4 serum creatinine levels was prespecified in order to sufficiently calculate creatinine CV in the period prior to each event. AKI50% episodes were defined using the Kidney Disease Improving Global Outcomes (KDIGO) criteria,40 whereby the maximum creatinine level was >1.5 times greater than the minimum creatinine level during the 6 months prior to the event (stage I AKI). Hospital admissions where an AKI episode or PICU admission was recorded in the research database were also identified.
2.4 Data analyses
The objectives of the analyses were to determine the prevalence of AI and identify risk factors and adverse effects potentially associated with AI diagnosis or low MC levels. A secondary objective was to evaluate the utility of a threshold level of 240 nmol/L for MC testing as an indicator of AI risk. Descriptive statistics were summarized as follows: non-normally distributed continuous data were reported as median and interquartile range (IQR), normally distributed continuous data were reported as mean and standard deviation (SD), and categorical data were reported as frequency and proportion (%). The Shapiro–Wilk test was applied to test continuous variables for normality.
All statistical analyses were performed with RStudio Version 4.1.0 for Windows.41 Significance was assigned for p < .05.
2.4.1 Comparisons between patients categorized by AI diagnosis
Only one event per patient was used to classify a diagnosis of AI. If a patient had multiple ACTH stimulation or MC tests linked with them, the first sample that either confirmed or excluded a diagnosis of AI was selected. Patients with an unresolved diagnosis based on a screening MC (240–399 nmol/L) were deemed to be indeterminate. Univariate analysis was used to explore differences in risk factors and adverse effects between the AI-confirmed and AI-excluded groups. Tests for significance included t-tests for continuous variables, and chi-square or Fisher's exact tests for categorical variables, depending on the number of data elements in each category.
2.4.2 Associations with MC level on a continuum of risk
To determine the association of potential risk factors and adverse effects with MC level, linear mixed effects modeling was used to account for multiple MC samples taken over time linked with the same patient. An initial univariate model was used to identify covariates with a trend towards significant association (p < .1) with MC, which were then evaluated in a correlation matrix within each subgroup (i.e., corticosteroid exposure, medical complexity, and kidney function lability) to identify significant interactions.
2.4.3 Comparisons between MC sample groups categorized by AI risk
Risk factor and adverse effect variables that demonstrated a trend toward significance (p < .1) in the univariate linear mixed effects modeling were included in a comparison analysis between MC samples categorized based on AI risk. MC samples that were <240 nmol/L were defined as “High-risk,” and those that were ≥ 240 nmol/L were defined as “Not high-risk.” Logistic mixed effects modeling was used to account for repeat MC samples.
3 RESULTS
3.1 Cohort baseline transplant characteristics
A total of 61 PKT recipients were initially screened for inclusion, of which 51 patients were retained for analysis after exclusion (Figure 1). One patient was counted twice, as they had two transplants that both met the inclusion criteria. Baseline cohort transplant characteristics are summarized in Table 1. This cohort had a median age of 7.4 (IQR 3.8, 13.1) years, 60.8% (31/51) were male, and 45.1% (23/51) were white. End-stage kidney disease was due to a nonglomerular diagnosis in 58.8% (30/51) of patients. There were 23.5% (12/51) preemptive transplants, and the remainder (76.5% (39/51)) received dialysis for a median of 1.5 (IQR 1.0, 2.0) years. Four patients (7.8%, 4/51) had a previous transplant.
| Baseline transplant characteristics | n=51a |
|---|---|
| Age at transplant (years) | 7.4 (3.8, 13.1) |
| Male sex | 31 (60.8%) |
| Race | |
| White | 23 (45.1%) |
| Asian: Southeast, Far East, India, Philippines | 13 (25.5%) |
| Otherb | 15 (29.4%) |
| Primary diagnosis for kidney failure | |
| Glomerular | 21 (41.2%) |
| Nonglomerular | 30 (58.8%) |
| Preemptive transplant | 12 (23.5%) |
| Years on dialysis prior to transplant (n = 39)c | 1.5 (1.0, 2.0) |
| Type of dialysis (n = 39) | |
| Hemodialysis | 13 (33.3%) |
| Peritoneal dialysis | 26 (66.6%) |
| Previous transplant | 4 (7.8%) |
- a One of the 51 patients in the cohort was counted twice, as they had two transplants that both fit the inclusion criteria.
- b Race groups with <5 counts have been grouped as “Other.” Includes black/African American, Middle Eastern/Arabian, American Indian, Alaskan native, Latin American, multiracial, or other.
- c Reporting only on transplants with pre-transplant chronic dialysis. Values were reported as median (interquartile range) for non-normally distributed variables and count (%).
