Impact of length of donor ICU stay on outcome of patients after pediatric liver transplantation with whole and in situ split liver grafts
[Correction added 01 July, 2022, after first online publication: In the article title, “ex situ” has been corrected to “in situ” in this version.]
Abstract
Background
Patients who have a prolonged stay in the intensive care unit (ICU) are often excluded for organ donation because of supposed deleterious effects of a lengthy ICU stay. We aimed to determine the effects of a prolonged donor stay in the ICU on the outcome of liver transplantation (LT) in children.
Methods
Retrospective review of 89 pediatric LT patients, age 0–18 years, period 2003–2018, including patients having undergone whole organ or in situ split LT. The patients were divided into two groups according to the donor length of stay in the ICU. A prolonged stay was defined as >5 days. Recipient, graft, and donor characteristics were compared; outcome parameters included recipient and graft survival rates and postoperative complications.
Results
Group short (donor ICU stay <5 days) included 75 patients, group long (donor ICU stay >5 days) 14 patients. Baseline characteristics between recipients did not differ. Donors in group long had significantly more infectious complications and a higher gamma glutamyl transferase (gGT) the day of organ recovery. Incidence of biliary complications post-LT was significantly higher in group long (p = .029). Patient and graft survival rates did not differ significantly between groups.
Conclusions
Donors with a prolonged stay in the ICU should still be considered for liver donation if they fulfill most other selection criteria. Recipients from donors having stayed in ICU >5 days may be at increased risk of biliary complications.
Abbreviations
-
- ALAT
-
- alanine aminotransferase
-
- ASAT
-
- aspartate aminotransferase
-
- BMI
-
- body mass index
-
- CMV
-
- cytomegalovirus
-
- EBV
-
- Epstein-Barr virus
-
- ECMO
-
- extracorporeal membrane oxygenation
-
- gGT
-
- gamma glutamyl transferase
-
- ICU
-
- intensive care unit
-
- INR
-
- international normalized ratio
-
- IQR
-
- interquartile range
-
- LOS
-
- length of stay
-
- LT
-
- liver transplantation
-
- MOF
-
- multi-organ failure
-
- PELD
-
- pediatric end-stage liver disease
-
- POD
-
- postoperative day
-
- SD
-
- standard deviation
-
- Tx
-
- transplantation
1 INTRODUCTION
Extended donor criteria, including a longer ICU stay, are increasingly used in order to broaden the donor pool. However, donors after a prolonged ICU stay are still at risk of being refused more easily, out of concern for possible negative impact on recipient and graft outcome. Currently, there is not much literature either validating or refuting this practice.1 Nevertheless, in pediatric LT the clinical impression is that the influence of prolonged donor ICU stay on recipient outcome may be less important than what was previously assumed. Although the impact of prolonged ICU stay on patients in general has been extensively explored, there is little evidence-based literature on the topic of its influence on transplanted organs. The data currently available mainly address adult liver recipient outcomes.2
Generally, to assess ICU patients, mortality and quality of life are used as main outcome measures.3-9 There is a close correlation between prolonged ICU LOS and higher mortality. However, even though ICU LOS is an independent risk factor for higher mortality in all ICU patients, other patient characteristics are even more important risk factors for mortality. These include patient age (more than 60 years old), development of new organ dysfunction during ICU stay, and certain events in the ICU including hypoglycemia and benzodiazepine and steroid use.3, 8, 10 It is most likely that these factors—which then also might lead to a longer ICU stay—contribute to the credo that prolonged ICU stay in itself negatively impacts donor organ quality. It is important to note that there is no consistent definition of “prolonged” ICU LOS. Definitions vary from three to 21 days.6, 7, 11-13 This large range can be explained by the fact that the definition depends on the type of ICU and the patient's primary disease.2
What we do know is that donor ICU LOS is correlated directly with a higher risk of infection in those donors with cut-offs set between 3 and 5 days.14, 15 Main infectious risks are respiratory catheter related.16 Nevertheless, the transmission of pathogens to recipients seems very rare but was associated with higher mortality when it did occur. Elderly donors are considered a risk factor for donor to recipient transmission, but not ICU LOS.14
If the direct relationship between ICU LOS and outcome of Tx was scientifically proven, it would most certainly influence donor selection in pediatric LT. Should the donor ICU LOS be found to not negatively influence recipient outcome, expansion of donor selection criteria would be possible, thereby easing allocation and reducing wait-list time and mortality. We hypothesized in this study that donor ICU LOS stay does not have a significant impact on graft and patient outcome in a representative cohort of pediatric LT patients.
