Paraneoplastic pemphigus associated with post-transplant lymphoproliferative disorder after small bowel transplantation
Abstract
Background
PNP is a malignancy-associated autoimmune mucocutaneous syndrome due to autoantibodies against plakins, desmogleins, and other components of the epidermis and basement membrane of epithelial tissues. PNP-causing malignancies comprise mainly lymphoproliferative and hematologic neoplasms. PNP is extremely rare, especially in children.
Methods
Here, we present the first case of a child who developed PNP on a PTLD after small bowel transplantation because of a severe genetic protein-losing enteropathy.
Results
The patient in this case report had a severe stomatitis, striate palmoplantar keratoderma, and lichenoid skin lesions. In addition, she had marked esophageal involvement. She had lung pathology due to recurrent pulmonary infections and ventilator injury. Although we found no evidence of BO, she died from severe pneumonia and respiratory failure at the age of 12 years.
Conclusion
It is exceptional that, despite effective treatment of the PTLD, the girl survived 5 years after her diagnosis of PNP. We hypothesize that the girl survived relatively long after the PNP diagnosis due to strong T-cell suppressive treatments for her small bowel transplantation.
Abbreviations
-
- A2ML-1
-
- α2-macroglobulin-like-1
-
- AIHA
-
- autoimmune hemolytic anemia
-
- BMZ
-
- Basement membrane zone
-
- BO
-
- Bronchiolitis Obliterans
-
- BP180
-
- Bullous pemphigoid antigen 180 kDa
-
- BP230
-
- Bullous pemphigoid antigen 230 kDa
-
- CD
-
- Castleman disease
-
- CLL
-
- Chronic lymphocytic leukemia
-
- DGAT-1
-
- diacylglycerol-acyltransferase-1
-
- DIF
-
- direct immunofluorescence
-
- Dsg-1/3
-
- desmoglein-1/3
-
- DSP-I/II
-
- desmoplakins I and II
-
- EBV
-
- Epstein Barr Virus
-
- ECS
-
- epithelial intercellular surface
-
- ELISA
-
- enzyme-linked immunosorbent assay
-
- EP
-
- Envoplakin
-
- hrCT
-
- high-resolution computed tomography
-
- IFN-γ
-
- interferon-γ
-
- IIF
-
- indirect immunofluorescence
-
- PAMS
-
- paraneoplastic autoimmune multi-organ syndrome
-
- PET
-
- positron emission tomography
-
- PNP
-
- paraneoplastic pemphigus
-
- PP
-
- periplakin
-
- PTLD
-
- post-transplantation lymphoproliferative disorder
-
- PV
-
- pemphigus vulgaris
-
- WHO
-
- World Health Organisation
1 INTRODUCTION
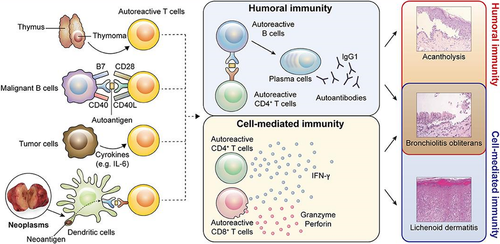
PNP is a severe autoimmune mucocutaneous syndrome characterized by severe stomatitis and mainly lichenoid skin involvement due to antibodies against plakins, desmogleins, and other components of the epidermis or basement membrane (Figure 1). PNP is also described with the more inclusive term PAMS, since multiple organs may be involved. PNP in children is most commonly induced by several lymphoproliferative and hematologic neoplasms.1 However, there are no case reports of PNP after solid organ transplantation. This is the first case of PNP in a child after solid organ transplantation associated malignancy PTLD.
2 PATIENT
We present a case of a girl that was born prematurely at 35 weeks of gestation. She was one of a monochorionic-monoamniotic twin. From birth, both girls had a severe intestinal failure with protein-losing enteropathy and were fully dependent on parenteral nutrition. Due to recurrent central venous catheter-related infections, one of the twins presented in this case underwent a small bowel transplantation at the age of 3.5 years. The post-operative period was uncomplicated, within 2 weeks she achieved complete enteral nutrition. Only years later, at the age of 9, both were found to have a DGAT-1 mutation, as was previous described by Van Rijn et al.2 Ten months after her transplantation our patient developed an EBV associated PTLD despite tapering down immune suppression (tacrolimus). The PTLD was classified as monomorphic plasmacytoma-like PTLD (according to the WHO classification).3 Within 6 months, she achieved complete remission after Rituximab and chemotherapy (Cyclophosphamide, Vincristine, and Prednisone). At the age of 7 years, she developed an AIHA and she increasingly suffered from painful red lesions on her tongue. At that moment, her maintenance therapy was tacrolimus at trough levels between 2.3 and 5.1 µg/L and low-dose prednisolone 7.5 mg/day (0.3 mg/kg/day).