3.2 Prevalence rate of AI in PKT cohort
The prevalence rate of AI was 25.5% (13/51) among all patients and 46.4% (13/28) among only patients who were definitively diagnosed or excluded for AI. There were four cases of AI identified on repeated screening in patients considered “at risk,” three of which had repeat testing indicated by an interval increase in steroid exposure and one case with indeterminate MC on prior screening. The remaining 23 patients (45.1% of cohort) were classified as “indeterminate” with MC between ≥240 and <400 nmol/L and based on the clinical protocol did not have confirmatory testing. Among patients with their most recent MC <240 nmol/L, 54.2% (13/24) were AI confirmed at a median 3.1 (IQR 1.5, 3.8) years post-transplant. Patients that were excluded for AI were tested at 2.0 (IQR 1.2, 6.4) years post-transplant (p = NS vs. years post-transplant for confirmed AI).
3.3 Comparisons of clinical factors based on confirmed AI diagnosis
Comparisons of clinical factors for patients confirmed and excluded for AI are reported in Table 2. The mean MC level for all patients was 237 ± 136 (range 11–628) nmol/L. MC level was lower for AI confirmed (98.5 ± 68.1; range 11–226 nmol/L) than in AI excluded patients (330 ± 146 nmol/L; range 110–628 nmol/L; p < .001). One patient did not have a valid MC level on or in proximity to the date of the ACTH stimulation test for comparison. Overall, the two groups demonstrated no significant differences in baseline transplant and contemporaneous risk characteristics, except for measures of corticosteroid exposure.
| Total number of patients (n = 51) | Definitively tested patients (n = 28) | AI excluded (n = 15) | AI confirmed (n = 13) | p-value | |
|---|---|---|---|---|---|
| Mean morning cortisol level (nmol/L) | 237 ± 136 | 219 ± 163 | 330 ± 146 | 98.5 ± 68.1 | <0.001 |
| (range 11–628) | (range 11–628) | (range 110–628) | (range 11–226) | ||
| Baseline transplant characteristics | |||||
| Age at transplant | 7.4 (3.8, 13.1) | 6.7 (3.0, 14.0) | 6.6 (3.0, 12.7) | 7.2 (3.2, 14.2) | 0.93 |
| Male sex | 31 (60.8%) | 18 (64.3%) | 9 (60.0%) | 9 (69.2%) | 0.71 |
| Race | 0.53 | ||||
| White | 23 (45.1%) | 15 (53.6%) | 9 (60.0%) | 6 (46.2%) | |
| Asiana | 13 (25.5%) | 6 (21.4%) | 2 (13.3%) | 4 (30.8%) | |
| Otherb | 15 (29.4%) | 7 (25.0%) | 4 (26.7%) | 3 (23.1%) | |
| Glomerular diagnosis | 21 (41.2%) | 8 (28.6%) | 4 (26.7%) | 4 (30.8%) | 1 |
| Preemptive transplant | 12 (23.5%) | 7 (25.0%) | 4 (26.7%) | 3 (23.1%) | 1 |
| Years on dialysis (n = 39 non-preemptive)c | 1.5 (1.0, 2.0) | 1.8 (1.0, 2.4) | 2.0 (1.2, 2.7) | 1.6 (1.0, 2.0) | 0.20 |
| Previous transplant | 4 (7.8%) | 3 (10.7%) | 1 (6.7%) | 2 (15.4%) | 0.58 |
| Contemporaneous characteristics | |||||
| Age (years) | 12.8 (8.4, 16.3) | 12.7 (7.9, 16.8) | 12.5 (7.6, 16.0) | 12.8 (8.3, 17.5) | 0.82 |
| Years since transplant | 2.1 (1.4, 5.9) | 2.4 (1.4, 5.6) | 2.0 (1.2, 6.4) | 3.1 (1.5, 3.8) | 0.84 |
| Height Z-score | −1.2 ± 1.4 | −1.4 ± 1.4 | −1.0 ± 1.5 | −1.7 ± 1.3 | 0.17 |
| BMI Z-score | 0.93 (−0.5, 1.5) | 0.98 (−0.5, 1.6) | 0.97 (−1.2, 1.4) | 0.99 (0.1, 1.7) | 0.21 |
| Corticosteroid exposure | |||||
| Current pred dosage (mg/m2) | 3.1 (1.4, 4.2) | 3.3 (1.4, 5.1) | 1.5 (1.1, 3.1) | 4.3 (3.4, 6.3) | 0.001 |
| Prior 6-month pred dosage (mg/m2) | 3.1 (1.5, 4.4) | 3.4 (1.8, 5.7) | 2.0 (1.1, 3.4) | 4.3 (4.0, 6.3) | 0.02 |
| Daily (vs. not dailyd) pred administration | 32 (62.7%) | 17 (60.7%) | 5 (33.3%) | 12 (92.3%) | 0.002 |
| Rejections since transplant | 1 (0, 2) | 1 (0, 2) | 0 (0, 1) | 2 (1, 4) | 0.001 |
| Months since last rejection | 15.7 (6.6, 33.1) | 16.6 (7.7, 33.0) | 17.4 (7.1, 45.2) | 15.7 (11.9, 22.9) | 0.15 |
- Note: All variables were measured at time of AI diagnosis or during 6 months prior unless otherwise stated. Values were reported as median (interquartile range) for non-normally distributed variables, mean ± standard deviation for normally distributed variables, or count (%). Significant results were bolded. Abbreviations: AI, Adrenal Insufficiency; BMI, Body mass index.
