Motor development in children 0-2 years pre- and post-LTX, a prospective study
Abstract
To determine prospectively gross and fine motor development of children <2 years of age, who undergo LTX.
In this prospective study, children aged <2 years who undergo LTX were tested using the motor scale of the Bayley Scales of infant and toddler development, 3rd edition Dutch version. Testing was done during screening pre- and post-LTX: at the time of hospital discharge (2-6 weeks), at 3 months, 6 months, and 1 year. Z-scores were calculated.
Twenty-nine children participated in this study, 14 boys, median age 6 months, at screening for LTX. Gross motor skills were delayed pre-LTX (Z-score −1.3). Fine motor skills were normal (Z-score 0.3). Immediately post-LTX, both skills reduced, and at 1 year post-LTX, gross motor skills Z-score was −1.0 and fine motor skills Z-score 0.0.
Both gross and fine motor skills Z-scores decline post-LTX and tend to recover after 1 year, gross motor skills to low normal and fine motor skills to normal levels. Monitoring of gross motor development and attention on stimulating gross motor development post-LTX remains important, to enable participation in physical activity and sport for health benefits later in life.
Abbreviations
-
- ALT
-
- alamine aminotransferase
-
- AST
-
- aspartate aminotransferase
-
- Bayley III NL
-
- Bayley Scales of infant development 3rd edition, Dutch version
-
- GT
-
- glutamyl transferase
-
- INR
-
- international normalized ratio
-
- IQR
-
- interquartile range
-
- LTX
-
- liver transplantation
-
- MUAC
-
- mid-upper arm circumference
-
- N
-
- number of valid observations
-
- PELD
-
- pediatric end-stage liver disease
-
- PT
-
- prothromin time
-
- SD
-
- standard deviation
-
- UMCG
-
- University Medical Center Groningen
1 INTRODUCTION
LTX is the standard care for children with a life-threatening liver disease. New surgical techniques and immune-suppressive medication have improved survival of these children.1 In the Netherlands the 5-year survival has increased in the last 2 decades from 71% to 83%. Living related LTX in the Netherlands has a 5-year survival of 95%.2 Given this high survival rate, it is important to focus on the long-term outcomes. Beside hypertension, atherosclerosis, reduced growth, obesity, lowered bone density, osteoporosis, increased cardiovascular risk factors, and reduced aerobic exercise capacity, a reduced motor development has been reported in these children.3-11 Children with liver diseases are at risk in all neurodevelopmental domains; cognitive, behavioral, and motor outcomes.11
Although most studies showed impaired motor development in children pre- and post-LTX,9, 10, 12-14 one study showed motor scores improved and children reached the norm for their age within 4 years post-LTX.15 In another study, 2 year follow-up showed low normal motor development scores following pediatric LTX.10 Studies do not always distinguish between gross and fine motor skills. In one study in children with biliary atresia pre-LTX, gross and fine motor skills were studied separately.12 It was shown that gross motor skills were delayed, while fine motor scores were relatively preserved.12 One can imagine that by scoring motor development as a single score low scores on gross motor skills may be compensated by better fine motor skill scores or vice versa.
Insight in the separate scores of gross and fine motor skills is needed pre- and post-LTX as motor skill development during early childhood may have health benefits on the short term as well as on the long term.16 In addition, for clinical relevance insight in longitudinal data of motor development after LTX is needed, in order to be able to refer more specifically to a pediatric physical therapist for stimulating motor development in case of a delayed motor development.
The aim of this study was to evaluate gross and fine motor development in children, aged 0-2 years, pre-LTX (screening), at the time of hospital discharge (2-6 weeks), and at 3 months, 6 months, and 1 year post-LTX, to determine the extent and the course of the motor development over time.
2 PATIENTS AND METHODS
All children aged 0-2 years, who were screened for LTX and put on the waiting list for a LTX at the UMCG, the only pediatric liver transplant center in the Netherlands, were eligible for this prospective study. Patients were included between May 2015 and November 2017.
Assessments of the motor development were performed pre-LTX at the time of screening and post-LTX around discharge (2-6 weeks), at 3 months, 6 months, and 1 year post-LTX. Assessments were combined with a visit to the outpatient clinic of the UMCG or during a short hospital stay for medical evaluation.
