T-cell infiltrate intensity is associated with delayed response to treatment in late acute cellular rejection in pediatric liver transplant recipients
Abstract
Background
Late acute cellular rejection (ACR) is associated with donor-specific antibodies (DSA) development, chronic rejection, and allograft loss. However, accurate predictors of late ACR treatment response are lacking. ACR is primarily T-cell mediated, yet B cells and plasma cells (PC) also infiltrate the portal areas during late ACR. To test the hypothesis that the inflammatory milieu is associated with delayed response (DR) to rejection therapy, we performed a single-center retrospective case-control study of pediatric late liver ACR using multiparameter immunofluorescence for CD4, CD8, CD68, CD20, and CD138 to identify immune cell subpopulations.
Methods
Pediatric liver transplant recipients transplanted at <17 years of age and treated for biopsy-proven late ACR between January 2014 and 2019 were stratified into rapid response (RR) and DR based on alanine aminotransferase (ALT) normalization within 30 days of diagnosis. All patients received IV methylprednisolone as an initial rejection treatment. Immunofluorescence was performed on archived formalin-fixed paraffin embedded (FFPE) liver biopsy tissue.
Results
Liver biopsies from 60 episodes of late ACR in 54 patients were included in the analysis, of which 33 were DR (55%). Anti-thymocyte globulin was only required in the DR group. The frequency of liver-infiltrating CD20+ and CD8+ lymphocytes and the prevalence of autoantibodies were higher in the DR group. In univariate logistic regression analysis, serum gamma-glutamyl transpeptidase (GGT) level at diagnosis, but not ALT, Banff score or presence of DSA, predicted DR.
Conclusions
Higher serum GGT level, presence of autoantibodies, and increased CD8+ T-cell infiltration portends DR in late ACR treatment in children.
Abbreviations
-
- ACR
-
- acute cellular rejection
-
- ALT
-
- alanine aminotransferase
-
- ANA
-
- anti-nuclear antibodies
-
- ASMA
-
- anti-smooth muscle antibodies
-
- CMV
-
- cytomegalovirus
-
- dnAIH
-
- de novo alloimmune hepatitis
-
- DR
-
- delayed response
-
- DSA
-
- donor-specific antibodies
-
- EBV
-
- Epstein-Barr virus
-
- FFPE
-
- formalin-fixed paraffin embedded
-
- GGT
-
- gamma-glutamyl transpeptidase
-
- LD
-
- living donor
-
- LLS
-
- left lateral section
-
- IgG
-
- immunoglobulin G
-
- MLVI
-
- medication level variation index
-
- PC
-
- plasma cell
-
- PTLD
-
- post-transplant lymphoproliferative disease
-
- rATG
-
- rabbit anti-thymocyte globulin
-
- RLL
-
- reduced left lobe
-
- RR
-
- rapid response
-
- RT
-
- room temperature
1 INTRODUCTION
Acute cellular rejection (ACR) is a common complication of pediatric liver transplantation, occurring in ~50% of pediatric liver transplant recipients within the first post-transplant year.1-8 In contrast to early ACR (defined as occurring within the first 90 days post-transplant) which does not adversely affect graft or patient survival in the pediatric population, late ACR is associated with progression to chronic rejection and allograft loss.5, 8-14 Multiple clinical and laboratory-based assays are used to predict increased risk of late rejection, including medication level variation index (MLVI),15 presence of donor specific-antibodies (DSA),16-18 and the Pleximmune assay,19, 20 which measures upregulation of CD154 on circulating CD8 T cells in response to donor HLA. However, these assays do not accurately discriminate between rejection episodes that are easily treated and those that are not.