- a Including southeast and far east Asia, India, and the Philippines.
- b Race groups with <5 counts have been grouped as “Other.” Includes black/African American, Middle Eastern/Arabian, American Indian, Alaskan native, Latin American, multiracial, or other.
- c 39 transplants were done non-preemptively.
- d Patients on “not daily” administration schedule had three times a week (MWF) administration, except for one patient who was not on prednisone at the time of AI diagnosis. p-values were calculated using t-tests for continuous variables, and chi-square or Fisher's exact tests for categorical variables, depending on the number of data elements in each category.
3.3.1 AI confirmed patients had greater corticosteroid exposure than AI excluded patients
Corticosteroid exposure was greater in AI confirmed patients than excluded patients across several measures (Table 2). One patient was not on prednisone at the time of definitive testing. There were significant differences in the following measures: (1) current prednisone dosage at the time of event (4.3 (IQR 3.4, 6.3) mg/m2 vs. 1.5 (IQR 1.1, 3.1) mg/m2, p = .001); (2) mean prednisone dosage during 6 months prior to event (4.3 (IQR 4.0, 6.3) mg/m2/day vs. 2.0 (IQR 1.1, 3.4) mg/m2/day, p = .02); (3) daily (vs. not daily) prednisone administration (12 (92.3%) vs. 5 (33.3%), p = .002); and (4) number of rejection episodes since transplant (2 (IQR 1, 4) vs. 0 (IQR 0, 1), p = .001). No difference in time since the last rejection episode was identified (p = .15).
3.4 Associations of potential risk factors with MC level
Univariate linear mixed effects modeling of risk factors potentially associated with MC level is summarized in Table 3. Each model was adjusted for repeated measures. A total of 83 valid MC samples (mean 1.6 ± 0.9 samples per patient) were evaluated at a median age of 11.8 (IQR 7.3, 15.7) years and 2.5 (IQR 1.1, 5.1) years post-transplant. The mean MC level for all samples was 246.1 ± 121.3 nmol/L. There were no significant associations found with MC level for baseline transplant or contemporaneous risk characteristics, other than measures of corticosteroid exposure.
| Total (n = 83) | β | 95% CI | R2 marginal | p-value | |
|---|---|---|---|---|---|
| Baseline transplant characteristics | |||||
| Age at transplant | 7.2 (3.3, 11.9) | 4.1 | [−2.3, 10.6] | 0.024 | .20 |
| Male sex | 51 (61.4%) | 13.5 | [−47.7, 74.6] | 0.003 | .66 |
| Race | |||||
| White | 38 (45.8%) | 6.9 | [−53.2, 66.9] | <0.001 | .82 |
| Asiana | 19 (22.9%) | 9.0 | [−61.8, 79.9] | <0.001 | .80 |
| Otherb | 26 (31.3%) | −15.7 | [−80.2, 48.7] | 0.004 | .63 |
| Glomerular diagnosis | 30 (36.1%) | 8.6 | [−53.0, 70.2] | 0.001 | .78 |
| Preemptive transplant | 19 (22.9%) | −28.0 | [−98.6, 42.5] | 0.009 | .43 |
| Years on dialysis (n = 64 non-preemptive)c | 1.7 (1.0, 2.2) | 0.06 | [−0.02, 0.13] | 0.04 | .14 |
| Previous transplant | 2 (2.4%) | 62.0 | [−112, 236] | 0.006 | .48 |
| Contemporaneous characteristics | |||||
| Age at event (years) | 11.8 (7.3, 15.7) | 1.1 | [−5.5, 7.6] | 0.001 | .75 |
| Years since transplant | 2.5 (1.1, 5.1) | −6.2 | [−15.1, 2.7] | 0.03 | .17 |
| Height (cm) Z-score | −0.60 (1.87, 0.20) | 9.3 | [−11.3, 29.8] | 0.01 | .37 |
| BMI Z-score | 0.76 (−0.59, 1.33) | −3.0 | [−24.8, 18.8] | 0.001 | .78 |
| Corticosteroid exposure | |||||
| Current prednisone dosage (mg/m2) | 2.6 (1.3, 4.1) | −16.8 | [−25.5, −8.1] | 0.15 | <.001 |
| Prior 6-month prednisone dosage (mg/m2) | 2.4 (1.5, 4.3) | −22.6 | [−35.7, −9.5] | 0.14 | .001 |
| Daily (vs. not dailyd) prednisone administration | 45 (54.2%) | −75.0 | [−127.8, −22.3] | 0.09 | .006 |
| Rejections since transplant | 1 (0, 1) | −54.7 | [−82.9, −26.6] | 0.18 | .003 |
| Months since last rejection | 15.1 (6.9, 38.1) | 0.3 | [−0.45, 1.07] | 0.009 | .42 |
- Note: All variables were measured at time of MC testing or during 6 months prior unless otherwise stated. Values reported as median (interquartile range) for non-normally distributed variables, mean ± standard deviation for normally distributed variables, or count (%). Significant results were bolded. Abbreviations: BMI, Body mass index; MC, Morning cortisol.