Exclusion criteria were related to secondary diagnosis that might intervene with the assessment not associated with LTX such as Down syndrome. The Medical Ethical Committee of the UMCG stated that this study fulfilled all requirements for patients’ anonymity and it is in agreement with regulations of the UMCG for publication of patient data (M19.227796).
2.1 Motor development
We assessed motor development using the motor scale of the Bayley Scales of infant and toddler development, 3rd edition (Bayley III),17 because it differentiates between gross and fine motor development. Besides Dutch norm values are available and we therefore used the Dutch version ((Bayley III-NL).18 The Bayley Scales of infant and toddler development is widely used in the clinical evaluation of young children with developmental delay and provides age-standardized composite scores for cognitive, language, and motor skills. Motor development is divided in gross and fine motor skills with a mean score of 10 and a SD of 3. The scores are interpreted as follows: normal motor development ≥7 (Z-score ≥−1) and delayed motor development <7 (Z-score <−1). The Bayley III-NL is a valid and reliable instrument.18
2.2 Patient characteristics
Weight (kilograms) and height (centimeters) were measured using an electronic scale and a stadiometer (Seca). MUAC was measured using measuring tape.
All the other study variables like type of liver disease, type, date and number of LTX, admissions pre-LTX, central venous catheter pre-LTX, length of hospitalization post-LTX, length of intensive care unit (days), medication, laboratory values (PT, INR, Bilirubin, Albumin, AST, ALT, gamma GT, and cholesterol), PELD score, pediatric physical therapy, or other treatment on stimulating motor development were asked for or retrieved from the medical files. No reliable information was available about the socioeconomic status of the families of these children.
2.3 Statistical analysis
2.3.1 Sample size
As all pediatric LTXs in the Netherlands are performed in our hospital (UMCG) all Dutch children that underwent LTX were eligible for this study. Data were checked for normality, and Z-scores for gross and fine motor development were calculated. Z-scores were calculated as (Value patient - Mean norm)/SD norm.
Differences in motor development between children with or without pediatric physical therapy and children with a living donor and children with deceased donors were calculated using the Mann-Whitney U test.
3 RESULTS
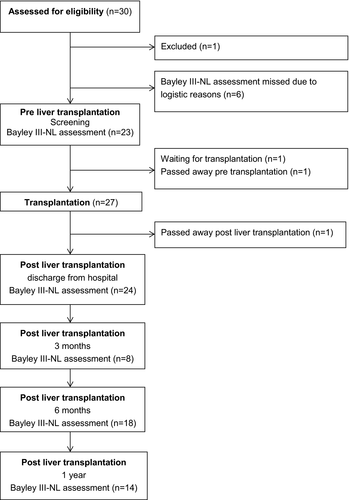
One child was excluded from the study because of the exclusion criteria. Twenty-nine children, 14 boys (48%), median age 6 months ([IQR] 4.0; 6.0), were eligible and participated in this study (Table 1). Of these 29 children, three were born premature (36 + 4 and 36 + 5), and 1 child was born at 32 weeks, but did not show abnormalities at a term age. One child had a tetralogy of Fallot, corrected at the age of 5 months, and 1 child (also premature born) had an esophagus atresia, corrected at day one, both without complications.
| Characteristics (n = 29) | |
|---|---|
| Type of liver disease | |
| Biliary atresia | 26 (90%) |
| Acute liver failure | 2 (7%) |
| Familiar hypercholesterolemia | 1 (3%) |
| Transplantation (n = 27) | |
| PELD pretransplantation | 12.0 [6.0; 16.0] |
| PELD LTX | 17.0 [12.0; 23.0] |
| Age at LTX (months) | 8.0 [6.0; 10.0] |
| Time between screening and LTX (months) | 3.0 [1.0; 3.0] |
| Type of LTX | |
| Partial living donors | 19 (70%) |
| Partial deceased donors | 7 (26%) |
| Full size | 1 (4%) |
| Number of LTXs | |
| 1 | 25 (93%) |
| 2 | 2 (7%) |
| Number of days on intensive care unit (days) | 10.0 [6.0; 15.5] |
Note
- Data are presented as numbers (percentages) or as medians and [IQR].