2 METHODS
We performed a single center retrospective cohort study. Patients were included if they were aged 0–18 years at the time of LT during the years 2003–2018. Patients who received a graft from a living donor, from ex situ liver reduction, or had undergone a multi-organ transplantation were excluded, as well as patients for whom the charts were incomplete. Of note, the majority of patients at our hospital who received a partial liver received an in situ split graft and only some patients with a monosegment received an ex situ split organ, thus they were excluded to avoid bias. Patients were divided into two groups according to donor ICU LOS. Based on our clinical experience and the literature available on the topic, we chose a cut-off of 5 days for prolonged ICU stay. Therefore, group short included patients who received an organ from a donor with ICU LOS ranging from 0 to 5 days, group long had donors with ICU LOS of more than 5 days.
The following characteristics were collected from donors and recipients: demographic, history, and laboratory features and values. Outcome criteria were the following: levels of factor V and total bilirubin on day 0, 5, 10, and 30 post-LT; patient survival; cause of death; patient survival time; graft survival time; incidence of graft loss and reason for graft loss (a graft was lost when there was a need to retransplant or when the recipient passed away); time to graft loss; early rejection (which means rejection of the graft during the first month post-LT); vascular complications during the first month post-LT (defined as arterial, portal, and venous thromboses or stenoses); and biliary complications during the first year post-LT (defined as bile leak needing drainage), cholestasis (defined as the elevation of gGT and conjugated bilirubin beyond normal values) unrelated to medical reasons such as rejection or viral infection, dilatation of the intrahepatic bile ducts, and stenosis needing an intervention; the one patient with diffuse cholangiopathy after arterial thrombosis was excluded from the analysis between groups regarding biliary complications, since this event was deemed directly related to the arterial thrombosis.
2.1 Statistical analysis
Continuous data are expressed as mean/SD or median/IQR according to the normality of distribution assessed by the Shapiro–Wilk test. To test for differences between groups, the Mann–Whitney U test was used for data of non-normal distribution and the independent t-test for data of normal distribution. For categorical variables, the chi-square test was applied. In case assumptions for the chi-square test were not met, we used the Fisher's exact test. Mean patient and graft survival were analyzed using Kaplan–Meier survival curves and the testing for significant differences in the survival distributions between the groups was done performing the log rank test. Regression analysis was used to test for associations between donor ICU LOS and certain continuous outcomes using the Spearman correlation. The statistical analysis was performed by SPSS software version 25 (IBM Corporation). Statistical significance was indicated by p < .05 and all significance testing was two-sided.
The study was approved by the local Ethics Committee (CER 11-01OR/MATPED 11-004R).
3 RESULTS
3.1 Study population
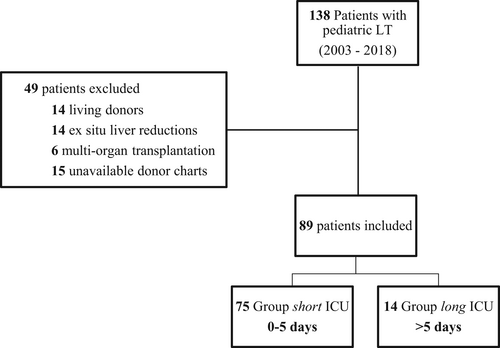
Of the 138 patients having undergone pediatric LT during the investigated time period, 89 were included in the study; group short included 75 patients (median ICU LOS 2 days, range 0–5 days), group long consisted of 14 patients (median ICU LOS 6 days, range 6–13 days) (Figure 1). There were five cases of Re-Tx in our cohort, which were all early Re-Tx (i.e., within the first month of initial LT); baseline characteristics with first time LT did not differ.