- a Including southeast and far east Asia, India, and the Philippines.
- b Race groups with <5 counts have been grouped as “Other.” Includes black/African American, Middle Eastern/Arabian, American Indian, Alaskan native, Latin American, multiracial, or other.
- c 64 samples were taken from patients who were transplanted non-preemptively.
- d Samples on “not daily” administration schedule were taken from patients who had three times a week (MWF) administration, except for one sample taken from a patient who was not on prednisone at the time of MC testing. Linear mixed effects modeling adjusted for repeated measures was conducted to report results.
3.4.1 Increased corticosteroid exposure is associated with low MC level
Increased corticosteroid exposure was associated with lower MC levels across several measures (Table 3, Figure 2): 1) current prednisone dosage (β = −16.8; CI = −25.5, −8.1; p < .001); 2) mean prednisone dosage in the 6 months prior (β = −22.6; CI = −35.7, −9.5; p = .001); 3) daily (vs. not daily) prednisone administration (β = −75.0; CI = −127.8, −22.3; p = .006); and 4) number of rejection episodes since transplant (β = −54.7; CI = −82.9, −26.6; p = .003). These measures were highly intercorrelated (all p ≤ .001; Table S1). Time since the last rejection episode was not significantly associated with a low MC level (p = .42).
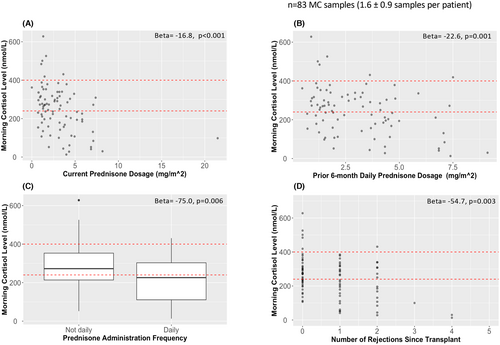
3.4.2 Excess corticosteroid exposure is associated with high risk for AI
MC samples were categorized as “high-risk” (<240 nmol/L) and “not high-risk” (≥240 nmol/L) for evaluation according to the clinical decision-making framework used. A similar number of samples fell into the high-risk (n = 39; 47%) and not high-risk (n = 44; 53%) categories. The primary comparison was made between the high- and not high-risk groups for the four corticosteroid exposure measures identified (Table 4). Greater corticosteroid exposure was identified in the high-risk group for 3 of the 4 measures: (1) current prednisone dosage (β = 0.44; CI = 0.12, 0.76; p = .008); (2) mean prednisone dosage in the 6 months prior (β = 0.36; CI = 0.065, 0.66; p = .017); (3) number of rejection episodes since transplant (β = 0.71; CI = 0.70, 0.72; p < .001).
| Corticosteroid exposure | Not high-risk (n = 44) | High-risk (n = 39) | β | 95% CI | R2 marginal | p-value |
|---|---|---|---|---|---|---|
| Current prednisone dosage (mg/m2) | 1.8 (1.3, 3.2) | 3.7 (1.9, 4.1) | 0.44 | [0.12, 0.76] | 0.28 | .008 |
| Prior 6-month prednisone dosage (mg/m2) | 1.8 (1.3, 3.3) | 3.7 (1.9, 4.6) | 0.36 | [0.065, 0.66] | 0.12 | .017 |
| Daily (vs. not dailya) prednisone administration | 21 (47.7%) | 24 (61.5%) | 0.69 | [−0.36, 1.73] | 0.030 | .20 |
| Rejections since transplant | 0 (0, 1) | 1 (0, 2) | 0.71 | [0.70, 0.72] | 0.10 | <.001 |
- Note: All variables were measured at time of MC or during 6 months prior unless otherwise stated. Values reported as median (interquartile range) for non-normally distributed variables or count (%). Significant results were bolded. Abbreviation: MC, Morning cortisol.
- a Samples on “not daily” administration schedule were taken from patients who had three times a week (MWF) administration, except for one sample taken from a patient who was not on prednisone at the time of MC testing. Logistic mixed effects modeling adjusted for repeated measures was conducted to report results. MC samples with <240 nmol/L were defined as high-risk, ≥240 nmol/L as not high-risk. Only variables that demonstrated a trend towards significance (p < .1) in the univariate modeling with MC level were included.