In total, six assessments of the Bayley III-NL were missing pre-LTX because of logistic reasons. Pretransplant ascites was present in 13 out of 29 children (45%), six out of 29 children (21%) had a central venous catheter pre-LTX, and 18 out of 29 children (62%) had 1 or more admissions pre-LTX with a median duration of 27.5 days (IQR 7.0; 40.0). At time of analyzing this study, one child was waiting for a LTX and 1 child died on the waiting list for LTX. In total, 27 children had a LTX. One child died post-LTX (Figure 1). Two children needed retransplantation, one child because of ischemic type of biliary lesions and the other because of hepatic artery thrombosis. In total, 23 children were assessed at time of screening for LTX (Table 2).
|
Pre-LTX (screening) median (IQR) n = 23 |
Post-LTX (discharge) median (IQR) n = 24 |
Post-LTX (3 mo) median (IQR) n = 8 |
Post-LTX (6 mo) median (IQR) n = 18 |
Post-LTX (1 y) median (IQR) n = 14 |
|
|---|---|---|---|---|---|
| Gender, boys (%) | 11 (48%) | 13 (54%) | 4 (50%) | 9 (50%) | 9 (64%) |
| Age (months) | 6.0 (4.0; 6.0) | 9.0 (7.3; 11.8) | 11.5 (11.0; 15.0) | 13.5 (12.8 ;16.0) | 20.0 (19.8; 24.8) |
| Height | 65.0 (62.0; 67.0) | 74.0 (68.0; 78.0)a | 76.8 (72.6; 83.4) | 78.5 (75.3; 82.5)b | 86.5 (81.0; 89.3) |
| Z-score | −0.3 (−1.2; 0.4) | -0.1 (−0.7; 0.8)a | 0.2 (−0.2; 0.8) | -0.1 (−1.0; 0.6) b | -0.3 (−1.2; 0.4) |
| Weight | 7.5 (6.3; 8.0) | 8.9 (8.4; 10.8) | 9.9 (9.6; 11.9) | 10.4 (9.7; 10.9)c | 12.1 (11.4; 14.2) |
| Z-score | 0.1 (−0.3; 0.6) | 0.2 (−0.4; 0.6) | 0.0 (−0.4; 0.7) | -0.3 (−0.5; 0.5)c | -0.1 (−1.3; 0.5) |
| Weight for height Z-score | 0.7 (−0.1 ;1.3) | 0.5 (−0.4; 1.0) | 0.1 (−0.3; 0.8) | 0.0 (−0.5; 0.5) b | -0.1 (−0.7; 1.3) |
| Muac | 13.0 (12.4; 13.5)d | 14.1 (13.8; 15.0)a | 15.9 (15.0; 16.5) | 15.6 (14.9; 16.3)c | 15.4 (13.8; 16.7) e |
| Z-score | −1.0 (−2.0; −0.5)d | −0.5 (−1.0; 0.0)a | 0.0 (−0.9; 1.2) | 0.2 (−0.6; 0.6)c | −1.0 (−2.1; 0.3)e |
| Physical therapy (%) | 1 (4%) | 10 (42%) | 5 (63%) | 9 (50%) | 6 (43%) |
| Frequency | |||||
| <1 × (week) | 1 (20%) | 2 (22%) | 3 (50%) | ||
| 1 × (week) | 5 (50%) | 3 (60%) | 7 (78%) | 3 (50%) | |
| 2 × (week) | 1 (100%) | 5 (50%) | 1 (20%) | ||
| Laboratory value | |||||
| PT | 11.9 (11.4; 13.8) | 12.0 (10.5; 13.4)f | - | - | 11.6 (11.1; 12.1)g |
| INR | 1.1 (1.1; 1.3) | - | - | - | 1.1 (1.0; 1.2)g |
| Total bilirubin (umol/L) | 144.0 (115.0; 220.0) | 6.5 (5.3; 9.0) | 6.0 (5.3; 11.5) | 7.5 (6.0; 10.8) | 5.5 (3.0; 8.5) |