New treatment strategies for rejection are needed because ACR and chronic rejection remain the top two leading causes of late graft loss even in the most recent tacrolimus era.6 To date, treatment protocols for acute rejection have been dictated by center preference and may include high-dose pulse IV methylprednisolone with or without an oral prednisone taper, anti-thymocyte globulin (ATG), combined with increased dosing of calcineurin inhibitors (CNI): approaches that are identical for both early and late rejection.21 Indeed, clinical trials of methylprednisolone safety and efficacy in treating rejection have not been conducted in either pediatric or adult liver transplant recipients. Of note, prolonged use of steroids and CNI have been associated with stunting, renal dysfunction, and steroid-induced diabetes in pediatric liver transplant recipients.22 ATG is effective in treating steroid-resistant rejection in single-center studies, but overall is under-utilized due to historical association with post-transplant lymphoproliferative disease (PTLD) and infections.23-25 Although eosinophilic portal infiltrates are associated with severe rejection,26 there are currently no clinical predictors or histologic features of rejection which are adequately able to predict treatment response, including the Banff score.27
The primary objective of this study was to identify clinical and histologic predictors of treatment response in late ACR. We performed a retrospective single-center study of the biopsy-proven late ACR episodes occurring over a 5-year period (January 2015–2019) in patients undergoing isolated initial liver transplant at <17 years of age. Patient demographics, allograft type, infectious complications, and surgical complications related to the transplant were assessed by review of the electronic medical record. Based on our center's protocol for ACR treatment (which involves IV methylprednisolone burst and taper in combination with increased CNI trough goals for 30 days after ACR diagnosis), we arbitrarily identified two subsets of patients: those who responded rapidly to standard anti-rejection therapy with ALT (alanine aminotransferase) <50 within 30 days of diagnosis (rapid responders; RR) and those with a delayed response (delayed responders; DR), who took on average up to 109 days to reach ALT <50. In order to identify potential predictors of rejection treatment response, we obtained data from the electronic health record of study subjects pertaining to transplant indication and complications as well as rejection-related variables including Banff score, donor-specific antibody status, and variables associated with potential de novo alloimmune hepatitis (dnAIH), a condition known for its poor response to treatment.28-31 Increased serum gamma-glutamyl transferase (GGT) in DR patients was the sole marker statistically significant in DR compared to RR groups. To test the hypothesis that the inflammatory milieu in the liver biopsy at the time of late ACR diagnosis was associated with treatment response, we performed multiparameter immunofluorescence staining to jointly identify and quantify CD4+ and CD8+ T cells, CD138+ plasma cells (PCs), CD20+ B cells, and CD68+ macrophages (a cell type associated with dnAIH30, 31) in proximity to CK+ bile ducts. This powerful technique was initially developed in cancer immunophenotyping and allows high-resolution staining of cellular markers on formalin-fixed tissue.32, 33 Using univariate logistic regression, a trend toward increased CD8+ T-cell infiltration in portal areas at the time of ACR diagnosis was associated with delayed treatment response.
2 PATIENTS AND METHODS
2.1 Study design
All children who received an isolated liver transplant at <17 years of age and who were treated for biopsy-proven late ACR between January 2014 and January 2019 at Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio, USA were included in the study. Late ACR was defined as occurring minimum of 6 months after transplantation. The patients were identified from our center's transplant registry utilizing ICD-10 codes for ACR and billing codes associated with the liver biopsy procedure. Patients who received a second liver transplant or a dual organ liver transplant (such as liver-kidney) were excluded from the study. Data were retrieved from the hospital-based digital patient medical records. In total, 60 biopsies from 54 patients were included in the analysis, with 1–3 episodes of late ACR occurring per patient during the study period. Patients were treated per center protocol with methylprednisolone 5 mg/kg I.V for 3 days, followed by a daily taper of 1 mg/kg each day along with increased tacrolimus trough level goals to target a range of 10–15 ng/ml. If liver tests improved sufficiently, the patient was discharged on oral prednisone at a dose of 1 mg/kg, to be tapered by 25% per week until discontinuation at week 4 post-ACR diagnosis. If liver tests did not improve, repeat methylprednisolone burst or ATG was administered per clinical judgment. If liver tests normalized, tacrolimus trough levels returned to post-transplant baseline trough goals of 3–8 ng/ml by 1 month post-ACR diagnosis.
2.2 Clinical and laboratory recorded variables
For each study subject, the following transplant-related variables were obtained via review of the electronic health record: recipient and donor age/race/ethnicity/sex, indication for transplant, recipient race/ethnicity, allograft type (whole, left lateral segment, right lobe, living donor [LD]), serostatus of Epstein-Barr virus (EBV) and cytomegalovirus (CMV) infections at the time of transplant, and history of biliary or vascular complications.
For each study subject, the following rejection-related variables were obtained via review of the electronic health record: age of recipient at rejection, time between transplant and rejection, ALT, total bilirubin, and GGT at the time of rejection diagnosis, time to ALT normalization, use of repeat methylprednisolone pulse or rabbit anti-thymocyte globulin (rATG), Banff score at the time of rejection, DSA, presence of autoantibodies, immunoglobulin G (IgG) level, history of prior or subsequent PTLD, and concurrent infection at the time of rejection. MLVI, which has been associated with risk of late ACR, was calculated by the standard deviation of tacrolimus trough measurements using at least 3 values over a 12-month time period.15 Subjects were followed for a minimum of 2 years, with a median follow-up duration of 4.5 years.
