Optimizing medication adherence with home-monitoring – A feasibility study using capillary microsampling and mHealth in solid organ-transplanted adolescents
Abstract
Background
Reliable methods to detect and reduce medication nonadherence in solid organ-transplanted (SOT) adolescents are warranted. We aimed to evaluate the feasibility of combining a medication-manager application (TusenTac®-app) with home-sampling of tacrolimus (Tac) in young SOT recipients.
Methods
Kidney and combined SOT recipients between 14 and 25 years were included. During an 8-week intervention period, the participants were instructed to use the transplant-specific, age-adapted TusenTac®-app daily and to perform weekly at-home Tac trough finger-prick microsampling. Microsample Tac concentrations were controlled against timed venous samples twice. Medication implementation and persistence adherence were measured with BAASIS© questionnaires, TusenTac®-registrations, Tac trough concentration coefficient of variation (CV%) and self-reporting by interview. For comparison, venous Tac trough CV% were obtained from the year before and after the short-term intervention.
Results
Twenty-two recipients were included, two withdrawals, leaving 20; median age 17.9 (14.5–24.8) years, 12 females (60%). The participants registered their dosage intake 88% (1502/1703) of the expected times, and 90% (106/118) of the microsamples were obtained correctly. At inclusion, 11 recipients (55%) were nonadherent assessed with BAASIS© questionnaire, four of these (36%) turned adherent during the intervention period. At the end, 70% reported improved timing-adherence at the interview. There was no significant change in TacCV% from the year before to the year after the short-term intervention. Home-sampling was reliable and measured Tac concentrations accurately.
Conclusions
Home-monitoring, combining Tac finger-prick microsampling and a medication-manager app, is feasible in adolescent SOT recipients with 70% perceived improvement in medication timing-adherence. There were no significant long-term changes in TacCV% confirming the need for continuous use and individualized interventions.
Abbreviations
-
- App
-
- application
-
- BAASIS©
-
- Basel Assessment of Adherence to Immunosuppressive Medication Scale
-
- CI
-
- confidence interval
-
- CKD
-
- Chronic kidney disorder
-
- CKD–EPI
-
- Chronic Kidney Disease–Epidemiology Collaboration
-
- CV
-
- coefficient of variation
-
- dnDSA
-
- de novo donor specific antibodies
-
- DSA
-
- donor specific antibodies
-
- eGFR
-
- estimated glomerular filtration rate
-
- eHeatlh
-
- electronic health
-
- ESPACOMP
-
- The International Society for Medication Adherence, former European Society for Patient Adherence, Compliance, and Persistence
-
- GFR
-
- glomerular filtration rate
-
- IPNA
-
- International Pediatric Nephrology Association
-
- mHealth
-
- mobile health
-
- OUS-RH
-
- Oslo University Hospital-Rikshospitalet
-
- PRA
-
- panel reactive antibodies
-
- SOT
-
- solid organ transplanted
-
- Tac
-
- tacrolimus
-
- TSD
-
- services for sensitive data
-
- USIT
-
- Center for Information Technology at University of Oslo
-
- VAMS™
-
- volumetric absorptive microsampling
-
- VAS
-
- Visual Analogue Scale
-
- WHO
-
- World Health Organization
1 INTRODUCTION
During the last decades, there have been important advances in the surgical and medical treatment and post-transplant care of patients in need of solid organ transplantation (SOT) resulting in significantly improved short-term outcomes.1 Still, the 5- and 10-year graft survival rates remain unaltered with the worst long-term SOT graft outcomes for the adolescents and young adults.2-6 Following a transplantation, adherence to life-long immunosuppressive treatment is mandatory to avoid acute rejections, development of de novo donor-specific antibodies (dnDSA) that may induce graft dysfunction and in worst case, that leads to graft loss.7-11 In the literature, the prevalence of medication nonadherence among pediatric and adult SOT patients ranges from 2% to 67%.7, 11-13 The vast prevalence indicates a wide diversity in methods and definitions used in nonadherence assessment. In a recently published large national registry study, we identified medication nonadherence to be the reason for 58% of the graft losses among kidney transplanted adolescents and young adults aged 14–26 years.13 Unfortunately, nonadherence to the medical regimen among adolescents and young adults is too common, and there is an extensive potential for improvement through individualized treatment and self-management. Results from different interventions have been somewhat conflicting.14, 15 To optimize clinical outcomes multimodal interventions have been suggested16, 17 and are recommended by the IPNA guidelines published in 2011.18 The consensus report from the American Transplant Congress published in 2018 emphasizes that tools to detect nonadherence should be combined with tools for improvement of nonadherence.19
Mobile health (mHealth) offers an attractive and novel alternative to address modifiable barriers to medication nonadherence, including lack of routines/forgetfulness, which are some of the most common barriers to optimal adherence in adolescents and young adults.20, 21 Following a SOT, there is, in addition to medication adherence, a constant need for therapeutic drug monitoring of immunosuppressive medication. Our team designed a novel medication manager application (app), TusenTac®, to monitor and promote medication adherence with a special focus on tacrolimus (Tac), being the cornerstone in current SOT immunosuppressive treatment.22 Dosing of Tac can be challenging due to high pharmacokinetic intra- and interpatient variability, narrow therapeutic window, and relatively short half-life that requires close monitoring and adherent patients. Volumetric absorptive microsampling (VAMS™) has recently gained clinical acceptance and can be used to monitor Tac.23-26 Microsampling can be performed by the patients themselves through a simple finger-prick taken at home.