Dermatological examination revealed an erosive cheilitis, ulcerations on the oral mucosae with white plaques and a lichenoid appearance on the buccal mucosae (Figure 2A), a striate keratoderma on the flexor surface of the fingers (Figure 2B), nodules with a keratotic surface on the extensor parts of the fingers (Figure 2C) and erythematosquamous papules and plaques on extremities and trunk (Figure 2D). Histopathology of tongue biopsies showed an erosion with chronic inflammation and suprabasal acantholysis (Figure 3A). DIF of perilesional tissue (tongue) showed complement C3 and immunoglobulin IgG and IgA depositions at the BMZ and IgG depositions in an epithelial cell surface pattern (Figure 3B). IIF of serum and monkey esophagus sections as substrate demonstrated anti-ECS IgG antibodies, without anti-BMZ antibodies, and IIF on rat bladder showed binding to the urothelium (Figure 3C). IgG enzyme-linked immunoassays (ELISA) detected IgG antibodies to Dsg-3 (index 142; cut off >19, MESACUP-2 test, MBL Nagoya Japan), but not to Dsg-1 (MESACU-2 test, MBL Nagoya Japan). IgA against Dgs-1 and Dsg-3 was negative. Immunoblotting was negative for EP, PP, BP230, BP180, desmoplakin I and II, plectin, plakophilin 3, laminin-332, and desmocollin 1–3 (for the methodology see reference4). Immunoprecipitation did not reveal antibodies to the protease inhibitor alpha-2-macroglobulin-like-1 (for the methodology see reference5). She was diagnosed with PNP based on the clinical features, the DIF, and the positive serology on rat bladder in combination with the history of PTLD.

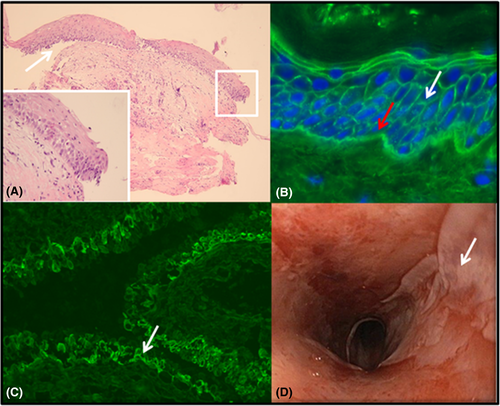
Because PNP is associated with lymphoproliferative and hematologic neoplasms we determined whether the PTLD had returned or whether other malignancies were present. Diagnostics investigations showed no recurrence of PTLD or signs of other malignancies (multiple PET scans and endoscopy of colon and small bowel). Quantitative EBV viral load was low. Because of the oral lesions, we performed an endoscopy to see if there was any gastrointestinal involvement. This showed multiple erosions over the complete esophageal tract with a positive Nikolsky sign (Figure 3D). Histology, DIF, and serology (IIF) of the biopsy material confirmed the diagnosis of esophageal involvement of the PNP. The treatment of the PNP consisted of prednisolone 2 mg/mg/day and a single dose (subsequent administrations failed due to severe pneumonia) of rituximab (950 mg/m2) to down-regulate her cellular and humoral immune response. After the rituximab infusion, she remained permanently depleted in B cells. She received immunoglobulins periodically.
After her small bowel transplantation, maintenance treatment consisted of tacrolimus (trough levels 3–7 µg/L). We tapered down the prednisolone on the basis of clinical symptoms to 0.7–1.1 mg/kg/day. Anti-Dsg-3 IgG levels decreased but remained slightly positive. IgG anti-ECS antibodies to rat bladder were alternately positive or negative. Her cutaneous and mucosal PNP was still active without excessive pain symptoms. In the 5 years from PNP diagnosis till her death, she mainly suffered from pulmonary recurrent infections, bronchiectasis, and as a result progressive decline in pulmonary function. At the age of 12 years, she developed a severe pneumonia again, based on an opportunistic multi-resistant pseudomonas aeruginosa. Antibiotics were not effective. Because of increasing discomfort and dyspnea, a palliative treatment was started with morphine at home, where she eventually died.
