Clinical conundrums in pediatric kidney transplantation: What we know about the role of angiotensin II type I receptor antibodies in pediatric kidney transplantation and the path forward
Abstract
Antibodies to angiotensin II type 1 receptor (AT1R-Abs) are among the most well-studied non-HLA antibodies in renal transplantation. These antibodies have been shown to be common in pediatric kidney transplantation and associated with antibody-mediated rejection (AMR), vascular inflammation, development of human leukocyte donor-specific antibodies (HLA DSA), and allograft loss. As AT1R-Ab testing becomes more readily accessible, evidence to guide clinical practice for testing and treating AT1R-Ab positivity in pediatric kidney transplant recipients remains limited. This review discusses the clinical complexities of evaluating AT1R-Abs given the current available evidence.
Abbreviations
-
- ACR
-
- acute cellular rejection
-
- AMR
-
- antibody-mediated rejection
-
- ARB
-
- angiotensin receptor blocker
-
- ATG
-
- anti-thymocyte globulin
-
- AT1R
-
- angiotensin II type 1 receptor
-
- DSA
-
- donor specific antibody
-
- eGFR
-
- estimated glomerular filtration rate
-
- ETAR
-
- endothelin-type A receptor
-
- FSGS
-
- focal segmental glomerulosclerosis
-
- GPCR
-
- G protein-coupled receptor
-
- HLA
-
- human leukocyte antigen
1 INTRODUCTION
The role of non-HLA antibodies in kidney allograft injury has been increasingly recognized. Antibodies to the angiotensin II type 1 receptor (AT1R-Abs) are among the most well-studied non-HLA antibodies in renal transplantation. The AT1R is a that is the primary mediator of the actions of angiotensin II. These include the classical function of raising blood pressure in addition to important pathophysiological roles in cardiovascular remodeling and inflammation.1, 2 AT1R-Abs bind to the second extracellular loop of the AT1R and provoke a sustained activation of the receptor. The tissue-specific downstream signaling following AT1R activation is complex and has been recently reviewed.2 Though AT1R-Abs are from the complement activating subclasses of IgG1 or IgG3, they are typically not associated with complement activation on biopsy.3-6 AT1R-Abs are closely associated with endothelin type A receptor antibodies (ETAR-Abs) in both the adult7, 8 and pediatric9, 10 kidney transplant populations. There is some suggestion that these antibodies may behave synergistically9, 10; however, the nature of this relationship remains an open question.
AT1R-Abs were first described by Dragun et al. in patients with a clinical presentation of antibody-mediated rejection (AMR), thrombosis, and severe hypertension.3 AT1R-Abs have subsequently been associated with AMR and vascular inflammation without hypertension, as well as elevated serum cytokines, development of human leukocyte donor-specific antibodies (HLA DSA), and allograft loss in kidney transplant recipients.6, 11-20 It is notable that these findings are not universal; there have also been studies showing no association between AT1R-Abs (specifically pre-transplant AT1R-Ab) and poor allograft outcomes.21-23
Elevated AT1R-Ab levels are significantly more common in pediatric patients than in adults.17, 24, 25 As awareness regarding non-HLA antibodies rises in the transplant community, more tests for AT1R-Ab are being performed. The interpretation and clinical response to the results, however, remain quite complex, particularly in the pediatric population. In this review, we will briefly highlight some of the complexities in managing an AT1R-Ab-positive pediatric kidney transplant recipient.
2 AT1R-AB TESTING
AT1R-Ab testing in a pediatric kidney transplant recipient is helpful in specific clinical contexts. ETAR-Ab testing is not currently available as a clinical test. As of now, there is not enough evidence to support universal screening of patients for AT1R-Ab as part of the pre-transplant evaluation or routine post-transplant care.26, 27 AT1R-Ab testing may be considered during the pre-transplant evaluation in patients with marked hypertension, history of autoimmunity, significant HLA sensitization, or history of allograft loss from HLA DSA-negative AMR. The test may add value to the overall immunologic risk assessment and peri-operative planning given there is some evidence of synergy between AT1R-Ab and HLA DSA6, 11, 15, 28 and that intervention with angiotensin receptor blockers (ARBs) may be helpful.29 However, there is currently an absence of robust evidence to support this.27
Non-HLA testing is suggested in the context of kidney transplant biopsy findings consistent with AMR when HLA DSA are negative.27, 30 It is notable that this is the only scenario with at least moderate quality evidence to support testing for AT1R-Ab per the Sensitization in Transplantation: Assessment of Risk 2022 Working Group.27 Hypertension in the context of AMR or unexplained arterial changes on biopsy may also be special circumstances that warrant AT1R-Ab testing. Outside of these scenarios, when to test for AT1R-Abs is even less clear.