In this study, we implemented a novel medication manager app (TusenTac®) and home-sampling of Tac in the patients daily routine to facilitate behavioral changes and empowerment. We aimed to evaluate the feasibility of this combined intervention and the possible impact on medication adherence in SOT adolescents and young adults.
2 MATERIALS AND METHODS
2.1 Study design and patient population
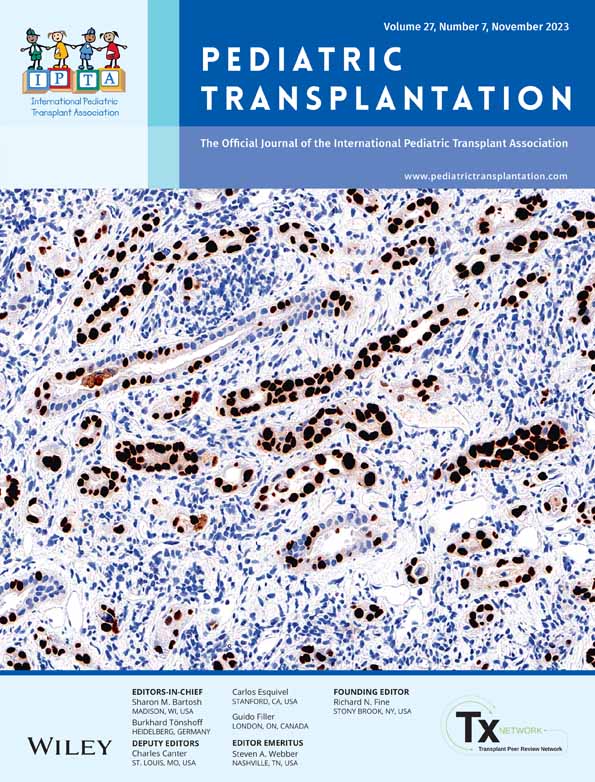
In this single-center, observational feasibility study, we recruited SOT recipients (kidney and liver, single or combined, recipients) between 14 and 25 years. Participation involved 8 weeks use of a novel age-adapted and transplant-specific medication manager app (TusenTac®) in combination with at-home Tac sampling using VAMS™ (Figure 1). The recipients received information (oral and written) and were invited to participate during a standard follow-up appointment at the out-patient clinic at Oslo University Hospital, Rikshospitalet (OUS-RH) at both the pediatric and the adult nephrology department. In Norway, the actual transfer from pediatric to adult primary care is when the patient turns 18 years old. Included participants needed to have Tac as part of their immunosuppressive regimen, to be minimum 6 months post-transplant, have a stable graft function, and live within a certain proximity to the hospital. No specific exclusion criteria were set besides this. The enrollment period was from January 2021 through June 2021 with follow-up data collected until June 2022. Venous Tac trough concentrations obtained at the hospital were retrospectively collected up to 1 year before inclusion and prospectively 1 year following the home-monitoring period. The study was conducted in accordance with ethical principles in the Declaration of Helsinki and Good Clinical Practice. It was approved by the regional committee for medical research ethics in Norway (REK number: 71776). A written informed consent was obtained from each participant, or the participant's parent/guardian when below 16 years, before subjected to any study-specific procedure.