2.3 Independent pathologist review
All biopsies which had tissue available (n = 57 of 60) underwent independent review by a pathologist (D.L.) who was blinded to the initial pathology report and treatment response group. The Banff score was independently calculated for each biopsy, as were each of its components (portal inflammation: 0–3; ductulitis: 0–3; venulitis: 0–3). The degree of fibrosis was assessed by trichrome stain. Ductopenia was assessed by CK7 immunostain. C4d staining was performed in only six biopsies and was negative in all. Biopsies were uniformly assessed in a blinded fashion by D.L. for interface hepatitis (as a feature of dnAIH) and this was not observed. The degree of PC or eosinophil infiltrates in late ACR liver biopsy tissue was assessed by the following scale: 0–1 = none/rare, 2 = occasional, 3 = prominent.
2.4 Antibodies and reagents
Ten times Tris-buffered saline, Tween-20, and ProLong Gold antifade mountant with DAPI were from ThermoFisher Scientific. The Manual Opal 7-color IHC Kit was obtained from Akoya Biosciences. The following primary antibodies were used for immunofluorescence: CD4 (clone EP204, CellMarque, 1:1250 dilution, antigen retrieval in AR9 buffer), CD8 (clone SP57, Ventana, undiluted, antigen retrieval in AR9 buffer), CD20 (clone L26, Ventana, 1:2 dilution, antigen retrieval in AR6 buffer), CD68 (clone PG-M1, Abcam, 1:250 dilution, antigen retrieval in AR9 buffer), CD138 (clone B-A38, Ventana, 1:2 dilution, antigen retrieval in AR6 buffer), pan-CK (PA1-27114, Invitrogen, antigen retrieval in AR9 buffer, 1:500 dilution), AF750 goat anti-rabbit Ig (clone Ab175733, Abcam, 1:200 dilution).
2.5 Multiparameter immunofluorescence
Five-micrometer tissue sections of formalin-fixed paraffin embedded (FFPE) liver biopsy tissue were incubated overnight at 55°C to melt the paraffin. Slides were deparaffinized with sequential incubations in Histoclear (National Diagnostics, Fisher Scientific) for 10 min at room temperature (RT) × 3, then rehydrated in ethanol, and washed in ultrapure water for 5 min at RT with gentle rotation. Antigen retrieval was performed by microwaving slides at 20% power for 15 min in the appropriate antigen retrieval buffer. Following antigen retrieval, the slides were passively cooled to RT and subsequently rinsed with ultrapure water for 5 min. Slides were incubated with a blocking reagent to inhibit nonspecific antibody binding according to the manufacturer's protocol for 10 min at RT with gentle rotation. The primary antibody was diluted in blocking reagent and incubated at 4°C overnight (in the case of anti-CD20, -CD138, -CD4, -CD68, and -CK antibodies) or for 1 h at RT (anti-CD8) in a humidified slide chamber in the dark with gentle rotation. Slides were washed twice with TBST for 5 min at RT with gentle rotation. Excess liquid was removed from each slide. Tissue sections were incubated with either anti-mouse/rabbit HRP conjugated secondary antibody for 10 min at RT, followed by two washes with TBST × 5 min. Tissues were then incubated with Opal detection fluorophores according to the manufacturer's instructions: Opal 520 (panCK), Opal 570 (CD4), Opal 650 (CD138), or Opal 690 (CD20, CD68). In the case of CD8 staining, slides were incubated for 1 h at RT with AF750-conjugated goat anti-rabbit antibody diluted 1:200 in antibody diluent. Slides were initially stained for either CD20, CD68, or CD138, followed by serial staining for CK, CD4, and CD8. Slides were coverslipped with ProLong Gold DAPI, dried, and stored at 4°C for up to 48 h until imaging.
Images were captured using a Nikon Eclipse TiE widefield microscope affixed with an Andor Xyla 4.2 megapixel 12-bit sCMOS monochromatic camera. Automated tile scanning of liver sections was done using a fully encoded motorized stage. High-speed multi-channel fluorescence imaging was acquired using a Lumencor SpectraX LED light engine. Image analysis was performed using NIS Elements Advanced Research software (Nikon).
2.6 Statistical analysis
For CD4, CD8, CD20, CD68, and CD138 cell counts, a minimum of seven portal tracts per biopsy slide were imaged. Cells were enumerated using the automated NIS Elements Advanced Research software. Mean cell counts/high-powered field (hpf) were generated for each cell type per biopsy. A total of 21 RR and 24 DR biopsies were included in the final quantification, with the remainder (6 RR and 9 DR) excluded because they did not contain a sufficient number of portal tracts for analysis. Two-tailed Student's t-test was used to determine the statistical significance between rejection response groups and immune cell counts with a p-value < .05.