3.2 Recipient data
Recipient data are summarized in Table 1. Baseline characteristics between recipients in the two groups did not differ significantly, with the exception of age at diagnosis where children in group long were younger than in group short (p = .015).
| Total | Donor ICU stay 0–5 days | Donor ICU stay >5 days | p-value | |
|---|---|---|---|---|
| N | 89 | 75 | 14 | |
| Age at Tx (months), median (IQR) | 35 (121) | 41 (127) | 19.5 (45) | .29 |
| Gender, % (No.) | ||||
| Male | 56% (50) | 56% (42) | 57% (8) | .90 |
| Female | 44% (39) | 44% (33) | 43% (6) | |
| Weight (kg), median (IQR) | 12.7 (29) | 13.6 (31) | 9.95 (9.9) | .26 |
| Height (m), median (IQR) | 0.91 (0.7) | 0.92 (0.7) | 0.79 (0.4) | .26 |
| BMI, median (IQR) | 16.2 (3.5) | 16.5 (3) | 16.1 (3.6) | .58 |
| Diagnostic, % (No.) | ||||
| Biliary atresia | 46% (41) | 45% (34) | 50% (7) | .75 |
| Other neonatal cholestasis | 20% (18) | 20% (15) | 21% (3) | .58 |
| Metabolic disease | 19% (17) | 20% (15) | 14% (2) | .47 |
| Fulminant hepatitis | 4% (4) | 4% (3) | 7% (1) | .50 |
| Tumor | 6% (5) | 7% (5) | 0% (0) | .42 |
| Rejection +Re-Tx | 4% (4) | 4% (3) | 7% (1) | .50 |
| Primary Tx or Re-Tx, % (No.) | ||||
| Primary Tx | 94% (84) | 95% (71) | 93% (13) | .58 |
| Re-Tx | 6% (5) | 5% (4) | 7% (1) | |
| Priority, % (No.) | ||||
| Super-urgencya | 37% (31) | 36% (25) | 46% (6) | .46 |
| Hospitalized | 8% (7) | 8% (6) | 7% (1) | .70 |
| Waiting at home | 54% (45) | 56% (39) | 46% (6) | .53 |
| Age at diagnosis (months), median (IQR) | 2.5 (24.1) | 3 (38) | 1.4 (1.3) | .015 |
| Factor V (%), median (IQR) | 69 (51) | 67 (56) | 81.5 (42) | .39 |
| Total bilirubin (μmol/L), median (IQR) | 67 (276) | 67 (293) | 57.5 (225) | .89 |
| Creatinine (μmol/L), median (IQR) | 31 (29) | 31 (28) | 33.5 (30) | .57 |
| Thrombocytes (G/L), median (IQR) | 107 (114) | 108 (121) | 81.5 (72) | .53 |
| Albumin (g/L), median (IQR) | 31 (9) | 31 (10) | 29.5 (7) | .89 |
| INR, median (IQR) | 1.3 (0.3) | 1.24 (0.3) | 1.315 (0.4) | .53 |
| Growth failure, % (No.) | 14% (12) | 14% (10) | 14% (2) | .60 |
| Preoperative ascites, % (No.) | 44% (37) | 43% (31) | 46% (6) | .84 |
| PELD, median (IQR) | 8 (18) | 7 (19) | 11 (11) | .22 |
| CMV+, % (No.) | 58% (52) | 63% (47) | 36% (5) | .06 |
| EBV+, % (No.) | 44% (39) | 46% (34) | 36% (5) | .48 |
| Peri-Tx blood transfusion (ml), median (IQR) | 400 (590) | 400 (560) | 500 (700) | .20 |
Note
- Significance of Age at diagnosis is shown in bold.
- Abbreviations: BMI, body mass index; CMV, cytomegalovirus; EBV, Epstein-Barr virus; ICU, intensive care unit; IQR, interquartile range; PELD, pediatric end-stage liver disease; Tx, transplantation.
- a A patient whose transplantation has been deemed very urgent due to poor health condition, and who is therefore prioritized in organ allocation.
3.3 Graft and donor data
Graft characteristics are summarized in Table 2. One patient in the whole cohort (group long) received a monosegment split liver which led to a significant difference between the groups (p = .02). No other significant difference was observed. Of note, we have pediatric as well as adult (≥18 yo) donors in both groups. More precisely, 53% of all donors in the cohort were adults and 47% were pediatric donors. Of those adult donors, 29% donated a whole liver and 71% a split liver. Among the pediatric donors, the distribution was 68% whole to 32% split liver.