3.5 Comparisons of potential adverse effects based on AI diagnosis
Comparisons of potential adverse complications of AI related to medical complexity, hemodynamic stability, metabolic control, and kidney function lability between AI confirmed and excluded patients are reported in Table 5. No significant differences were identified.
| Total number of patients (n = 51) | Definitively tested patients (n = 28) | AI excluded (n = 15) | AI confirmed (n = 13) | p-value | |
|---|---|---|---|---|---|
| Medical complexity | |||||
| Number of medications | 8.0 (6.0, 10.0) | 9.0 (6.0, 10.3) | 6.0 (5.5, 10.0) | 10.0 (7.0, 12.0) | 0.15 |
| Prior 6-month hospitalizations | 0 (0, 1) | 0 (0, 1) | 0 (0, 1) | 0 (0, 1) | 0.47 |
| Prior 6-month hospitalization days | 0.0 (0.0, 3.0) | 0.0 (0.0, 3.3) | 0.0 (0.0, 3.0) | 0.0 (0.0, 7.0) | 0.94 |
| Prior 6-month PICU hospitalizationsa | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0.34 |
| Hemodynamic | |||||
| Systolic BP percentile | 0.68 (0.43, 0.89) | 0.68 (0.41, 0.88) | 0.57 (0.36, 0.79) | 0.71 (0.55, 0.93) | 0.24 |
| Diastolic BP percentile | 0.78 (0.50, 0.91) | 0.79 (0.52, 0.90) | 0.77 (0.48, 0.80) | 0.89 (0.52, 0.96) | 0.43 |
| Metabolic | |||||
| Serum glucose (mmol/L) | 5.0 (4.8, 5.7) | 5.0 (4.7, 5.8) | 5.1 (4.8, 5.9) | 4.9 (4.7, 5.2) | 0.17 |
| On Insulin | 4 (7.8%) | 4 (14.3%) | 2 (13.3%) | 2 (15.4%) | 1.0 |
| Serum sodium (mmol/L) | 138 (136, 140) | 138 (136, 139) | 138 (137, 140) | 138 (136, 139) | 0.95 |
| Kidney function lability | |||||
| eGFR (mL/min/1.73 m2)b | 62.8 (44.8, 83.7) | 61.6 (37.2, 73.2) | 65.1 (55.5, 84.6) | 41.0 (35.8, 63.3) | 0.09 |
| Prior 6-month CV of creatinine | 10.6 (8.7, 13.7) | 10.4 (9.5, 12.6) | 10.3 (9.4, 11.4) | 10.5 (9.6, 15.7) | 0.62 |
| Prior 6-month AKI50%c | 15 (29.4%) | 8 (28.6%) | 4 (26.7%) | 4 (30.8%) | 1.0 |
| Prior 6-month AKI hospitalizations | 0 (0, 1) | 0.0 (0.0, 0.25) | 0 (0, 0) | 0 (0, 1) | 0.84 |
- Note: All variables were measured at time of AI diagnosis or during 6 months prior unless otherwise stated. Values reported as median (interquartile range) for non-normally distributed variables, mean ± standard deviation for normally distributed variables, or count (%). Significant results were bolded.
- Abbreviations: AI, Adrenal Insufficiency; AKI, Acute kidney injury; BP, Blood pressure; CV, coefficient of variation; eGFR, Estimated glomerular filtration; PICU, Pediatric Intensive Care Unit.
- a Hospitalizations with admission to pediatric intensive care unit (PICU).
- b eGFR calculated using the bedside Schwartz equation.
- c Experienced an AKI50% during 6 months prior to event, which was defined as having a 50% increase in creatinine from baseline level. p-values were calculated using t-tests for continuous variables, and chi-square or Fisher's exact tests for categorical variables, depending on the number of data elements in each category.
3.6 Associations of potential adverse effects with MC level
Adverse clinical events that may be associated with AI were tested for associations with MC level using linear mixed effects modeling adjusted for repeated measures (Table 6).
| Total (n = 83) | β | 95% CI | R2 marginal | p-value | |
|---|---|---|---|---|---|
| Medical complexity | |||||
| Number of medications | 7 (6, 9) | −16.3 | [−25.6, −7.0] | 0.14 | <.001 |
| Prior 6-month hospitalizations | 0 (0, 1) | −15.5 | [−36.5, 5.5] | 0.03 | .15 |
| Prior 6-month hospitalization days | 0 (0, 3.5) | −3.3 | [−5.9, −0.7] | 0.08 | .013 |
| Prior 6-month PICU hospitalizationsa | 0 (0, 0) | −121.8 | [−255.9, 12.4] | 0.04 | .075 |
| Hemodynamic | |||||
| Systolic BP percentile | 0.68 (0.44, 0.89) | 22.3 | [−72.1, 116.7] | 0.003 | .64 |
| Diastolic BP percentile | 0.74 (0.55, 0.90) | −5.2 | [−117.6, 107.3] | <0.001 | .93 |
| Metabolic | |||||
| Serum glucose (mmol/L) | 5.0 (4.7, 5.5) | 6.3 | [−19.4, 32.1] | 0.003 | .63 |
| On Insulin | 3 (3.6%) | −83.0 | [−231.0, 65.0] | 0.02 | .27 |
| Serum sodium (mmol/L) | 138.9 ± 2.6 | 0.95 | [−9.5, 11.4] | <0.001 | .86 |
| Kidney function lability | |||||
| eGFR (mL/min/1.73 m2)b | 67.1 ± 26.8 | 0.91 | [−0.1, 1.9] | 0.04 | .080 |
| Prior 6-month CV of creatinine | 9.9 (6.7, 14.4) | −2.4 | [−4.4, −0.3] | 0.06 | .025 |
| Prior 6-month AKI50%c | 28 (33.7%) | −70.6 | [−123.9, −17.2] | 0.08 | .010 |
| Prior 6-month AKI hospitalizations | 0 (0, 1) | −16.2 | [−41.2, 8.8] | 0.02 | .20 |
- Note: All variables were measured at time of MC or during 6 months prior unless otherwise stated. Values reported as median (interquartile range) for non-normally distributed variables, mean ± standard deviation for normally distributed variables, or count (%). Significant results were bolded. Abbreviations: AKI, Acute kidney injury; BP, Blood pressure; CV, Coefficient of variation; eGFR, Estimated glomerular filtration rate; MC, Morning cortisol; PICU, Pediatric Intensive Care Unit.