Repeated measure analysis in a generalized linear mixed effect logistic regression model was conducted to determine the relationship between rejection response type and demographic factors. For stepwise variable selection, the predictors of CD4 count, CD8 count, CD20 count, CD138 count, and peak ALT were log-transformed and analyzed via a univariate logistic regression model along with the predictors of age at transplant, sex at birth, indication for transplant, history of biliary or vascular complications, recipient race, graft type, MLVI, Banff score, DSA/autoantibody presence, and time between transplant and rejection. Concurrent infection with EBV or CMV at the time of rejection was not included in the variable selection due to >50% missing data. Odds ratios, confidence intervals, and p-values were reported. A two-sided p-value < .05 was used to determine the significance of variables in all analyses. Data were analyzed using SAS.
3 RESULTS
3.1 Patient demographics and cohort characteristics
Patients who had received an isolated liver transplant at <17 years of age and treated for biopsy-proven late ACR between January 2014 and 2019 were stratified into rapid responders (RR) or delayed responders (DR) based on ALT normalization within 30 days of diagnosis (RR) or longer than 30 days (DR). A total of 60 rejection episodes in 54 patients were included in the analysis with 1–3 episodes of rejection per patient. Thirty-three rejection episodes were DR (55%) and the remainder were RR. There were no statistically significant differences in baseline immunosuppression usage between the DR and RR groups, with the majority of patients on tacrolimus monotherapy and only eight patients on sirolimus monotherapy at the time of ACR (not shown). All patients were followed for a minimum of 2 years. There was one graft loss in the RR group for biliary stricture resulting in re-transplantation, and one patient in the DR group died due to tumor recurrence. The DR group reached the threshold value of ALT <50 on an average of 102 days after treatment, while the RR group normalized ALT by an average of 19 days post-diagnosis (Table 1). There were no statistically significant differences between DR and RR rejection episodes in age of donor or recipient or time between transplant and ACR, although there was a trend toward patients with DR being older at the time of rejection with a longer time between transplant and rejection (Table 1). Similarly, there were no differences between DR and RR patients in the categories of recipient sex, donor/recipient race, or transplant indication with the majority of subjects transplanted for biliary atresia (BA) in both groups (Table 1), and only one patient was transplanted for autoimmune hepatitis. Although both vascular and biliary complications have been associated with increased risk for rejection, these surgical complications occurred at similar rates in both DR and RR rejection episodes. As expected, DR rejection episodes were more likely to be treated with prolonged courses of IV steroids (8 DR vs. 3 RR), and all of the steroid-resistant rejection episodes requiring treatment with rATG (n = 3) occurred in the DR group (Table 1). No association was identified between rejection treatment response and CMV or EBV seroconversions, which were previously reported to confer risk for rejection (Table 1).34 Although ACR episodes predispose to future rejection episodes, there were no statistically significant differences in prior rejection episodes or rejection episodes observed during the follow-up period in DR versus RR subjects (Table 1).
| Delayed response (n = 30) | Rapid response (n = 24) | p-Value | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Age (years) | |||
| At transplant | 6.5 (6.1) | 4.2 (4.4) | .049 |
| Donor age | 18.9 (12.3) | 14.9 (9.5) | |
| Age at rejection | 12.3 (7.8) | 8.03 (5.6) | |
| Time between transplant and ACR | 6.1 (5.6) | 4.4 (3.9) | |
| Days to ALT < 50 | 102 (73) | 19 (7.6) | <.001 |
| N (%) | N (%) | ||
|---|---|---|---|
| Recipient sex | |||
| Female | 13 (43.3) | 14 (58.3) | .273 |
| Male | 17 (56.7) | 10 (41.7) | |
| Race | |||
| Recipient | |||
| Black | 7 (23.3) | 6 (25) | .939 |
| Other | 3 (10) | 3 (12.5) | |