2.2 Smartphone application development
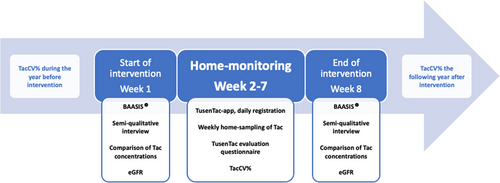
The smartphone medication manager, TusenTac®, was developed in collaboration with Center for Information Technology (USIT) at University of Oslo, and this study follows the guidelines on reporting from eHealth intervention.27 The app is available, free of charge, at App Store®/GooglePlay™. It is the first Norwegian app with tailored design for SOT recipients. The users set the dosing time for immunosuppressants, and the app gives automatic predefined medication reminder alerts (default settings); beginning 15 min before scheduled time with continuous alerts every 15 min until the user registers the medication as “taken” in the app. After five reminders with no “dose-taken” registration, the alerts are automatically silenced. It was not possible to silence the alerts without simultaneously register the medication as “taken”. If the phone was unavailable at dosage time, it was possible to retrospectively submit the specific time the dose was taken. The information registered in the app regarding medication intake (dose taken, the scheduled time to take the medication, the actual time of dosing), was uploaded to Services for sensitive data (TSD), at the University of Oslo. TSD is a secure platform for data collection and storage where researchers can analyze sensitive data.28 Subsequently, the data collected on medication intake functions as self-report and allowed us to use the app as an evaluation tool of medication implementation adherence, i.e., if the medication was taken as prescribed. In addition to serve as an immunosuppressive medication monitor, the TusenTac®-app serves as a fully fledged medication manager. The user can create a medication schedule with reminders for all medications, and it acts as a medication diary. The age-adapted information pamphlet that all patients receive when newly transplanted was integrated to enhance the importance of and ease the accessibility to important transplant knowledge. In addition, the app keeps track of selected patient variables with a focus on Tac whole blood concentrations, plasma creatinine concentrations, blood pressure, and weight, up to the discretion of the user. To stimulate continuous use, the app was age-adapted with different fun facts appearing after a medication registration and a tailored “transplant designed” gamification system with challenges, e.g., “take 5 Tac-doses on time 5 days in a row” etc. Participants were able to follow their own degree of medication adherence displayed in percentages and visualized in a graph with smileys (Figure 2). At the first clinical study appointment, the app was installed with a personal participant code, main app functions were demonstrated, and the individual scheduled time each participant was to take his/her Tac medication was registered. The feasibility of the app was measured by calculating number of daily registrations done related to expected registrations considering whether the participant had once daily or twice daily regimen, i.e., (actual registrations /expected registrations) × 100%, with >80% indicating feasibility. A user feedback questionnaire was integrated as a part of the app with a 1–10 rating scale where 1 indicates “Overall, I do not like the app” and 10 indicates “Overall, I like the app very much”. Participants were invited to answer this during the second week of the home-monitoring period to indicate feasibility (average score >8.0) and give user feedback of the app. If the questionnaire was not filled out at the end of the second week, each participant received a personal text message, including a link to a web-based version of the questionnaire. If not answered after 3 days, the participants received an automatic reminder. After that no further reminders were given.
2.3 Tacrolimus finger-prick microsampling
Tac trough concentrations are currently monitored by a venous blood sample at the hospital before the intake of the morning dose. Novel technology allows the patient to use volumetric absorptive microsampling (VAMS™) as a therapeutic drug monitoring method. The Mitra® Microsampler (Neoteryx) is a micro-sampling device that absorbs an exact amount of capillary blood (e.g., 10 μL), independent of hematocrit, through a finger-prick and can be used in home-monitoring of Tac concentration measurements. The technique has previously been validated at our center for use in both adult26 and pediatric SOT recipients.23 All participants received a short microsampling training-session at study inclusion by one of the authors (IAK) or a study nurse. They received a written individual participant plan, a capillary sample instruction displayed in pictures and a prefilled laboratory requisition as an example. Participants were instructed to obtain capillary blood samples from one finger with a lancet (Medlance 1.5 mm, HTL-STREFA) prior to the morning Tac dose (trough concentrations) once a week for six consecutive weeks. The rational for weekly sampling was to evaluate the feasibility of home-sampling. Both tips in the Mitra® cartridge were to be filled at each sampling time at home. The filled Mitra tips were sealed within the specimen bag containing the desiccant and sent with regular mail service to the laboratory for analysis. In addition, a Tac capillary microsample was collected by the patient at the hospital at the beginning and at the end of the study period giving a potential total of eight microsamples per patient. All samples were analyzed at the clinical laboratory of the Transplant center using liquid chromatography tandem mass spectrometry as previously described.26 The microsamples were kept at ambient temperature and analyzed within 1 week of sampling. The Tac concentration results were communicated either by a text (SMS) with no other information than the value being within target range or not, or a phone call, by preference of the participant. The lower limit of quantification for microsamples is 0.7 μg/L with an imprecision CV% of 6.2% at 1.9 μg/L and 5% at 29.6 μg/L.23, 26 The feasibility of home-sampling was evaluated as acceptable if >80% of the expected samples were obtained correctly and delivered to the laboratory.
2.4 Data collection of venous Tac concentrations
At the start and end of the home monitoring period, a phlebotomist at our outpatient laboratory collected a standard venous blood sample for comparison of trough Tac concentrations with the microsample. All the microcapillary samples obtained at the same time as the venous samples were taken by the patients except two, one by one of the authors (IAK) and another by the mother (a trained nurse) of a participant. All venous Tac whole blood concentrations 12 months before and 12 months after the home-monitoring period were obtained from the medical records. Only trough concentrations collected while the patients were in steady-state conditions were included, i.e., stable Tac dosage for at least 5 days followed by ≥3 consecutive concentration measurements. Tac concentrations obtained during hospitalizations lasting >1 day were excluded to avoid values under influence from acute rejections, infections/antibiotics, or other intercurrent events. Target trough whole blood concentrations were individualized and in the overall range of 3–10 μg/L, depending on type of transplantation, time since transplantation, and rejection episodes, following the standard national protocols.