- a Hospitalizations with admission to pediatric intensive care unit (PICU).
- b eGFR calculated using the bedside Schwartz equation.
- c Experienced an AKI50% during 6 months prior to MC, which was defined as having a 50% increase in creatinine from baseline level. Linear mixed effects modeling adjusted for repeated measures was conducted to report results.
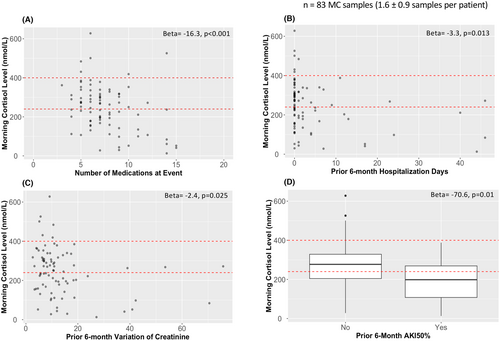
3.6.1 Medical complexity is associated with AI risk
The number of medications (β = −16.3; CI = −25.6, −7.0; p < .001) and the number of prior 6-month hospitalization days (β = −3.3; CI = −5.9, −0.7; p = .013) were associated with lower MC level (Table 6 and Figure 3). Number of medications was correlated with hospitalization days (rho = 0.34, p = .002), but not with PICU hospitalizations (Table S2). A significant association was also identified between the high-risk group (Table 7) and greater number of medications (β = 0.28; CI = 0.062, 0.50; p = .012).
| Not high-risk (n = 44) | High-risk (n = 39) | β | 95% CI | R2 marginal | p-value | |
|---|---|---|---|---|---|---|
| Medical complexity | ||||||
| Number of medications | 6 (5, 8) | 8 (6, 10.5) | 0.28 | [0.062, 0.50] | 0.14 | .012 |
| Prior 6-month hospitalization days | 0 (0, 1) | 1 (0, 5.5) | 0.045 | [−0.010, 0.10] | 0.055 | .11 |
| Prior 6-month PICU hospitalizationsa | 0 (0, 0) | 0 (0, 0) | 0.84 | [−1.80, 3.48] | 0.007 | .53 |
| Kidney function lability | ||||||
| eGFR (mL/min/1.73 m2)b | 71.1 ± 24.7 | 62.6 ± 28.7 | −0.013 | [−0.032, 0.006] | 0.033 | .17 |
| Prior 6-month CV of creatinine | 9.3 (6.7, 11.2) | 11.6 (7.3, 16.0) | 0.015 | [−0.022, 0.053] | 0.011 | .42 |
| Prior 6-month AKI50%c | 10 (22.7%) | 18 (46.2%) | 1.08 | [0.075, 2.09] | 0.069 | .035 |
- Note: All variables were measured at time of MC or during 6 months prior unless otherwise stated. Values reported as median (interquartile range) for non-normally distributed variables or count (%). Significant results were bolded. Abbreviations: AKI, Acute kidney injury; CV, Coefficient of variation; eGFR, Estimated glomerular filtration rate; MC, Morning cortisol; PICU, Pediatric Intensive Care Unit.
- a Hospitalizations with admission to pediatric intensive care unit (PICU).
- b eGFR calculated using the bedside Schwartz equation.
- c Experienced an AKI50% during 6 months prior to MC, which was defined as having 50% increase in creatinine from baseline level. Logistic mixed effects modeling adjusted for repeated measures was conducted to report results. MC samples with <240 nmol/L were defined as high-risk, ≥240 nmol/L as not high-risk. Only variables that demonstrated a trend towards significance (p < .1) in the univariate modeling with MC levels was included.
3.6.2 Greater kidney function lability is associated with AI risk
Serum creatinine levels (median 11 (7.5, 15) creatinine samples per MC sample) were used to calculate the coefficient of variation (CV) in the 6 months prior to each event. The 6-month serum creatinine CV (β = −2.4; CI = −4.4, −0.3; p = .025) and occurrence (vs. not) of an AKI50% episode (β = −70.6; CI = −123.9, −17.2; p = .01) were significantly associated with lower MC level (Table 6 and Figure 3). A significant association was also identified between the high-risk group (Table 7) and occurrence of AKI50% (β = 1.08; CI = 0.075, 2.09; p = .035). Prior 6-month serum creatinine CV and AKI50% were highly correlated (p < .001) (Table S3), but neither was correlated with eGFR.