| White | 20 (66.7) | 15 (62.5) | |
| Donor | |||
| Black | 6 (26.1) | 4 (21.1) | .361 |
| Other | 2 (8.7) | 0 (0.00) | |
| White | 15 (65.2) | 15 (78.9) | |
| History of biliary complications | |||
| No | 22 (73.33) | 20 (83.3) | .380 |
| Yes | 8 (26.67) | 4 (16.7) | |
| History of vascular complications | |||
| No | 24 (80.00) | 17 (70.8) | .434 |
| Yes | 6 (20.00) | 7 (29.2) | |
| Transplant indication | |||
| BA | 18 (54.6) | 15 (55.6) | .714 |
| Malignancy | 3 (9.1) | 2 (7.4) | |
| Metabolic/genetic | 4 (12.1) | 6 (22.2) | |
| Other | 8 (24.2) | 4 (14.8) | |
| Allograft type | |||
| LLS | 6 (18.2) | 7 (25.9) | .956 |
| RLL | 11 (33.3) | 8 (29.6) | |
| Whole | 16 (48.5) | 12 (44.4) | |
| Living donor | 1 (3.03) | 1 (3.7) | |
| EBV status | |||
| Donor | |||
| Negative | 7 (43.8) | 3 (20.00) | .26 |
| Positive | 9 (56.3) | 12 (80.00) | |
| Recipient | |||
| Negative | 16 (53.3) | 9 (33.3) | .097 |
| Positive | 14 (46.7) | 18 (66.7) | |
| CMV status | |||
| Donor | |||
| Negative | 21 (77.8) | 11 (52.4) | .138 |
| Positive | 6 (22.2) | 10 (47.6) | |
| Recipient | |||
| Negative | 17 (56.7) | 15 (55.6) | .821 |
| Positive | 13 (43.3) | 12 (44.4) | |
| Rejection treatment | |||
| Prolonged steroids | |||
| No | 25 (75.76) | 24 (88.9) | .25 |
| Yes | 8 (24.24) | 3 (11.1) | |
| Rabbit ATG | |||
| No | 30 (90.9) | 27 (100) | 1 |
| Yes | 3 (9.1) | 0 (0.00) | |
| Prior rejection | |||
| No | 19 (57.58) | 16 (61.54) | .707 |
| Yes | 14 (42.42) | 10 (38.46) | |
| Rejection during follow-up period | |||
| No | 18 (54.55) | 16 (61.54) | .678 |
| Yes | 15 (45.45) | 10 (38.46) | |
- Abbreviations: LLS, Left lateral section; RLL, reduced left lobe.
3.2 Serum GGT level, but not ALT, bilirubin, Banff score, or MLVI, is associated with delayed rejection treatment response
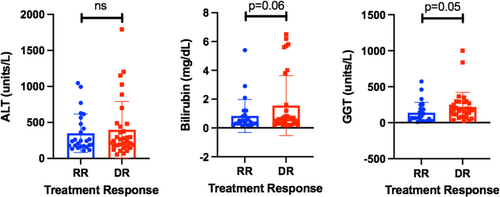
Rejection Activity Index (RAI) or Banff score was developed in adult liver transplant recipients undergoing early rejection, with three factors graded on a scale of 0–3: portal inflammation, bile ductulitis, and venulitis.27, 35 Similar to previous work,27 the Banff score on the original histopathology report achieved similar severity scores in both DR and RR groups (OR 0.953, CI 0.657–1.381, p = .795, Table 2). There were no statistically significant differences found in Banff total, Banff sub-score, or fibrosis score between DR and RR subjects (Table 3). Although centrilobular infiltrates have been associated with late ACR,10 there were no statistically significant differences in either the presence or extent of centrilobular infiltrates in DR versus RR subjects (Table 3). Surprisingly and in contrast to prior studies,35 the log-transformed peak ALT value (a biomarker of rejection severity) was also not significantly associated with treatment response (Table 2, OR 1.130). Because cholestatic hepatitis is associated with steroid-refractory rejection,24 we analyzed total bilirubin and GGT levels at diagnosis in DR versus RR subjects. There was a trend in increased bilirubin in DR subjects compared to RR, although this was not statistically significant (p = .06; Figure 1). However, serum GGT mean levels (Figure 1; p = .05 by Student's t-test) and log-transformed GGT values (Table 2) were increased in DR compared to RR subjects, with each 10-fold increase in GGT level resulting in OR 5.242 of being DR (p = .03).
| Predictors | Odds ratio estimate (DR vs. RR) | 95% Confidence limits | p-Value | |
|---|---|---|---|---|
| MLVI | 0.597 | 0.346 | 1.031 | .064 |
| BANFF score | 0.953 | 0.657 | 1.381 | .795 |
| Peak ALT (log transformed) | 1.130 | 0.108 | 11.767 | .894 |
| GGT level (log transformed) | 5.242 | 1.182 | 23.253 | .030 |
| Total bilirubin (log transformed) | 2.377 | 0.591 | 9.569 | .218 |
| Detectable DSA | 1.683 | 0.433 | 6.537 | .445 |
| DSA > 5000 MFI | 1.937 | 0.382 | 9.832 | .358 |
| Autoantibody (ANA, ASMA) | 9.136 | 0.876 | 95.228 | .064 |
| Age at transplant | 1.087 | 0.967 | 1.223 | .160 |
| Indication for transplant—BA | 0.569 | 0.113 | 2.861 | .487 |
| History of biliary complications | 1.547 | 0.354 | 6.757 | .556 |
| History of vascular complications | 0.620 | 0.149 | 2.579 | .505 |
Note
- Bold values are statistically significance level is p < 0.05.