2.5 Adherence
Based on the ESPACOMP criteria, we define medication adherence as “the extent to which a patient takes the medication as prescribed”.29, 30 It includes three phases: initiation, implementation, and continuation, and all three are required for optimizing clinical outcomes. In our study, medication adherence was assessed by app-monitored registrations of Tac dose and time of dosing, measurement of trough Tac variability before, during, and after the study period, and patient self-report (BAASIS©-questionnaire and interview) before and after the study period.
The app-monitored medication adherence rate was measured by calculating the actual registrations done within ±2 h related to expected registrations based on the participants medication regimen, i.e., (actual registrations/expected registrations) × 100%.
The intrapatient Tac coefficient of variation (CV%) calculated as (mean/SD) × 100% was assessed by including all Tac trough concentrations, at least three per patient, fulfilling steady-state conditions on an unchanged dose during each phase of the study (before, during, after). The prespecified cut-off value for assessing a patient as adherent was a Tac CV < 30%,31, 32 but cut-off values of 25% and 20% were also investigated.
Self-reported measure of adherence was done with the Norwegian translation of the Basel Assessment of Adherence to Immunosuppressive Medication Scale (BAASIS©).33, 34 BAASIS© measures both implementation and continuation of immunosuppressive treatment in SOT recipients. It contains 4-item questions related to both timing and taking adherence. The patient was categorized as nonadherent if an affirmative answer was given to any of the four items: missed a dose (taking), immunosuppressive medication taken more than ±2 h from the set dosing time (timing), dose changed without it being prescribed or if the patient stops taking the medication completely (persistence). The last part of BAASIS© is a visual analogue scale (VAS) where the patients rate their own adherence from 0 (“I never take my medication as prescribed”) to 100 (“I always take my medication as prescribed”). It was an integrated part of the app, and the answers were stored in TSD.
In addition, information on adherence was gathered from patient self-reports through a semistructured interview at the beginning and at the end of the intervention period (all interviews performed by IAK). The interviews lasted 30–45 min with three main areas of focus: (1) adherence-related questions following the structure of the BAASIS© questionnaire both at the beginning and at the end, and at the end of the study period, (2) app-related questions and (3) microsampling-related questions. The adherence-related questions included how many missed doses during the last month/study period, how many doses taken with a 2-h deviation from the scheduled time and if the participant perceived an improvement in adherence with regards to both timing and dosing due to the app. The participants were categorized as nonadherent if they either had missed ≥1 dose or taken ≥1 dose more than 2 h too late or too early. They were categorized as “perceived improved timing-adherence” if they either spontaneously told the interviewer this as one of the main advantages of the app or they answered confirmative to a direct question. In addition, barriers to medication adherence were included by asking: “Can you give some examples of what gets in the way for not taking your medication?” The feasibility of home-monitoring was also evaluated at the interview at the end of the intervention period by asking: “How did you find home-monitoring?” and “How do you prefer to have the regular follow-ups organized?”
2.6 Graft function
Data on graft function, including creatinine, estimated glomerular filtration rate (eGFR), formation of dnDSA, and rejection episodes, were retrospectively obtained from the medical records the year before the home-monitoring period and collected during the 1-year follow-up period. The calculations of eGFR were done using the CKD–EPI (Chronic Kidney Disease–Epidemiology Collaboration) equation with age-adjusted creatinine values to make it applicable to evaluate both the pediatric and the young adult population.35
2.7 Primary and secondary outcomes
Our primary outcome was to evaluate the feasibility of self-management with the TusenTac®-app in combination with at-home sampling of Tac concentrations with VAMS™. The feasibility was evaluated by combining patient satisfaction, number of correctly obtained capillary microsamples, and the use of the TusenTac®-app according to the instructions given. In the secondary outcome, we aimed to evaluate improvement in immunosuppressive medication adherence after daily use of the app and at-home sampling of Tac concentrations, measured by BAASIS©, intrapatient Tac through concentration variability and self-report through interviews.
2.8 Statistical analysis
SPSS software (IBM Corp. Released 2021. IBM SPSS Statistics for Macintosh, Version 28.0: IBM Corp) was utilized for statistical analysis. Baseline characteristics were tabulated descriptively. Chi-square test/McNemar's test and Fisher exact test were used to compare the prevalence of adherence assessed with BAASIS© and interview pre and postintervention due to non-normal distribution/small samples. Mann–Whitney U was used to compare the distribution of Tac CV% pre, during and postintervention between adherent and nonadherent participants assessed with BAASIS© and interview at baseline. Wilcoxon signed rank test to compare the median of differences in eGFR pre and post intervention. p-Values <.05 were considered significant.