| Albumin (g/L) | 35.0 (32.0; 39.0) | 36.5 (32.0; 39.0) | 41.0 (37.0; 42.8) | 40.5 (36.8; 41.3) | 43.0 (41.8; 44.0) |
| AST (U/L) | 218.0 (156.0; 343.0) | 41.5 (33.0; 52.0) | 56.5 (49.5; 97.8) | 52.0 (42.3; 63.8) | 47.5 (39.8; 55.3) |
| ALT (U/L) | 184.0 (100.0; 210.0) | 48.0 (35.8; 65.8) | 103.5 (54.3; 110.8) | 45.5 (36.8; 65.3) | 31.5 (23.0; 39.8) |
| Gamma GT (U/L) | 427.0 (199.0; 536.0) | 154.5 (91.0; 246.0) | 72.0 (21.0; 140.8) | 41.0 (22.8; 92.5) | 22.0 (15.0; 48.3) |
| Cholesterol | 4.4 (3.6; 7.1)d | 2.9 (2.5; 4.2)h | 3.1 (2.7; 4.6)h | 3.2 (2.8; 4.0)i | 3.2 (2.7; 3.6) |
Note
- Norm values for Z-scores for height, weight and MUAC by TNO.24
- a n = 23 valid observations.
- b n = 16 valid observations.
- c n = 17 valid observations.
- d n = 21 valid observations.
- e n = 8 valid observations.
- f n = 19 observations.
- g n = 13 valid observations.
- h n = 7 valid observations.
- i n = 14 valid observations.
The median time of the assessment of the Bayley III-NL at discharge was 3.5 weeks (IQR 2.0; 5.8). At 3 months post-LTX, not all the children were seen in our outpatient clinic due to a short period between discharge and this evaluation moment or evaluation in a local hospital and therefore not all Bayley III-NL scores were available (Table 2).
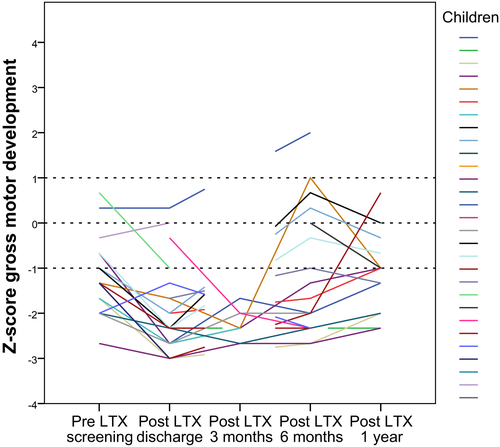
Gross motor development was delayed pre-LTX, Z-score −1.3, and reduced post-LTX, and reduced further 3 months post-LTX (Table 3 and Figure 2). After 6 months, Z-scores were still lower compared to pre-LTX and 1 year post-LTX gross motor skill Z-scores were low normal (Z-score −1.0). Trajectories of individual children on gross motor Z-scores over time are shown in Figure 3.
|
Pre-LTX screening n = 23 |
Post-LTX discharge n = 24 |
Post-LTX 3 mo n = 8 |
Post-LTX 6 mo n = 18 |
Post-LTX 1 y n = 14 |
|
|---|---|---|---|---|---|
| Gross motor development | |||||
| Standard score | 6.0 (5.0; 8.0) | 3.0 (2.0 ;5.0)a | 3.0 (2.3; 4.0) | 4.5 (3.0; 9.3) | 7.0 (4.0; 8.3) |
| Z-score | −1.3 (−1.7; −0.7) | −2.3 (−2.7; −1.7)a | −2.3 (−2.6; −2.0) | −1.8 (−2.3; −0.3) | −1.0 (−2.0; −0.6) |
| Fine motor development | |||||
| Standard score | 11.0 (8.0; 13.0) | 9.0 (7.0; 10.0) | 8.0 (7.0; 12.0) | 8.5 (6.8; 10.3) | 10.0 (8.8; 11.5) |
| Z-score | 0.3 (−0.7; 1.0) | -0.3 (−1.0; 0) | -0.7 (−1.0; 0.7) | -0.5 (−1.1; 0.1) | 0.0 (−0.4; 0.5) |
- a n = 23 valid observations.