Testing for AT1R-Abs is now performed most frequently by sandwich ELISA test that uses plates coated with extracts retaining AT1R in its native configuration.31, 32 This method has allowed for wider availability and access to testing. It is important to have the test performed in an experienced immunology laboratory where quality and consistency of reagents are routinely tested. In vitro functional assays were used in the discovery of AT1R-Abs, but were impractical for widespread clinical use.3 A more recent article reported on findings of another functional assay, however, it did not correlate well with the validated ELISA.33 Further, there have also been concerns raised about other molecules interfering with the ELISA,34 though these findings were controversial.35 Thus, testing for these antibodies should be limited to experienced laboratories with demonstrated expertise.
Interpretation of test results also requires expertise and experience. A range between 2.5 and 40 U/mL is generally reported, and 10–17 U/mL is defined as “at risk,” while >17 U/mL is regarded as positive. In the literature, positive cutoffs associated with adverse outcomes range from 9 to 17 U/mL.14 Based on our receiver operating characteristic curve analysis and the known association between younger age and high AT1R-Ab levels, we generally use >17 U/mL as a positive cutoff for our pediatric kidney transplant recipients.17 However, in different contexts, levels as low as 9.5 U/mL may be relevant.19 The standard test is done at 1:100 dilution; however, for patients with levels >40 units/mL, testing at higher dilutions at 1:500 or 1:1000 is helpful to obtain a quantitative result for monitoring the efficacy of immunotherapy.
Future studies should focus on developing standardized reference reagents to validate AT1R-Ab measurements across test platforms and establish their precision, sensitivity and specificity, and diagnostic and prognostic validity.
3 AT1R-AB IN PRE-TRANSPLANT PATIENTS
There are several case reports of significant early rejection in pediatric kidney transplant patients with high levels of pre-transplant AT1R-Ab.36-38 On the other hand, there are studies showing that pre-transplant AT1R-Ab positivity is quite common in pediatric patients, and the majority do not necessarily go on to develop early acute complications.17, 22 A recent meta-analysis provides a full table of studies in adults and pediatric patients on this topic with annotation of studies looking at pre- and post-transplant AT1R-Abs.14 Despite the availability of these studies, the question of how to treat patients with a positive pre-transplant test is a controversial one.
One study in adult transplant recipients compared a preemptive treatment strategy based on pre-transplant screening for AT1R-Ab to historical controls. Patients with positive AT1R-Ab (>17 U/mL) were treated with anti-thymocyte globulin induction and early candesartan. Peri-operative plasmapheresis was added in patients with AT1R-Ab >25 U/mL. This protocol resulted in improvement in rejection and allograft outcomes when compared to historical controls.29 In a patient with previous allograft loss, particularly if AT1R-Ab is the suspected cause, a positive test prior to re-transplant would suggest the benefits of this protocol may outweigh the risks. Desensitization using standard protocols is also a consideration in these cases39; however, data to support this specifically for AT1R-Abs are sparse.
In other pediatric patients with pre-transplant AT1R-Ab positivity, it is reasonable to monitor closely for signs of AMR and consider early introduction of ARB therapy, as this has limited risk. However, there is not yet clinical trial evidence to support the benefits of this approach. The use of more aggressive preemptive treatment protocols as described above may be reasonable depending on the patient's vascular access and overall clinical status. Nevertheless, randomized controlled trials are needed to validate this, as well as alternate approaches.
4 AT1R-AB AND ANTIBODY-MEDIATED REJECTION
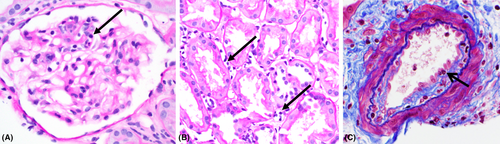
AT1R-Ab-associated AMR appears to have a distinct phenotype consisting of microcirculatory and arterial inflammation, that is, vasculitis, lack of C4d deposition, and increased expression of endothelial activation/injury-associated transcripts.6, 40 In practice, this translates to C4d-negative AMR with a higher likelihood of intimal arteritis (Figure 1). In patients presenting with early active AMR and AT1R-Ab positivity, proceeding with treatment with plasmapheresis, IVIG, and an ARB is warranted.3, 37, 38, 41, 42 It should be noted that AT1R-Abs are also pro-thrombotic,3, 36 so depending on individual risks and benefits in the patient, anticoagulation could be considered. Some cases may be fulminant, and allograft loss has been reported even with aggressive treatment.36 Even in patients who are HLA DSA-positive, if the patient has significant hypertension associated with the rejection episode, testing for AT1R-Ab may be considered.37 If the patient is both HLA DSA- and AT1R-Ab-positive, an ARB may be a helpful addition to the AMR treatment regimen. Use of various AMR treatment protocols in the treatment of non-HLA antibodies in solid organ transplants has been recently reviewed. This review provides a compressive table of the available literature on the treatment of AT1R-Ab-positive AMR.42 Importantly, data in pediatric recipients are as yet lacking.