3 RESULTS
A total of 22 recipients were recruited by convenience when attending the outpatient clinic at Oslo University Hospital, Rikshospitalet. There were three withdrawals during the first study week due to fear of needles, leaving 20 patients to complete the study. Demographics are shown in Table 1. The median age was 17.9 (14.5–24.8) years, n = 12 (60%) participants were female and n = 16 (80%) kidney recipients. Ten of the patients (50%) were on a once daily Tac dosing regimen.
| n = 20 | |
|---|---|
| Age at study start (median years, range) | 17.9 (14.5–24.8) |
| Male sex, n (%) | 8 (40%) |
| Type of solid organ transplantation | |
| Kidney, n (%) | 16 (80%) |
| Combined or liver, n (%) | 4 (20%) |
| Age at transplantation (median years, range) | 13.1 (0.4–23.2) |
| Time since transplantation (median years, range) | 6.3 (0.6–16.3) |
| Tacrolimus formulation | |
| Twice daily, n (%) | 10 (50%) |
| Once daily, n (%) | 10 (50%) |
| Current education/employment status | |
| High school, n (%) | 13 (65%) |
| University/College, n (%) | 3 (15%) |
| Employed, n (%) | 1 (5%) |
| Unemployed/Other, n (%) | 3 (15%) |
| eGFRa, at study start (median mL/min/1.73 m2, range), n = 17 | 56 (23–92) |
| eGFRa, at study end (median mL/min/1.73 m2, range), n = 17 | 61 (30–99) |
| Number of medications (median number, range) | 5 (3–8) |
- a Estimated glomerular filtration rate calculated using the CKD–EPI equation with age-adjusted creatinine values.35
3.1 Tacrolimus concentrations
In total, 118 capillary microsamples were obtained at home by the patients themselves, except one patient for whom the mother collected all samples, resulting in a median of 5 (range: 3–7) samples per patient. Two of the samples were rejected at the laboratory after visual inspection due to incomplete filling of the Mitra® tip, three samples were not obtained as trough samples, and seven samples were not obtained under steady-state conditions, leaving 106 samples eligible for Tac CV% analysis during the 8-week intervention, i.e., in total 90% of the expected number of samples. The median Tac concentration was 5.1 μg/L (range: 2.5–9.5 μg/L). The median intrapatient capillary microsample Tac CV% was 20% (5%–45%) in the samples taken at home, 90% of the patients had Tac CV% <30%. In total, 59 (56%) of the laboratory requisition forms were filled out correctly by the patients, i.e., included information on both dosage time and sampling time. The microsamples, with an accompanying completed form, were collected with a median of 11 min (range: 0–218 min) from the scheduled dosage time.
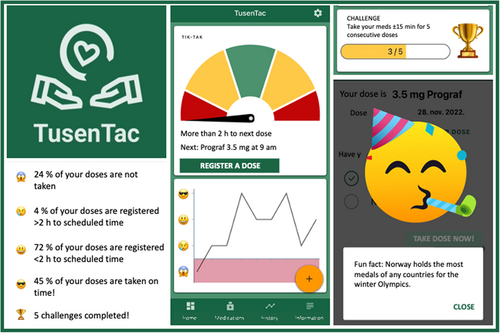
In total, 44 venous and capillary microsample pairs were obtained from 21 patients for comparison of trough Tac concentrations at the beginning and at the end of the home-monitoring period. Two patients provided each one extra pair for analysis at the end of the period, one microsample did not pass the visual inspection due to underfilling of the tip and one sample pair was excluded due to medication intake just before sampling, leaving 42 sample pairs eligible for analysis: 20 pairs from the start- and 22 pairs from the end of the home-monitoring period. The time difference between the sampling of the venous and the capillary microsample was 25 (3–150) minutes. The mean difference in Tac concentrations between venous samples and capillary microsamples was 0.01 ± 0.8 μg/L, and the relative mean difference between the venous and capillary microsamples was 2.1% (95% confidence interval [CI], −2.3%–6.6%). In total, 88% (n = 37) of the sample pairs had differences within ±20%. There was no difference in the distribution of the relative mean difference when comparing the samples obtained pre monitoring period vs. post monitoring period (Figure 3). Data on corresponding hemoglobin levels were available for 93% (n = 39) of the sample pairs, median 12.7 g/dL (range 9.2–15.2 g/dL). There was no significant correlation between the relative difference in the venous and microcapillary Tac measurements and the corresponding hemoglobin level (Spearman's rho was 0.17, 95% CI −0.18% to +0.44%, n = 39 sample pairs), indicating that the hemoglobin levels did not affect the measured Tac levels.
For comparison, we included venous Tac concentrations from the year before each patient's home-monitoring period (n = 96) and the year after the monitoring period (n = 97), with a mean of 10 (5–15) samples/participant. Comparisons of Tac CV% from the samples collected from the year before and the year after the home-sampling period, showed no significant difference in the Tac CV% using Wilcoxon signed-rank test (p = .20), with median CV% was 20% (2%–34%) pre-monitoring and 20% (7%–44%) post-monitoring.