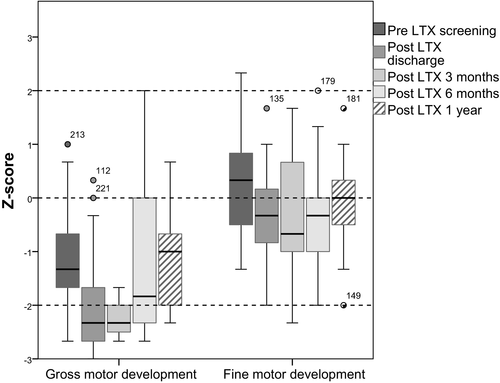
Fine motor development was normal pre-LTX, Z-score 0.3 (Table 3 and Figure 2). Z-scores reduced post-LTX around discharge, at 3 and 6 months post-LTX, but were 1 year post-LTX on the level of pre-LTX (Z-score 0.0).
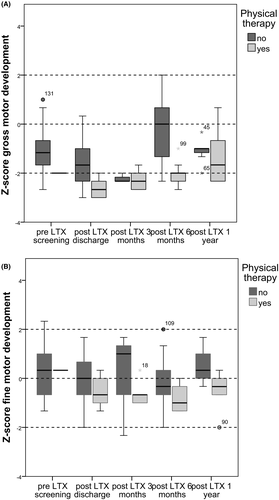
Pre-LTX 1 child received pediatric physical therapy and post-LTX 10 out of 24 children. The children who received pediatric physical therapy was aimed at improving motor development. Children receiving pediatric physical therapy more often showed significant lower gross motor scores compared to children without pediatric physical therapy (Figure 4A,B). Post-LTX around discharge gross motor skills were significantly lower (P < .01) in the pediatric physical therapy group, and at 6 months post-LTX, gross motor skills were still significantly lower in this group (P .02). At all other evaluation moments, no significant differences were found between the group with or without pediatric physical therapy. No significant differences were found in motor development scores between children with transplants of living donors and deceased donors (details not provided, available upon request to corresponding author).
Boxplots of potential risk factors for delayed gross motor development are provided in Appendix 1, pre-LTX (Figure A1A-D and direct post-LTX Figure A2A-D.
4 DISCUSSION
This study showed that children pre-LTX had delayed gross motor skills and normal fine motor skills. Both Bayley III-NL Z-scores on gross and fine motor skills reduced post-LTX and at 1 year post-LTX motor development tend to recover; gross motor skills to low normal and fine motor skills stayed within the normal range.
Our cohort included all but one child and therefore showed a true reflection of the Dutch LTX population. Our findings of delayed motor development pre-LTX and recovering of motor development to low normal post-LTX was also found previously in a study in children with liver based metabolic disorders.10 In that study, low normal motor development scores were found 2 years post-LTX,10 but motor development was assessed with the Bayley scales of infant development 2nd edition, where no distinction is made in gross and fine motor skills and motor development scores are a combination of both. As found in our study, but also previously, fine motor skills scores pre-LTX were within normal values.12 In that study, motor development was assessed with the Mullen Scales of Early Learning but no longitudinal analysis was performed.12 A delayed gross motor development might not be recognized when gross and fine motor development is presented as a combined score.