| Total | Donor ICU stay 0–5 days | Donor ICU stay >5 days | p-value | |
|---|---|---|---|---|
| N | 89 | 75 | 14 | |
| Graft type, % (No.) | ||||
| Whole liver | 46% (41) | 48% (36) | 36% (5) | .40 |
| Left lateral segment | 46% (41) | 44% (33) | 57% (8) | .37 |
| Left liver | 4% (4) | 5% (4) | 0% (0) | .50 |
| Monosegment | 1% (1) | 0% (0) | 7% (1) | .16 |
| Right liver | 2% (2) | 3% (2) | 0% (0) | .71 |
| Type of biliary anastomosis, % (No.) | ||||
| Bilio-enteric | 92% (82) | 91% (68) | 100% (14) | .59 |
| Duct-to-duct | 8% (7) | 9% (7) | 0% (0) | |
| Graft weight (g), median (IQR) | 320 (225) | 320 (185) | 385 (358) | .06 |
| Cold ischemia time (min), mean (SD) | 344 (107) | 341 (105) | 357 (116) | .62 |
| Warm ischemia time (min), median (IQR) | 55 (19) | 55 (19) | 61.5 (25) | .09 |
| Total ischemia time (min), mean (SD) | 394 (120) | 389 (120) | 420 (118) | .37 |
- Abbreviations: ICU, intensive care unit; IQR, interquartile range; SD, standard deviation.
Donor data are shown in Table 3. All donations took place after brain death. Donors in both groups showed to have been in overall good health before their death, showing minor or even no history of major diseases, smoking, or alcoholism. Yet, during their ICU stay, donors in group long were significantly more often subject to infectious complications and had more episodes of fever than donors in group short. Donors mostly had excellent biological markers, yet gGT and fibrinogen were significantly elevated in group long (p = .01). Factor V activity also significantly differed with an activity on the lower spectrum for group short and on the upper limit for group long (p = .026). Donors in group short had a total bilirubin that was significantly elevated compared to group long, however and of note, values stayed within a normal range for both groups (p = .026). There was no evidence of steatosis on ultrasound in any of the donors.
| Total | Donor ICU stay 0–5 days | Donor ICU stay >5 days | p-value | |
|---|---|---|---|---|
| N | 89 | 75 | 14 | |
| ICU LOS (d), median (IQR) | 2 (2) | 2 (2) | 6 (2) | |
| Age (years), mean (SD) | 26 (19) | 27 (19) | 24 (16) | .65 |
| Gender, % (N) | ||||
| Male | 56% (50) | 59% (44) | 64% (9) | .69 |
| Female | 44% (39) | 41% (31) | 36% (5) | |
| Weight (kg), median (IQR) | 64 (40) | 65 (44) | 57 (24) | .71 |
| Height (m), median (IQR) | 1.66 (0.35) | 1.65 (0.40) | 1.68 (0.20) | .95 |
| BMI, mean (SD) | 21.1 (5) | 21.0 (5) | 20.0 (4) | .42 |
| Cause of death, % (N) | ||||
| Traumatic brain injury | 48% (43) | 52% (39) | 29% (4) | .15 |
| Asphyxia | 33% (29) | 31% (23) | 43% (6) | .37 |
| Infection | 6% (5) | 4% (3) | 14% (2) | .17 |
| Road accident | 13% (12) | 13% (10) | 14% (2) | .60 |
| History of heart disease, % (N) | 5% (4) | 4% (3) | 8% (1) | .50 |
| History of hypertension, % (N) | 6% (5) | 6% (4) | 8% (1) | .58 |
| History of lung disease, % (N) | 5% (4) | 6% (4) | 0% (0) | .50 |
| History of diabetes I or II, % (N) | 0% (0) | 0% (0) | 0% (0) | |
| History of liver disease, % (N) | 0% (0) | 0% (0) | 0% (0) | |
| History of pancreatic disease, % (N) | 0% (0) | 0% (0) | 0% (0) | |
| History of kidney disease, % (N) | 1% (1) | 0% (0) | 8% (1) | .16 |
| History of infectious disease, % (N) | 5% (4) | 4% (3) | 8% (1) | .50 |
| History of cancer, % (N) | 0% (0) | 0% (0) | 0% (0) | |