3.2 Adherence
Six participants classified themselves as nonadherent at the first interview vs. four at the end of the home-monitoring period. With the BAASIS©-questionnaire 11 were classified as nonadherent before home-monitoring, vs. 7 at the end, i.e., 36% of the nonadherent converted to adherent (Table 2). However, an exact McNemar's test did not demonstrate a significant difference in the proportions of patients classified as nonadherent vs. adherent either by interview or with the BAASIS©-questionnaire, before and at the end of the home-monitor period, p = .5 and p = .13, respectively. A Wilcoxon signed-rank test showed that the 8-week use of the app and Tac home-monitoring did not result in a statistically significant change in the VAS score (0–100) of the BAASIS©-questionnaire, median score 97 (range: 50–100) before and 100 (range: 55–100) at the end, respectively, p = .12. One of the two patients who withdrew the first week was categorized as nonadherent by BAASIS© and interview.
| Nonadherence measurement method | Year before intervention, n = 18 | Start of intervention, n = 20 Week 1 | Home-monitoring, n = 20 Week 2–7 | End of intervention, n = 20 Week 8 | Year after intervention, n = 18 |
|---|---|---|---|---|---|
| BAASIS®a assessed nonadherence, n (%) | 11 (55%) | 7 (35%) | |||
| Interview-assessed nonadherence, n (%) | 6 (30%) | 4 (20%) | |||
| Tacrolimus CV% b , n (%) | |||||
| >30% | 4 (20%) | 3 (15%) | 4 (20%) | ||
| >25% | 6 (30%) | 6 (30%) | 3 (15%) | ||
| >20% | 9 (45%) | 9 (45%) | 9 (45%) | ||
| App monitored nonadherence % (±SD) | 27% (±22) | ||||
| Improved timing adherence at interview, n (%) | 14 (70%) |
- a Basel Assessment of Adherence to Immunosuppressive Medication Scale.
- b Coefficient of variation.
Four patients (20%) showed a venous Tac CV% >30% the year before the home-monitoring period, vs. 4 (20%) during the year following the at-home intervention period (McNemar's test, p = 1.00). Neither of the other venous Tac CV% cut-off values we tested (25%, 20%) showed significant change in the number of nonadherent patients. The number of nonadherent patients changed from 6 to 3 and from 9 to 9, respectively (p = .69 and p = 1.00). There was a significant difference in Tac CV% the year before the home-monitoring period between the nonadherent and the adherent groups defined by interview (Mann–Whitney U test, p < .01), but not significant when using the results of the BAASIS©-questionnaire as grouping variable (p = .09). During the home-monitoring period there was also a significant difference in Tac CV% between the nonadherent vs. adherent (assessed by interview/BAASIS©-questionnaire), both p < .05. There was no significant difference between the groups during the post-intervention follow-up (p = .78 and p = .39). One patient was transplanted 6 months prior to the home-monitoring period, another three patients had <3 consecutively measured Tac concentrations and are consequently not included in the before-after comparisons of Tac CV%.
During the home-monitoring period, there were a total of 1502 registrations in the app by 18 of the participants (median 83, range: 42–132 per patient). The total amount of expected registrations was 1703, i.e., the registration adherence rate was 88%. The app registrations for two of the participants were not correctly transferred to the secure platform at the beginning of their home-monitoring period; hence, these registrations are excluded from the analyses. Seventy-three percent (n = 1099) of the doses were registered within and 4% (n = 66) outside ±2 h deviation from the customized scheduled timepoint with a median of 10 (range: 0–686) min deviation, and 22% (n = 337) were not registered as taken at all. The app-monitored medication nonadherence rate during the home-period was 27% (±22%), including both taking and timing nonadherence.
By interview, 70% (n = 14) of the participants perceived improvement in medication timing adherence during the home-monitoring period, and 50% (n = 7) stated the alerts as an important factor for improvement. All, but one, 95% (n = 19), would recommend the app to their peers. In general, there was a high degree of satisfaction expressed during the interview and the participants were overall in support of interactive health technology and smartphone as the preferable platform. Home-sampling was feasible and preferred by all compared to attending the outpatient clinic after medication adjustments. However, one of the participants commented that it might not be preferable when newly transplanted, “then it feels safer being at the hospital”. The participants scored the app on “how well they overall liked it” to an average of 8.1 (range: 1–10) in the anonymous evaluation questionnaire. Eighty percent (n = 16) of the participants stated that they wanted to continue using the app, and 65% continued to use it the first 2 months after the home-monitoring period.
3.3 Graft function and rejections
There were no rejections during the intervention period. The overall graft function in the kidney-transplanted recipients (n = 17) was stable with no change in eGFR when comparing the period 1 year prior to intervention, at start of intervention, end of intervention and 1 year after intervention (67 (range: 25–110) mL/min/1.73 m2 vs 56 (range: 23–92) mL/min/1.73 m2 vs 61 (range: 30–99) mL/min/1.73 m2 vs 57 (range: 29–102) mL/min/1.73 m2). One patient experienced a steroid sensitive acute rejection prior to the intervention period and dnDSA was detected during the intervention period. One participant experienced a steroid sensitive acute rejection during the 1-year period after intervention. The function of all liver-grafts was stable during the study period.