3 DISCUSSION
This is the first report of a pediatric case of PNP associated with lymphoproliferative disease after solid organ (small bowel) transplantation. After solid organ transplantation, PTLD is the most frequently seen lymphoproliferative and hematologic neoplasms (5-year incidence 0.2%–2.3% in adults, 2.2%–15% in children).6 However, an association between PNP and PTLD has not been described thus far.
PNP in children is very rare and is in the majority of cases caused by CD of which 32 cases are described between 1997 and 2016.7 In addition, other PNP-associated tumors were inflammatory fibrosarcoma,8, 9 Hodgkin lymphoma,10 and T-cell lymphoblastic lymphoma.11
Based on 163 cases, PNP in adults is most frequently (84%) associated with hematologic related neoplasms: non-Hodgkin lymphoma (38.6%), CLL (18.4%), CD (18.4%), thymoma, Waldenstrom's macroglobulinemia, Hodgkin lymphoma, and monoclonal gammopathy. Carcinoma, sarcoma, and melanoma are non-hematological causes that are associated with 16% of PNP cases.12 There are no cases of PNP after solid organ transplantation and/or PTLD. There is only one adult case who developed PNP after successful autologous bone marrow transplantation as treatment for his non-Hodgkin's lymphoma.13 No cases of PNP due to allogenic stem-cell transplantation have been reported.
The presence of mucositis is mandatory for the diagnosis of PNP. Besides the common oral mucositis, the nasopharynx, conjunctiva, perineal and urogenital area can be involved. Secondly, skin lesions mostly develop after the mucosal abnormalities. The skin presentations are very variable: pruritic purple-red papules (resembling lichen planus), targetoid erythematous plaques on trunk, soles, and palms (like erythema multiforme or graft-vs-host-disease) and erosions, and crusts as can be seen in PV and pemphigus foliaceus. Finally, internal organs like the lungs, gastrointestinal tract, thyroid, kidneys, and smooth muscles may be involved.
Because of the different (muco)cutaneous presentations in addition to the heterogeneous systemic involvement, the more inclusive term “PAMS” has been postulated. PAMS is the combination of multi-organ involvement and the original criteria for PNP by Anhalt et al. (revised by Camisa and Helm).1 According to this revised criteria, PNP may be diagnosed by the presence of either all three major or two major with two minor criteria (Table 1).14
| Major |
| Polymorphic mucocutaneous eruption |
| Concurrent internal neoplasia |
| Serum antibodies with a specific immunoprecipitation pattern |
| Minor |
| IIF positive cytoplasmic staining of rat bladder epithelium |
| DIF staining of perilesional tissue showing intercellular and BMZ immunoreactants |
| Histological signs of acantholysis in biopsy specimen from at least one anatomic site of involvement |
- PNP diagnostic criteria (presence of either all three major or two major with two minor criteria).
Our case met two of three major criteria of (1) polymorphic mucocutaneous eruption and (2) a concurrent neoplasia (PTLD). She did not meet the third criterion: the presence of specific PNP antibodies. These are the combination of the 210-kDa EP and the 190-kDa PP and/or the 170 kDa protease inhibitor A2ML-1. In individual cases, other antigens may be recognized too including the BP230, BP180, 250- and 210-kDa DSP-I and DSP-II, the 500-kDa plectin, plakophilin, laminin-332, and desmocollins 2 and 3. However, these antibodies are not specific to PNP as they are also present in other autoimmune bullous diseases.15-21 Most patients also have antibodies to the pemphigus antigen Dsg-3, sometimes in combination with Dsg-1. However, these anti-Dsg-3 antibodies alone do not discriminate between PV and PNP.5, 14 The positive rat bladder, which does not express Dsg-1and Dsg-3, suggests that our patient has another yet undiscovered antigen.
In our patient, the diagnosis PNP was confirmed with the presence of all three minor criteria: histological acantholysis, a positive DIF, and a positive serology on rat bladder epithelium.