| History of malignant melanoma, % (N) | 1% (1) | 1% (1) | 0% (0) | .85 |
| Cigarette smoking, % (N) | 40% (29) | 37% (23) | 55% (6) | .53 |
| Pack-year, median (IQR) | 0 (3.5) | 0 (4) | 0 (2) | .77 |
| History of moderate alcohol consumption, % (N) | 6% (5) | 6% (4) | 8% (1) | .58 |
| ASAT (U/L), median (IQR) | 68 (84) | 75 (102) | 50 (39) | .11 |
| ALAT (U/L), median (IQR) | 42 (79) | 39 (89) | 57 (57) | .66 |
| gGT (U/L), median (IQR) | 28 (44) | 23 (37) | 67 (46) | .001 |
| Total bilirubin (μmol/L), median (IQR) | 10 (8) | 10 (8) | 8 (5) | .026 |
| Creatinine (μmol/L), median (IQR) | 67 (41) | 70 (41) | 48 (39) | .32 |
| Hemoglobin (g/L), median (IQR) | 112 (31) | 113 (36) | 103 (32) | .16 |
| Leukocytes (G/L), median (IQR) | 13.2 (10) | 13.2 (12) | 11.5 (6) | .18 |
| Thrombocytes (G/L), median (IQR) | 164 (112) | 156 (112) | 199 (90) | .08 |
| Factor V (%), median (IQR) | 76 (37) | 70 (26) | 145 (5) | .026 |
| INR, median (IQR) | 1.1 (0) | 1.1 (0) | 1.1 (0) | .05 |
| Fibrinogen (g/L), median (IQR) | 3.7 (3) | 3.5 (2) | 6.0 (2) | .001 |
| Sodium (mmol/L), median (IQR) | 146 (7) | 147 (6) | 144 (60) | .09 |
| Norepinephrine infusion, % (N) | 38% (33) | 42% (31) | 15% (2) | .06 |
| Blood transfusion (U), median (IQR) | 0 (1) | 0 (1) | 0 (0) | .70 |
| Infectious complications: Blood, % (N) | 10% (6) | 6% (3) | 33% (3) | .037 |
| Infectious complications: Lung, % (N) | 26% (15) | 19% (9) | 67% (6) | .003 |
| Infectious complications: Urine, % (N) | 2% (1) | 0% (0) | 11% (1) | 0,15 |
| Fever, % (N) | 11% (9) | 7% (5) | 29% (4) | .038 |
| Duration of intubation, days (IQR) | 2.6 (1) | 2 (2) | 6 (0) | .000 |
| ECMO, % (N) | 4% (3) | 4% (3) | 0% (0) | .63 |
| Steatosisa (%), median (IQR) | 0 (0) | 0 (0) | 0 (0) | .87 |
| EBV+, % (N) | 85% (63) | 84% (52) | 92% (11) | .49 |
| CMV+, % (N) | 48% (38) | 50% (34) | 33% (4) | .36 |
Note
- Significance of gGT, Total Bilirubin, Factor V, Fibrinogen, Infectious complications: Blood, Infectious complications: Lung, Fever, Duration of intubation are shown in bold values.
- Abbreviations: ALAT, alanine aminotransferase; ASAT, aspartate aminotransferase; BMI, body mass index; CMV, cytomegalovirus; EBV, Epstein-Barr virus; ECMO, extracorporeal membrane oxygenation; gGT, gamma glutamyl transferase; ICU, intensive care unit; INR, international normalized ratio; IQR, interquartile range; LOS, length of stay; SD, standard deviation; Tx, transplantation.
- a Estimated on ultrasound and/or CT scan as well as by the recovery surgeon.
3.4 Outcomes
Outcome data are summarized in Table 4. Of all examined outcome parameters, the only significant difference between group long and short was the occurrence of biliary complications with significantly more events in group long (p = .008). Biliary complications were stratified into four categories. The most frequent biliary complication in both groups was cholestasis unrelated to medical reasons such as rejection or viral infection; no significant difference was observed between sub-types of biliary complications between groups. In 32% of patients, biliary complications occurred after a whole organ transplant, while in 35% after having received a split liver, that difference was not significant (p = .71). Duct-to-duct anastomosis led to 29% biliary complications, and bilio-enteric anastomosis to 34%, without a significant difference (p = .77).