4 DISCUSSION
The primary finding of our study was that home-monitoring with a medication manager app in combination with home-sampling of Tac concentrations in adolescent and young adult SOT is feasible. The participants registered their dosage intake in the app 9 out of 10 times, and 90% of the capillary microsamples were obtained correctly during the home-monitoring period. Furthermore, when compared to ordinary out-patient hospital consultations the self-management intervention was preferred by all participants. Seventy percent of the participants perceived an improvement in medication timing adherence during the home-monitoring period. This was supported by the BAASIS© assessment, in which 11 of 20 included participants were nonadherent at the start of the intervention period and 4 of these became adherent during the intervention. Some recipients will however need additional, individualized interventions for improvement and optimizing of their long-term medication adherence.
To our knowledge, this is the first and largest study where adolescent and young adult SOT's have performed at-home sampling of Tac concentrations. Some of the critiques against capillary microsampling have been the user difficulties and a high total error in Tac concentrations compared with venous samples. We have previously validated VAMS™ in adolescents.23 In the present study we also compared Tac trough concentrations in standard venous samples with capillary microsamples obtained by the participants. (n = 42) at the start and end of the home-monitoring period. The sample pairs demonstrated good concordance (relative mean difference of 2.1% (95% confidence interval [CI], −2.3% to 6.6%, data not shown). This demonstrates that the self-managed home-sampling of Tac is reliable and measures concentrations accurately. A combination of different assessment methods, as in our study, is recommended by Schäfer Keller et al. to increase the validity of adherence measurements.36 Intrapatient variability assessed by repeated Tac though measurements are, by many, considered as one of the most accurate indirect methods for identifying nonadherence.17 In our study, there was no significant reduction in Tac CV% when comparing the year before, the intervention period and the year after, even though 70% of the patients clearly expressed increased timing-adherence during the intervention assessed by interview.
As identified by WHO, nonadherence is influenced by multiple factors.37 Nonintentional medication nonadherence as a barrier is more common among young, transplanted patients and is characterized by lack of routines and forgetfulness as a contrast to the intentional medication nonadherence that is characterized by beliefs about not needing the medication or wanting to avoid potential side effects.38, 39 In our multimodal intervention we intended to address both intentional, as well as nonintentional medication nonadherence. Our focus was primarily the implementation phase. The app acts as an ongoing reinforcement of desired behavior, i.e., taking the medication. The low median Tac CV% of 19% during the home-monitoring period may be a result of such reinforcement but cannot be separated from the weekly instead of monthly Tac monitoring. The participants admitting to nonadherence supported this reinforcement theory, by admitting to nonadherence in the interviews, but simultaneously and quite intentional demonstrating good adherence in relation to the microsampling or the visits at the outpatient clinic. Optimizing self-management and adherence among SOT adolescents have been in focus for some decades, with the results being somewhat conflicting and published effective interventions remain scarce.40-42 McGillicuddy et al.43 were one of the first and few to show a significant reduction in Tac CV% in their 12 months long study using mHealth app with text messages for enhancing adherence, although they used CV% <40% as nonadherence cut-off in contrary to the more established 30%. In our study, we found a significant difference in Tac CV% prestudy (p < .01) and during the home-monitoring period (p < .05) between the nonadherent patients vs. the adherent patients (assessed by interview). This difference was however lost during the follow-up period when the app was free to use. Improved self-management has been shown to reduce the number of hospitalizations among patients with chronic disease and delay the progress of CKD, as well as improved quality of life.44 Among our participants only one participant experienced a transplantation-related hospitalization the following year after the home-monitoring period (data not shown). The study was not powered to detect changes in eGFR, but graft functions appeared to be stable during the 2-year observation period, with no significant change during the home-monitoring period (p = .52). One patient developed detectable dnDSA during the intervention-period. Retrospectively, this was likely due to a steroid-sensitive acute rejection episode just prior to intervention period.