In patients with late active AMR or chronic active AMR without significant dysfunction, treatment decisions may be less straightforward. Functional changes may be subacute or even subclinical, particularly when vascular inflammation is only identified as part of a protocol biopsy. The decision to place a plasmapheresis catheter in an immunosuppressed child with relatively stable renal function is complex. There is risk of central line infection and injury to precious future vascular access. Considerations in this decision include the size of the child, the severity of the biopsy findings (including both acute vascular inflammation and chronic changes), and the trend in estimated glomerular filtration rate (eGFR). For these reasons, alternative treatment approaches that do not include plasma exchange are urgently needed.
In these more difficult cases, the use of center protocols with or without a course of plasmapheresis for the treatment of AMR may be justified, but evidence to support the best treatment protocols is unavailable.42 Success of treating ongoing AT1R-Ab-positive AMR with increasing the dose of ARB alone has been reported.38 Though the evidence is still limited, tocilizumab, an IL6 signaling inhibitor, has the most robust evidence available for the treatment of AT1R-Ab-associated chronic active AMR. In one series, 13 adult patients with HLA DSA and AT1R-Ab-positive chronic active AMR were treated with tocilizumab. A reduction in microvascular inflammation and AT1R-Ab levels were observed.43 Another case series reported successful use of tocilizumab in the treatment of AT1R-Ab-associated AMR in three adults.44 In a pediatric case series, outcomes of 6 refractory AMR patients with no HLA DSA and positive AT1R-Ab at the time of tocilizumab initiation are reported. Tocilizumab stabilized renal function and reduced peritubular capillaritis and C4d scores in this cohort. It is notable that all six patients received concurrent IVIG and that 3/6 received an ARB.45 It is not clear when, how, and whether to wean or discontinue tocilizumab, and the risks of long-term augmented immunosuppression are not trivial. Patients should be carefully monitored for viral infections, transaminitis, hypogammaglobulinemia, and bowel complications.45
5 AT1R-AB IN POST-TRANSPLANT PATIENTS WITHOUT AMR
There is evidence that AT1R-Abs are associated with elevated inflammatory cytokines and decline in eGFR the first 2 years post-transplantation in pediatric patients.17 This occurs even in patients without rejection. AT1R-Abs have also been associated with recurrence of focal segmental glomerulosclerosis (FSGS).46, 47 Given these are activating autoantibodies, it is possible that additional non-classical mechanisms of antibody-mediated injury are relevant. Treatment in these contexts has not been established; but as noted above, ARBs have been examined in preliminary studies. To make matters more complicated, some patients with AT1R-Abs are normotensive and cannot tolerate ARB treatment.
More research is needed to understand the role of ARBs in the treatment of AT1R-Ab-positive patients without AMR. Further, the role of immunosuppression regimens in modulating outcomes in AT1R-Ab-positive patients is an area in great need of exploration. For example, recent interesting work has shown activation of mTOR signaling in endothelial cells exposed to AT1R and ETAR-Abs.48 However, it is unclear whether mTOR inhibitors would be a benefit to patients in vivo. There is also limited evidence that ATG induction, tacrolimus use, and elevated tacrolimus levels may put patients at risk for developing de novo AT1R-Ab.49 This finding is particularly interesting when put into the context of literature demonstrating upregulation of the AT1R receptor as a mechanism of calcineurin inhibitor toxicity.50, 51 Further study of this interplay is needed. As belatacept-based, tacrolimus-sparing, immunosuppression regimens are examined in children, the impact on AT1R-Ab levels and associated outcomes will be of significant interest.
6 CONCLUSIONS
Despite the wealth of literature published on AT1R-Ab in transplantation over the last 15 years, many questions remain on how to incorporate testing and treatment into clinical care. Moving forward, it is critical to be able to distinguish which patient endotypes are at highest risk for complications associated with AT1R-Abs. Work identifying the role of AT1R allograft expression and signaling, genetic polymorphisms, and concurrent clinical risk factors such as HLA DSA will help identify patients who have the most to benefit from treatment. This is a clinical problem of importance in the pediatric transplant community given our patients are at higher risk of both AT1R-Ab positivity and immunologic complications over the course of their lifetimes.
FUNDING INFORMATION
This work was supported by the National Institute of Allergy and Infectious Diseases Grant 5K23AI139335 (M.H.P); Ruth L. Kirschstein National Research Service Award T32 DK104687 UCLA Translational Research Grant in Pediatric Nephrology Program (M.H.P); the National Kidney Foundation (M.H.P); the American Society of Nephrology (M.H.P); and the Casey Lee Ball Foundation (M.H.P).
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.