| Total | Donor ICU stay 0–5 days | Donor ICU stay >5 days | p-value | |
|---|---|---|---|---|
| N | 89 | 75 | 14 | |
| Factor V (%), day 0, median (IQR) | 52 (34) | 52.5 (35) | 45 (23) | .40 |
| Factor V (%), day 5, median (IQR) | 100 (21) | 100 (22) | 100 (13) | .60 |
| Factor V (%), day 10, median (IQR) | 100 (14) | 100 (12) | 86 (31) | .06 |
| Factor V (%), day 30, median (IQR) | 100 (14) | 100 (12) | 100 (23) | .90 |
| Total bilirubin (μmol/L), day 0, median (IQR) | 73 (87) | 73 (83) | 84 (106) | .57 |
| Total bilirubin (μmol/L), day 5, median (IQR) | 42 (29) | 46 (87) | 36 (58) | .96 |
| Total bilirubin (μmol/L), day 10, median (IQR) | 24 (34) | 24 (32) | 24 (50) | .68 |
| Total bilirubin (μmol/L), day 30, median (IQR) | 15 (11) | 15 (11) | 13 (5) | .58 |
| Patient alive, % (N) | 91% (81) | 93% (70) | 79% (11) | .08 |
| Death, % (N) | 9% (8) | 7% (5) | 21% (3) | .11 |
| Cause of death, % (N) | (8) | (5) | (3) | |
| MOF | 13% (1) | 20% (1) | 0% (0) | .63 |
| Septic shock | 50% (4) | 40% (2) | 67% (2) | .50 |
| Cerebral hemorrhage | 13% (1) | 0% (0) | 34% (1) | .38 |
| Unknown | 25% (2) | 40% (2) | 0% (0) | .46 |
| Patient survival (months), mean (SD) | 69 (52) | 69 (52) | 65 (53) | .10 |
| Graft survival (months), mean (SD) | 66 (53) | 66 (53) | 64 (54) | .41 |
| Graft loss, % (N) | 15% (13) | 13% (10) | 21% (3) | .42 |
| Reason for graft loss, % (N) | (13) | (10) | (3) | .56 |
| Death | 54% (7) | 50% (5) | 67% (2) | |
| Re-Tx | 46% (6) | 50% (5) | 33% (1) | |
| Duration to graft loss (months), median (IQR) | 2 (6) | 2 (6) | 2 (47) | .49 |
| Early reject, % (N) | 20% (18) | 21% (16) | 14% (2) | .73 |
| Vascular complications, % (N) | (89) | (75) | (14) | |
| Arterial thrombosis | 3% (3) | 4% (3) | 0% (0) | .60 |
| Portal thrombosis | 0% (0) | 0% (0) | 0% (0) | |
| Hepatic vein thrombosis | 0% (0) | 0% (0) | 0% (0) | |
| Biliary complications, % (N) | 34% (30) | 28% (21) | 64% (9) | .008 |
| Types of biliary complications, % (N) | (30) | (21) | (9) | |
| Leak | 23% (7) | 24% (5) | 22% (2) | .63 |
| Cholestasisa | 33% (10) | 29% (6) | 44% (4) | .43 |
| Dilatation | 20% (6) | 24% (5) | 11% (1) | .64 |
| Stenosis | 23% (7) | 24% (5) | 22% (2) | .63 |
Note
- The only outcome which was significantly different between groups was biliary complication with significantly more events in group long.
- Significance of Biliary complications is shown in bold.
- Abbreviations: ICU, intensive care unit; IQR, interquartile range; MOF, multi-organ failure; SD, standard deviation; Tx, transplantation.
- a Unrelated to medical reasons such as rejection or viral infection.