Due to an incomplete knowledge of the pathogenesis of PNP, it is difficult to establish a targeted treatment protocol for PNP. First of all, it is important to detect an underlying malignancy before starting empirical immune suppression. However, in both malignant and benign (CD or benign thymoma) malignancies, PNP may persist after complete resection of the tumor.14
High-dose glucocorticoids are the first line of treatment. Although the cutaneous lesions often respond well, the oral mucosal lesions are mostly resistant to steroid therapy, similar to our case. A second step in the treatment may be rituximab. Rituximab can be used as monotherapy or in combination with other agents such as corticosteroids, intravenous immunoglobulin, or cyclosporine. Results of rituximab are highly variable. When Rituximab is not sufficient cyclophosphamide, mycophenolate mofetil, or azathioprine may cause an additional response. Alemtuzumab, a CD52 monoclonal antibody, showed promising results in adult population with PNP and B-lymphocyte CLL.22
Despite options for treatment, the mortality of PNP is high (up to 90%) despite the above mentioned treatment options. The primary cause of death is often respiratory failure (BO), evolution of the underlying neoplasm, sepsis, or multi-organ failure.23 This pulmonary injury is caused by acantholytic changes due to cellular and a humoral immune reaction directed against plakin proteins in the bronchial epithelium, with subsequent occlusion of distal alveoli with debris.
In a French adult study, 68% of 53 adult patients died and the 1-, 3-, and 5-year survival rates were 49%, 41%, and 38%, respectively.24 For children survival rates over time are unknown. In a series of Han et al., 21of the 30 children (70%) with CD died within 2 weeks to 3 years after diagnosis, mainly (17/21) due to respiratory failure. Four of these children had a biopsy-based diagnosis of BO.7 This is supported by other studies which report a high mortality (71%–79%) due to respiratory failure and/or BO in pediatric PNP patients.25-27
It was not possible to perform a bronchoscopy with biopsy or an expiratory hrCT to confirm the diagnosis of BO in our patient due to her compromised condition. Normal CT imaging showed bronchiectasis with a clear image of ventilator-induced lung injury, thus, we cannot fully exclude the presence of BO. However, the bronchiectasis was already present at an earlier age (at the age of 6 years) than pemphigus and the recurring infections can also be the cause of these radiological abnormalities. In addition, our patient had a relatively long survival, namely 5 years after the diagnosis of PNP, since most children with BO show a maximum survival of 3 years in the literature.7 We have two hypotheses for this finding.
The first hypothesis is that the PNP phenotype associated with a transplantation-related malignancy is different, compared to non-transplantation-related malignancies, because of the characteristics of the malignancy itself. However, we cannot support this hypothesis as, with the exception of one case of paraneoplastic pyoderma gangrenosum in PTLD, there is no literature of paraneoplastic phenomena in PTLD.28
A second hypothesis is that the combination of T-cell suppression (tacrolimus) and her B-cell depletion (due to rituximab) protected our patient against a fast progressive BO. Although autoantibody-mediated injury may be implicated in the development of BO, there are findings that also T-cell-mediated immunity plays an important role in the development of BO in PNP patients. For example, the transfer of Dsg-3-specific CD4+ cells into immune-deficient (RAG2−/−) mice is capable of causing comparable histopathological findings to those in patients with PNP. This is supported by the fact that lichenoid dermatitis (indicates a cellular immune mechanism) and CD8+ T-cell infiltration are frequently observed in the epidermis of PNP. Also in BO, it is very likely that this cellular immunity plays a crucial role in the pathogenesis.14, 29 Therefore, T-cell targeted therapy may add a new dimension to the current approach.
Despite both unsupported hypotheses, we presented a patient with a unique combination of PNP and other autoimmune phenomena after solid organ transplantation.
4 CONCLUSION
This article describes a unique case of PNP with skin and oral, an esophageal involvement, in association with a PTLD after solid organ transplantation. To our knowledge, this has never been described in adult and pediatric literature. Our patient survived for 5 years after the diagnosis PNP, which is exceptionally long because mortality rates are extremely high (70–80%) and mainly due to BO. We hypothesize that the strong T-cell suppressive treatment for her small bowel transplantation plays a role in this.
CONFLICT OF INTEREST
The authors have no conflict of interest related to this work.
AUTHORS’ CONTRIBUTIONS
Sander A. R. Fidder, Marieke C. Bolling, and Hubert P. J. van der Doef: Came up with the idea to write down the case; Sander A. R. Fidder, Marieke C. Bolling, and Hubert P. J. van der Doef: Wrote the first version; Sander A. R. Fidder, Hubert P. J. van der Doef, Gilles F. H. Diercks, and HD: Composed the figures; Dermatological and histological details have been written down by Marieke C. Bolling, Gilles F. H. Diercks, and Hendri H. Pas. All authors made corrections based on their expertise. All authors contributed to the final manuscript.