| Delayed response (n = 31), n (%) | Rapid response (n = 26), n (%) | |
|---|---|---|
| Rejection Activity Index (0–9) | ||
| Mild (3, 4) | 9 (29%) | 8 (31%) |
| Moderate (5–7) | 16 (52%) | 12 (46%) |
| Severe (8, 9) | 6 (19%) | 6 (23%) |
| Portal inflammation (0–3) | ||
| 1 | 6 (19%) | 7 (27%) |
| 2 | 19 (62%) | 13 (50%) |
| 3 | 6 (19%) | 6 (23%) |
| Venulitis (0–3) | ||
| 1 | 8 (26%) | 3 (12%) |
| 2 | 17 (55%) | 17 (65%) |
| 3 | 6 (19%) | 6 (23%) |
| Ductulitis (0–3) | ||
| 1 | 14 (45%) | 12 (46%) |
| 2 | 14 (45%) | 11 (42%) |
| 3 | 3 (10%) | 3 (12%) |
| Centrilobular inflammation | ||
| 0 | 10 (32%) | 7 (27%) |
| <50% | 13 (42%) | 12 (46%) |
| >50% | 8 (26%) | 7 (27%) |
| Fibrosis grade (0–4) | ||
| 0 | 13 (42%) | 4 (15%) |
| 1 | 15 (48%) | 19 (73%) |
| 2 | 2 (6.5%) | 3 (12%) |
| 3 | 1 (3.5%) | 0 (0%) |
| Plasma cell infiltrate (0–3) | ||
| 0–1 | 23 (74%) | 17 (65%) |
| 2 | 5 (16%) | 7 (27%) |
| 3 | 3 (10%) | 2 (8%) |
| Eosinophil infiltrate (0–3) | ||
| 0–1 | 21 (68%) | 18 (69%) |
| 2 | 9 (29%) | 6 (23%) |
| 3 | 1 (3%) | 2 (8%) |
Medication level variation index is calculated by averaging minimum of 3 tacrolimus drug levels over a minimum 12-month period to obtain the standard deviation.15 MLVI has been associated with medication non-adherence and is a risk factor for rejection.15 However, in our cohort, the log-transformed MLVI surprisingly showed a trend toward inverse relationship with rejection treatment response, with increased MLVI having OR 0.597 of being DR to treatment (p = .06, Table 2). These data suggest that MLVI may be inversely correlated with rejection treatment response.
3.3 Increased relative presence of autoantibodies and DSA correlate with rejection treatment response
Acute cellular rejection, especially when it occurs late after transplant, can be difficult to distinguish from dnAIH, an entity that is histologically defined by interface hepatitis and the presence of anti-nuclear (ANA) and anti-smooth muscle antibodies (ASMA) arising in patients without a history of autoimmune liver disease.29-31 DnAIH has more recently been re-defined as plasma-cell-rich rejection and tends to resolve much more slowly relative to T-cell-mediated rejection.9 Additionally, de novo DSA are a risk factor for pediatric late liver transplant rejection.11, 18 However, whether DSA are associated with rejection severity or treatment response in pediatric liver transplant recipients is not known. As such, we set out to determine whether the presence of autoantibodies or de novo DSA was associated with an increased risk of DR. As shown in Table 4, 55.17% of DR rejection episodes had DSA positivity measured within 3 months of rejection diagnosis as compared to 39% of RR rejection episodes. Similarly, nine DR rejection episodes were concurrently positive for autoantibodies (34%) as compared to one RR episode which was positive for autoantibodies (5%; Table 4). As shown in Table 2, patients with DSA measured within 3 months of rejection episode had a trend toward increased DR risk (OR 1.683, CI = 0.433–6.537, p = .358). Importantly, ANA and/or ASMA autoantibodies were more prevalent in patients with DR (Table 4). Univariate analysis showed a trend toward DR with an OR 9.136 (CI = 0.876–95.228, p = .064). Blinded pathologist review of DR versus RR liver biopsies did not reveal differences in the degree of PC infiltrates (Table 3) or interface hepatitis (not shown) between DR and RR rejection episodes, suggesting that DR subjects did not meet the criteria for dnAIH.