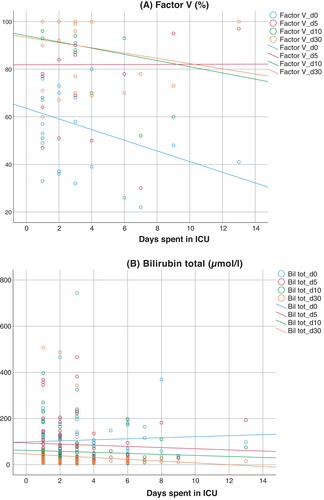
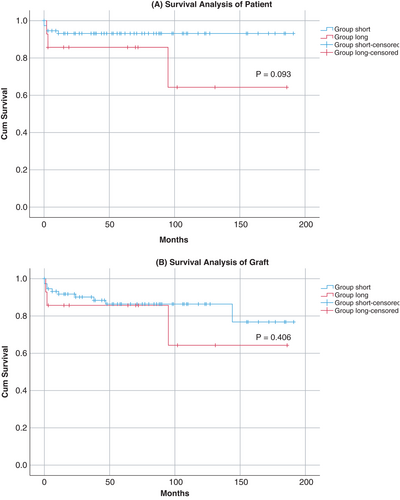
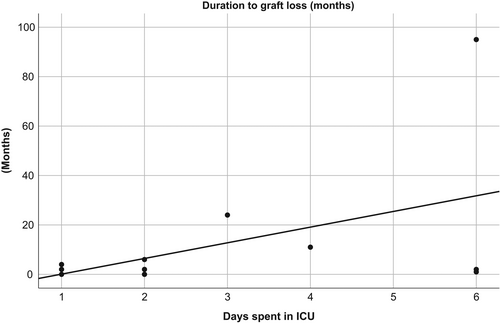
Biological markers (factor V and total bilirubin) kept normalizing in the course of the first 30 days after LT. Regression analysis between the donor's ICU LOS and those two laboratory parameters showed no significant association (Figure 2). Mean patient and graft survival time showed no significant difference between groups (Figure 3). There were more deaths in group long (21% compared to 7% in group short), however, this difference was not statistically significant (p = .11). There was no correlation between donor ICU LOS and time to graft loss (Figure 4).
4 DISCUSSION
This study of a cohort of pediatric LT patients indicates that donor ICU LOS, with a prolonged stay defined as more than five days, was associated with more biliary complications within the first year after LT.
There is little literature on the specific effect of donor ICU LOS on pediatric LT outcome. According to Devictor et al.,17 particular attention should be paid when selecting a donor for pediatric LT to donor age, cause of brain death, infections, hemodynamic stability and, indeed, ICU LOS. However, consistent data are lacking as to whether these donor characteristics actually do have an effect on the outcome of pediatric LT. In adult LT, several donor-related factors have been shown to have a negative influence on outcome, such as age over 50 years, moderate to marked steatosis of the liver, anoxia as cause of death, and possibly hypernatremia.18 Some older studies do include donor ICU LOS as a variable: Mor et al.19 found that a prolonged LOS in the ICU, defined as more than three days, did not affect early graft survival, even though there was a significantly increased rate of hepatocellular damage—defined as elevation of aminotransferases above 200 U/ml—in donors with longer LOS. In other studies, an increased donor's ICU LOS showed a possible relationship with primary non-function and initially poor function in univariate analysis, but this did not appear to be an independent risk factor for poor outcome after LT in multivariate analysis.20, 21 Strasberg et al.20 pointed out that ICU LOS did not represent a risk factor that directly influences outcome, but rather appeared to be a surrogate for other factors, explaining that with increasing LOS, donors might be subject to hypotensive episodes and low glycogen levels, both conditions being known to be factors that can lead to poorer outcome. Cuende et al.22 found an ICU stay of more than six days to be a moderate risk factor for lower graft survival; the authors explained this by the associated parenteral nutrition, presence of infections, and more aggressive hemodynamic management. In our cohort, survival and graft outcomes, as well as biological outcomes in the two groups were equal. Yet, the little number of deaths in each group made it difficult to analyze the different causes of death and their possible link to donor ICU LOS.
Recipient factors such as low weight and age, high PELD score, re-LT and LT listed as urgent/priority status are other factors that play a role in LT outcomes. Yet, these parameters did not significantly differ in our two groups. Graft characteristics and perioperative variables cannot be ignored either, since extended ischemia time and elevated blood transfusion rate are known to be poor prognostic factors.23, 24 Again, there was no significant difference between groups. Despite size matching, there was a significant difference between which type of allograft is donated depending on the donor's age (p = .001). However, since age distribution in our two groups is very similar with a median donor age of 27 years in group 1 vs. 24 years in group 2 (p = .65), this should not affect our outcome variables. We thus decided not to sub-analyze the groups.