Prior 6-month AKI50% exhibited correlation (p ≤ .01) with both measures of medical complexity (number of medications: r = 0.29, prior 6-month hospitalization days: r = 0.52). We conducted post-hoc bivariate regressions to explore their independence relative to MC level. The model including 6-month AKI and medication number was indeterminate (Table S4), with neither exhibiting independent association; however, the model with 6-month AKI (β = −82.9; CI = −144.9, −20.9; p = .009) and 6-month hospitalization days (β = −30.9; CI = −49.6, −12.2; p = .001) confirmed that each was independently associated with MC level (Table S5).
4 DISCUSSION
This is the largest study to date that reports on the prevalence, risk factors, and adverse effects of secondary AI in a PKT cohort. We found a high prevalence of AI (25.5%), which was greater (46.4%) when only definitively tested patients were considered. We identified that excess corticosteroid exposure was the primary risk factor for AI and medical complexity (i.e., medication burden and hospitalization days) and greater kidney function lability (i.e., serum creatinine lability and occurrence of AKI50%) were significantly associated with AI in this population.
The prevalence rate in this report is similar to the 31% prevalence reported by Bilavsky et al. but lower than the 85% reported by Revermann et al.16, 17 Bilavsky et al. included both pediatric liver and kidney transplant recipients, whereas Revermann et al. included only PKT recipients, similar to our study. Bilavsky et al. evaluated AI risk with a standard 250 ug ACTH stimulation test and used a higher stimulated cortisol threshold (<550 nmol/L) to diagnose AI, which would be expected to overestimate AI risk, compared with this cohort. The timing of AI testing post-transplant was not readily discernible in these two studies,16, 17 and the lower prevalence in our study may be due to screening for AI a relatively longer time post-transplant, leading to lesser ongoing steroid exposure in this cohort.
Similar to these two studies,16, 17 we identified corticosteroid exposure as the only significant risk factor for AI, as current prednisone dosage, prior 6-month average daily dosage of prednisone, daily administration of prednisone, and number of rejection episodes since transplant were all associated with AI. Bilavsky et al. only confirmed an association with prior 6-month dosage despite exploring multiple corticosteroid exposure-related variables and did not evaluate the effect of the current prednisone dose. Additionally, while our analysis found that most AI-confirmed patients had daily prednisone administration (12/13), Bilavsky et al. found that only 3/10 patients diagnosed with AI were on daily treatment; however, these contrasts may be due to their study lacking sufficient power to show weaker associations with other corticosteroid exposure-related measures they tested.16 In concordance with our findings, Tomkins et al. concluded that adult kidney transplant recipients with AI had a significantly greater daily prednisone dose and cumulative glucocorticoid exposure (including pretransplant, perioperative, and post-transplant exposure) compared to those without AI.42 Furthermore, multiple adult population studies have also reported corticosteroid exposure as the only risk factor for AI.9, 43, 44
This study also identified an association between corticosteroid exposure and MC level, which was not identified by Revermann et al.17 This stands in contrast to Sarna et al., where an association was identified between low MC level and corticosteroid exposure over time (area under serum methylprednisone time vs. concentration curve).18 They did not, however, identify an association with current methylprednisone dosage, whereas our analysis identified several measures of corticosteroid exposure associated with a lower MC level including current prednisone dosage, prior 6-month prednisone dosage, daily administration of prednisone, and number of rejection episodes, all of which were highly correlated with each other. It is possible that our larger sample size allowed us to detect significant associations, but the common finding is that a prolonged recent corticosteroid exposure (i.e., over 6 months) appears to be the consistent risk factor associated with risk of AI among multiple studies,16, 18, 42 and may be a preferred metric for risk evaluation and future research.
While our study did not focus on adrenal function recovery after corticosteroid exposure, the time since last rejection was not associated with AI nor low MC levels. This could indicate that recovery of adrenal function was not associated with how much time has passed after a steroid pulse; however, our results may be limited by the range of available follow-up data and by the fact that after treatment of rejection, most patients remained on daily prednisone. Nonetheless, this is concordant with Laulhé et al.'s findings, that delay between arrest of steroid treatment and time of ACTH stimulation testing did not predict adrenal function recovery.45
As proxies for medical complexity, number of medications30-34 and rates of hospitalization35-37 were both associated with a greater AI risk as reflected by lower MC level. Although causality cannot be inferred, the most likely explanation is that impaired cortisol responsiveness to stress contributes to a risk for more severe illness and sequelae.46-48 Although this has not been previously reported in PKT recipients, Worth et al. reported that 30% of their cohort (children with AI, n = 127) had a recorded emergency department visit due to acute illness,49 and Eyal et al. reported recurrent hospital admissions were significantly associated with adrenal crisis among children with AI.50 Increased rates of ICU admission, longer length of hospital stays, and higher rates of readmission have also been reported in adults with AI.51
AKI episodes are common after PKT and their frequency is associated with progressive allograft dysfunction and loss.52 This study identified an increased risk of AKI with risk for AI, which is likely related to hemodynamic instability and impaired renal perfusion with episodes of acute illness and adrenal crisis.53 Functional renal impairment is a typical laboratory feature of adrenal crisis.53 There have been multiple case reports demonstrating a diagnosis of AI or adrenal crisis presenting in AKI.54-57 Aside from the AKI, concurrent symptoms experienced by patients reported in these cases included lethargy, general weakness, nausea, poor oral intake, weight loss, light-headedness, and hypotension.54-57 This report is the first to identify association between AI risk and rates of AKI in PKT recipients. Perhaps unsurprisingly, AKI rates in this population are also associated with measures of medical complexity; however, we were able to confirm that the AKI risk as it relates to association with MC levels is independent of hospitalization days that may have occurred in the same period. The increased risk for AKI way be explained by exacerbated allograft hypoperfusion with adrenal insufficiency during otherwise typical intercurrent illnesses. Timely corticosteroid stress dosing in these scenarios, as is typically recommended for AI management, could also potentially mitigate the risk for AKI.