| Delayed response (N = 33) | Rapid response (N = 27) | p-Value | |
|---|---|---|---|
| Donor specific antibody (n, %) | |||
| Positive | 16 (55.17) | 9 (39.13) | .318 |
| Negative | 13 (44.83) | 14 (60.87) | |
| Missing | 4 (11.1) | 4 (14.8) | |
| Autoantibody (n, %) | |||
| Positive | 9 (34.62) | 1 (5.26) | .059 |
| Negative | 17 (65.38) | 18 (94.74) | |
| Missing | 7 (21.2) | 8 (29.6) | |
| IgG > 1500 (n, %) | |||
| Positive | 4 (14.81) | 7 (36.84) | .137 |
| Negative | 23 (85.19) | 12 (63.16) | |
| Missing | 6 (18.2) | 8 (29.6) | |
3.4 Inflammatory milieu at rejection diagnosis is associated with rejection treatment response
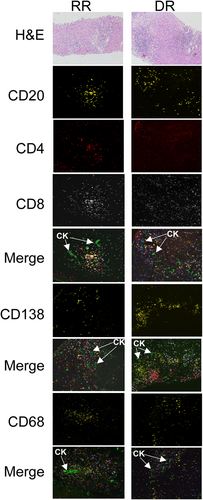
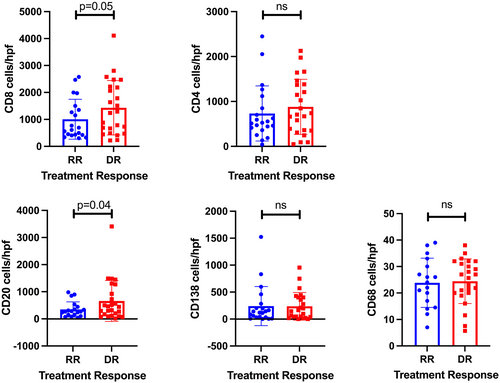
We next tested the hypothesis that the inflammatory milieu at the time of diagnosis of rejection was associated with rejection treatment response. Seven biopsies (RR = 3, DR = 4) were excluded from immunofluorescence analysis due to insufficient portal tracts (<7) for analysis from available archived FFPE liver tissue. A total of 21 RR and 24 DR FFPE 5 μm liver biopsy sections were stained sequentially with anti-CD20, -CD4, -CK, and -CD8 antibodies with the manual seven-color Opal IHC kit to allow simultaneous imaging of five parameters, including the DAPI nuclear stain. Serial sections from the same biopsy were used to image anti-CD138 and anti-CD68, respectively. A representative image from one RR and one DR liver biopsy is shown in Figure 2. As shown in Figure 3, increased portal infiltration by CD20+ and CD8+ cells occurred in DR rejection (p = .04, .05, respectively). There were no differences in CD4+ T cells, CD138+ PCs, or CD68+ macrophages in portal infiltrates in DR compared to RR biopsies (Figure 3). None of the cell counts reached a significance level in univariate analysis (Table 5).
| Predictors | Odds ratio estimate | 95% Confidence limits | p-Value | |
|---|---|---|---|---|
| CD4 | 2.053 | 0.409 | 10.304 | .3744 |
| CD8 | 2.897 | 0.397 | 21.142 | .2874 |
| CD20 | 1.159 | 0.391 | 3.433 | .7858 |
| CD138 | 1.069 | 0.462 | 2.477 | .8732 |
| CD4:CD8 ratio | 1.226 | 0.235 | 6.388 | .8266 |
The following terms were included in multiple regression analysis: log-scale CD4, CD8, CD20, and CD138 cell counts, log-scale peak ALT, age at transplant, recipient sex, transplant indication, history of biliary complications, and history of vascular complications. No significant term was identified at 0.10 level in the multiple regression model.
4 DISCUSSION
Late ACR is a risk factor for allograft loss, yet clinical and histologic predictors of rejection treatment response are lacking. In this single-center retrospective cohort study, we attempted to identify predictors of late ACR treatment response by subdividing patients into two cohorts: delayed (DR) and rapid response (RR) to treatment and performing a comprehensive review of clinical transplant- and rejection-related factors using the electronic health record. Surprisingly, the histologic and clinical criteria of Banff score, presence of PCs or eosinophils, ALT or bilirubin at diagnosis, and MLVI which have historically been used to predict rejection severity and response to treatment did not correlate with late ACR treatment response. In this study, we confirmed elevated GGT as a serum biomarker associated with difficult-to-treat rejection, and identified that the presence of anti-ANA or ASMA autoantibodies, rather than DSA, predicts DR (Table 2). For the first time in pediatric liver transplantation, we have employed the innovative Opal multiparameter immunofluorescence technique to analyze immune infiltrates in order to evaluate the co-localization of defined immune cell populations within inflamed portal tracts at the time of rejection diagnosis. Using this novel platform, we identified trends toward increased CD20+ and CD8+ lymphocyte counts within portal areas in DR late rejection. Although the p-values reached statistical significance only for the GGT level and would need to be confirmed in a larger cohort of patients, the trend suggests that CD20+ and CD8+ lymphocyte counts, GGT levels, and serologic markers of alloimmunity may serve as biomarkers for difficult-to-treat rejection.