The only outcome that showed a significant difference between groups was the incidence of biliary complications during the first year post-LT with more than twice as many biliary complications in group long. We also observed a trend toward higher mortality post-LT in group long, without it being significant. Of note, mean graft and patient survival were almost identical in both groups. As mentioned above, the types of biliary complications were bile leak, cholestasis unrelated to medical reasons such as rejection or viral infection, dilatation of the intrahepatic bile ducts, and stenosis. All biliomas were treated by external drainage, and patients presenting with evident stenosis were treated through balloon dilatation and internal–external drainage for six to twelve weeks; cholestasis was observed and it finally weaned in all cases within maximum four weeks. There was no significant difference between sub-types of biliary complications, most probably due to too small numbers, yet there was a trend of more cases of cholestasis in group long. Of note, of the three arterial thromboses seen and treated in our cohort, only one had a subsequent biliary complication. As such, this biliary complication was not included in the analysis due to its clear etiology unrelated to ICU LOS. Of note, upon analysis if there was a possible correlation between graft types and occurrence of biliary complications, there was no significant difference between whole and partial livers; also, no significant difference of the occurrence of biliary complications was seen for types of biliary anastomosis.
In an adult study, the donor ICU LOS did not have an impact on the biliary complications of the recipients.25 In their cohort, donors were double the age than in our cohort. Indeed, in our cohort donors were young, with low percentage of norepinephrine administration, normal sodium, no steatosis, low aminotransferase levels, low liver trauma percentage, and anoxia as cause of death was moderately present—and still our recipients had significantly more biliary complications in group long.
We have reason to believe that most biliary complications might be due to the same underlying reason, that is, prolonged donor LOS. Certainly, other factors can also lead to a higher rate of biliary complications, such as high donor age, high donor weight, donor–recipient sex mismatch, donation after circulatory death—but all of them not being attributable to our cohort. A factor that was significantly increased in group long was the donor's infection rate during ICU stay which, indeed, has been shown to have a negative influence on the outcome of LT, including on biliary complications.26, 27 Indeed, ICUs are the hospital wards with the highest rates of nosocomial infections and infections are often linked to the use of invasive devices: The difference of lung infections between the two groups might easily be explained by a longer duration of intubation.28, 29 Another factor, which might contribute to biliary complications, independently of the donor's LOS, is CMV mismatch, also present in our cohort. CMV infection is considered an independent risk factor for graft loss and death. However, due to the development of prevention and treatment strategies, CMV infection is no longer a major cause for morbidity or mortality.30, 31 Increased gGT was another interesting indication, that livers from group long showed already some degree of biliary suffering. If this was due to the increased rate of infection or other reasons such as altered microcirculation of the liver, that is, bile ducts, was not possible to be determined. Yet, it is another surrogate marker for the biliary future of the graft.
Although one of the first studies to look at the influence of donor ICU LOS on recipient outcome in pediatric LT, this study has several limitations, the main one being cohort size with Switzerland having only a limited number of LT cases per year. Given the current convictions, the numbers to study were especially small in group long, something which would benefit from pooling numbers from different centers. In a bigger cohort, multivariate analysis must be performed in order to take into consideration possible confounding factors. Furthermore, the fact that donors with a prolonged ICU LOS only get chosen if all other selection criteria are excellent, might be a possible selection bias, accounting for no significant differences in mortality and graft survival.
We showed in this study that prolonged donor ICU LOS, using whole organs or in situ split grafts, did not have a negative impact on the overall outcome of pediatric LT, except for significantly increasing the rate of biliary complications. Yet, since not all of the included biliary complications are severe, some even self-limited, we suggest that patients with an ICU LOS of more than five days should still be considered for liver donation if they fulfill all or most other donation criteria. But, transplant physicians must be aware that recipients from donors with prolonged ICU LOS might be more at risk of developing biliary complications than patients having received an organ from a donor with a shorter ICU LOS.
ACKNOWLEDGMENTS
We thank Simona Korff, PhD, for assisting with the LT database, as well as Lilian Penfornus and Pierre Guyon-Gellin for providing donor data. Open Access Funding provided by Universite de Geneve.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTION
All persons who meet authorship criteria are listed as authors, and all authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript. Furthermore, each author certifies that this material or similar material has not been and will not be submitted to or published in any other publication before its appearance in Pediatric Transplant. All persons who have made substantial contributions to the work reported in the manuscript but who do not meet the criteria for authorship are named in the Acknowledgments. A.M. and B.W. conceived the idea and the design of the study and carried out the acquisition of data. A.M. carried out the statistical analysis of the data and interpreted it with the help of B.W. A.M. drafted the manuscript with the help of B.W., V.M., and A.C. All authors participated in the critical revision of the article. Approval of the final version of the manuscript is to be published by all authors. B.W. supervised the project.
Open Research
DATA AVAILABILITY STATEMENT
Data are available on request from the authors.