For surveillance of AI risk, the MC level was associated with corticosteroid exposure on a continuum and the lack of a definitive threshold for diagnosing AI is similarly reflected in other reports.25, 27, 58 We evaluated the threshold set for our clinical program at the BCCH (<240 nmol/L) to indicate when confirmatory ACTH stimulation testing would be needed and observed that the majority (54.2%, 13/24) of those with an at-risk MC (<240 nmol/L) were diagnosed with AI, and the highest MC level reported among the AI diagnosed patients was 226 nmol/L, which is close to the 240 nmol/L, suggesting that this threshold is practically useful for AI screening in the transplant clinic. The utility of MC testing as an AI screening tool has been supported by studies in other populations. Tomkins et al. found that MC cutoff values of <130 nmol/L and > 288 nmol/L were optimal to rule in and rule out AI and could reduce the need for ACTH stimulation testing by 44%.42 Manosroi et al. concluded that ≤90 nmol/L and ≥ 380 nmol/L were optimal cutoff values for MC testing in adults and could reduce the need for ACTH stimulation testing by 10% and 30% (true positive and true negative rate, respectively).59 Despite this, the absence of consensus on threshold values and the lack of studies on MC testing utility in PKT recipients indicates need for further research.
Although this is the largest pediatric transplant cohort reported to date, the sample size limited the number of covariates we could reliably include and thereby the complexity of multivariable models for more refined analysis. The MC threshold for confirmatory ACTH stimulation testing was set by clinical consensus in our transplant program after close discussion with the division of endocrinology at BCCH, since there was insufficient prior data available. As a result, we could not fully model thresholds between 240 and 400 nmol/L, since for pragmatic reasons we could not justify clinical testing within this range.
Another potential limitation was that our study included MC samples taken up to 9 a.m. Cortisol secretion reaches morning peak at approximately 8 a.m., and therefore MC testing is usually conducted at this time.19, 20, 26 Due to some individuals having been screened with MC past this 8 a.m. peak, it is possible that our study overtested individuals who had an MC <240 nmol/L with a confirmatory ACTH stimulation test.
5 CONCLUSION
We identified that AI is relatively common in children who continue to receive maintenance corticosteroid dosing after kidney transplant, and the risk is associated with the magnitude of recent corticosteroid exposure. AI is consequential in this population, associated with increased medication complexity, hospitalization risk, and allograft AKI. Although this analysis did not evaluate the utility of stress dosing of corticosteroids during acute illness, it is recommended for the management of AI. These findings highlight the potential need for regular clinical surveillance in PKT recipients who have been treated with corticosteroids.
AUTHOR CONTRIBUTIONS
Hyunwoong Harry Chae, Shazhan Amed, Trisha Patel, and Tom D. Blydt-Hansen: conceptualized the study; Hyunwoong Harry Chae and Tom D. Blydt-Hansen: involved in data collection; Hyunwoong Harry Chae, Azim Ahmed, Jeffrey N. Bone, and Tom D. Blydt-Hansen: formally analyzed the study; Hyunwoong Harry Chae and Tom D. Blydt-Hansen: involved in methodology; Hyunwoong Harry Chae and Tom D. Blydt-Hansen: administered the project; Hyunwoong Harry Chae: wrote and led the manuscript preparation; Hyunwoong Harry Chae, Fatema S. Abdulhussein, Shazhan Amed, Trisha Patel, Tom D. Blydt-Hansen: reviewed and edited the manuscript.
ACKNOWLEDGMENTS
This project was supported in part by the UBC Faculty of Medicine via the Carman Crawford Browne Estate Fund for Medical Research. We thank the kidney transplant recipients and their families whose commitment to participate in research helped make this project possible. Special thanks to Candice Wiedman, Mike Guron, Monica Ho, and Ashleigh Ragutero from the Solid Organ Transplant research team for their support and assistance with data collection and the Clinical Research Support Unit at the BC Children's Hospital.
CONFLICT OF INTEREST STATEMENT
There are no conflicts of interest from all authors involved.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.