Our study has several limitations. First, this is a small single-center retrospective study that had several key variables measured as part of routine clinical care (including DSA and autoantibodies) rather than as part of a prospective study protocol. Therefore, there was often >10% missing data within several key predictors which may account for our lack of statistical significance in either univariate or multivariate logistic regression analysis. Power calculations confirm that an estimated n = 151 would be needed to achieve statistical significance in CD8+ T-cell infiltrates, a case number that could potentially be met with either a multi-center study or a larger cohort within our own institution. Our study had one episode of graft loss and one patient death, both from conditions unrelated to ACR or its treatment. Longer term follow-up, including documentation of the development of subsequent rejection episodes, ductopenia, chronic rejection, allograft fibrosis progression, and systematic measurement of C4d staining in all rejection biopsies (both DR and RR) may be needed to detect important clinical consequences of inadequate treatment of late rejection and increased steroid exposure.
Donor-specific antibodies are a risk factor for late rejection,11, 18, 36, 37 but are also present in some long-term liver transplant recipients with normal histology and normal liver tests.38, 39 In our study, DSA positivity at the time of rejection diagnosis showed a trend toward association with DR to treatment and was universally directed against HLA class II. Future studies utilizing banked serum from this patient cohort are planned to assess pathogenicity of the identified DSA, including ability of the DSA to bind to Fc receptors, IgG subclass, and the ability to bind to C1q.
Although autoantibody positivity showed a trend toward association with delayed treatment response in this study, surprisingly, PC infiltrates (quantified with immunofluorescence staining utilizing anti-CD138 antibodies, respectively) were not associated with rejection treatment response (Figure 3, Table 5). We also did not find convincing histologic evidence of antibody-mediated rejection or de novo AIH in DR biopsies based on histologic assessment by a blinded pathologist (Table 3), although this lack of association is likely hindered by small sample size and relatively short duration of follow-up (median 4.5 years). The association of de novo DSA positivity with rejection risk is well documented, with current evidence suggesting that DSA are a marker of inadequate immunosuppression rather than truly pathogenic.11, 18, 39-42 In our study, the concurrent findings of increased CD8+ lymphocyte infiltrates in individuals with concurrent DSA- and autoantibody-positivity raises the interesting possibility that inflammation associated with rejection may release allo- and auto-antigens from the inflamed liver to further sensitize the recipient.43, 44 Alternatively, the presence of DSA or autoantibodies may cause low-grade inflammation and chemokine production which drives T-cell tissue infiltration and/or release of antigens that primes CD8+ T cells.45 These hypotheses can be further tested directly in animal models of liver transplantation.46
In summary, we demonstrate that the degree of GGT elevation, CD20+ and CD8+ lymphocyte infiltration, and the presence of autoantibodies are associated with DR to rejection treatment in pediatric patients with late ACR. These data suggest that a combination of biochemical and serologic markers with targeted histologic assessment can identify late ACR episodes which may be more difficult to treat utilizing standard protocols of increased CNI and methylprednisolone. Future multi-center and prospective studies are needed to validate the associations identified in this study and determine whether novel ACR treatment protocols tailored to autoantibody serology status and the presence of CD20+ and CD8+ lymphocytes improves treatment response, shortens exposure to corticosteroids, and prolongs graft longevity.
AUTHOR CONTRIBUTIONS
Conception and design of the work: ALP, DH, and ESW. Data collection and analysis: ALP, MR, GB, and DL. Statistical analysis: QS and LF. Drafting the article: ALP and MR. Critical revision of the article and final approval of the published version: ALP, MR, GB, DL, QS, LF, DH, and ESW.
ACKNOWLEDGMENTS
This project was supported, in part, by NIH P30 DK078392 Pathology Research Core and Imaging Research Core of the Digestive Diseases Research Core Center in Cincinnati, OH and Cincinnati Children's Research Foundation. We would like to thank Dr. Matthew Kofron for assistance with imaging and analysis. A.L.P. was supported by NIH 5K12HD028827-28, 29 as well as funds from the Markham Family Liver Transplant Research Award (Cincinnati Children's Hospital Medical Center).
ETHICAL APPROVAL
This study was approved by the Institutional Review Board of Cincinnati Children's Hospital Medical Center, protocol #2019-0481.
Open Research
DATA AVAILABILITY STATEMENT
Data available on request from the authors.