Although our research highlights potential benefits for the young recipient and the feasibility of the implementation, the findings must be evaluated in the context of the limitations of the study. The home-monitoring period was short and longer intervention is warranted. There is obviously a potential bias in evaluating the short-term impact on adherence among the participants without having a control group. As Duncan et al. pointed out in their review from 2018, patients who are more adherent tend to participate in studies by default and interventions should concentrate on nonadherent patients.42 Although the medication nonadherent patient group before the study start was substantial and in line with the nonadherence prevalence in other large cross-sectional studies, this is a limitation of our study in addition to the short intervention time. The median Tac CV%, based on the Tac values obtained the year before entering the study, was 20% and this might be an indirect sign that the included participants overall were too adherent to get a measurable effect out of the intervention. Additionally, the results on perceived improved adherence might be an effect of participating in the study and not the intervention itself. We also recognize that it is not possible to separate the impact of home-monitoring from the impact of app-use on adherence. This multimodal design was our intention as recommended by the IPNA guidelines published in 2011.18 To manage some of the potential bias in doing the semistructured interviews, the questions were predefined and followed the same structure as the BAASIS© questionnaire. Another limitation is the sample-size given that this was a feasibility study. The study was on the other side conducted at the only transplant center in Norway, which gives us a good overview of the different patients, and we believe that they represent a wide range of socioeconomic and educational background. By random, the majority of our patients were female. A recent report indicates better self-reported adherence in females than in males.45 This may also have had an impact on our findings. The assessment of adherence using the app can also be viewed as a limitation. During the study, the app did not have an alternative to silence the alarm or to register the dosage as not taken, only the option to register doses ± the scheduled dosage time. Subsequently, it is possible to register the medication as taken in the TusenTac application without taken it, either with just silencing the alarm or afterwards falsely register the dosage as taken at a specific time. The same challenge is seen when electronic pillboxes are used as adherence measurement method.46 However, it is also possible to use the medication alerts in the app as a reminder to take the dosage without making a registration as well.
As we have experienced, promoting and especially measuring adherence can be a challenge. When the SOT children reach the vulnerable period between childhood and adulthood, they need to take responsibility and organize their own medical treatment, including medication and follow-up appointments without the same close involvement of the caregivers. The inevitable transfer to adult health care service may for many be a too sudden shift in responsibility, contributing to medication nonadherence and consequently, the highest graft loss rates among the adolescents and young adults.13 We believe an important issue in future research should be to detect, and direct, interventions including home monitoring/mHealth toward the patients who need it the most i.e., recipients that either already are nonadherent, subclinical nonadherent or those at risk for becoming nonadherent. Detecting these patients is a challenge. Medication adherence measurements should be addressed repeatedly in a validated and reliable manner. Implementing a regular use of different methods can be a useful tool in detecting the patients in need of intervention, as in combining a questionnaire/self-report, the clinicians' evaluation, and short-term self-registration in a medication app like TusenTac®. Our study highlights the feasibility of mHealth and home-monitoring as tools to not only detect, but to optimize medication adherence in the transition phase. It also elaborates the importance of multimodal interventions. In contrast to similar studies, we only lost 2 patients (9%) during the first study week with our multimodal design, due to needle scare. In a meta-analysis of app-based interventions for chronic diseases, the pooled dropout rate was found to be as high as 43%.47 Monitoring Tac variability in samples taken at home can be a cost-effective and more flexible way to monitor the implementation phase of medication adherence and to objective identify patients at risk for adverse outcomes.48 The long-term engagement in using the app and microcapillary sampling of Tac concentrations remains unknown. However, we believe home-monitoring can be an important addition to the existing post-transplantation care programs with the potential to optimize medication adherence.
Our original intention was to have a bilateral communication with the participants through the app with measured Tac concentrations and eventual dose changes where to be displayed directly in the app. Due to national data privacy regulations, this was not possible during the study. A medication manager app with a reminder function might function as ongoing positive reinforcement of a desired behaviour while it is in use, regardless of different supplemental features which at the end is not measurable. As part of an individual treatment and follow-up plan, we believe medication manager app's can be a tool to improve nonadherence for some patients, addressing each patient's different barriers to medication nonadherence.49 We also believe that home-monitoring is an option to facilitate a partnership between health care workers, caregivers, and patients with the aim to reduce the barriers and increase medication adherence through increased patient activation and empowerment. As we experienced in our study, making behavior change to sustain over time is difficult. Furthermore, during the period of emerging adulthood the need, motivation, and skills for medication adherence interventions and improvements are constantly changing. Consequently, we believe future interventions and applications need to be more personalized and dynamically adjusted. Throughout the time the transplanted adolescents were part of the home-monitoring program, they reported a perceived improved adherence, which indicates the importance of a personalized “hands-on follow-up”.
We conclude that home-monitoring with the combination of a mobile manager app and at-home Tac capillary microsampling is feasible and led to improved perceived medication timing adherence among 70% of the transplanted adolescents during the intervention. There was however no overall significant difference in Tac CV% from the year before to the year after the home-monitoring period. Self-managed Tac microsampling demonstrated good concordance with the venous samples. Based on these preliminary promising results, larger controlled studies promoting tailored multimodal interventions with long-term follow-up are warranted.
ACKNOWLEDGMENTS
The patients who participated in this study are highly acknowledged. We are also grateful for the excellent patient recruitment done by Margunn Høyvik Sæten at the Department of Pediatric and Adolescent Medicine. We express our gratitude to all the employees at the laboratory for Renal Physiology, Oslo University Hospital–Rikshospitalet for organizing sample collection. This feasibility study was supported by a grant from The Research Council of Norway.
CONFLICT OF INTEREST STATEMENT
The authors have nothing to disclose.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.