Another study showed no improvement of motor scores over time post-LTX,9 while yet another study showed improvement of motor scores to normal within 4 years post-LTX.15 In that study, the Griffiths Mental Ability Scales (Griffiths-II) was used to determine motor development, but this assessment tool seems to give higher motor scores compared with the Bayley scales of infant development, 2nd edition.19 When parents, of children with a LTX, score their children, they also score significantly more motor developmental problems compared to norm values.14
Delayed motor development in children pre-LTX can be understood due to their illness. These children also have growth failure, abdominal distension, and therefore are less in prone position.15, 20 One might expect that 1 year post-LTX children catch up on their motor development as there are fewer limitations, but unfortunately, they do not fully recover. In our study, median Z-scores of height, weight, and weight for height were not below −2. Children are referred to our hospital at a young age, and we have an aggressive nutritional therapy regime. Tube feeding is applied with a low threshold, and this regime might explain the low incidence of pretransplant growth failure. MUAC Z-score pre-LTX and 1 year post-LTX were low normal. Although gross motor skill Z-scores are −1.0, 1 year post-LTX, one might find this within the low normal range, but still 50% of these children had a delayed gross motor development. Trajectories of individual children on gross motor Z-scores over time showed that most of the children recovered to their pretransplantation scores (Figure 3). Some scored better, and some children declined in their Z-scores between 6 months and 1 year post-LTX but patterns differed. Of the ones declining in their gross motor skill Z-scores, most of them did not receive physical therapy.
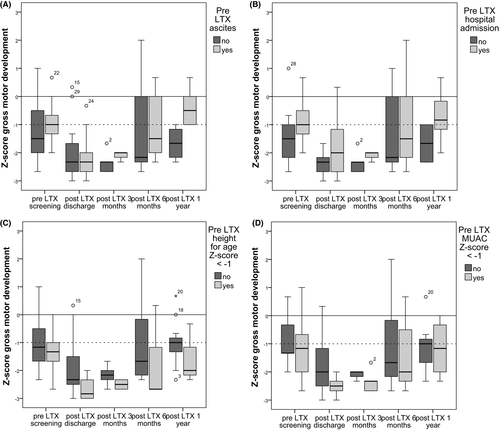
Based on inspection of the boxplots in Appendix 1 height for age (Z-scores <−1) pre-LTX seems to be a risk factor for delayed gross motor development. Remarkably, children with pre-LTX ascites and hospital admission had higher gross motor development scores. We are unable to come up with a logical explanation for this finding. A study of four malnourished, pretransplant children showed that aggressive nutritional support resulted in an improved mid-arm muscle and fat area and improved motor scores in 3 children. These results suggest that aggressive nutritional support may prevent developmental delay.15
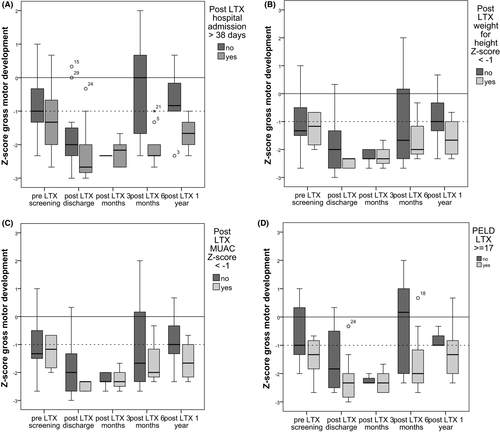
Admission more than 38 days post-LTX, weight for height Z-score < −1 post-LTX and a PELD score > 17 at transplantation seemed to be risk factors for delayed gross motor development. Appendix Figures A1 and A2, however, should be interpreted with caution because they are based on a limited number of available observations (Figure 3) and longitudinal multivariate analysis cannot be performed. Because we missed observations at the appointed times we do not have, for each child, observations at each time. Since it was not our primary goal to identify risk factors, we have chosen to elaborate on some factors (growth factors, hospital admission, PELD, and ascites) and to illustrate trends. Further research is needed with larger sample size and a saturated dataset to draw conclusions about potential risk factors on gross motor development.
It has been suggested that educating parents regarding appropriate developmental expectations (both cognitive and motor) might increase the parents compliance with developmental interventions as parents often believe and wish their children will be normal post-transplantation.13
In our study, children receiving pediatric physical therapy showed lower Z-scores on gross motor skills. Probably, only the children who are delayed in their motor development were referred for pediatric physical therapy. The percentage of children receiving pediatric physical therapy increased post-LTX. Based on clinical considerations, pediatric physical therapy was advised when motor development was delayed which often occurs, post-LTX. No reliable information was available about socioeconomic status of families of these children, but in the Netherlands physical therapy and health care is accessible to every child.
Gross motor scores post-LTX around discharge were probably underestimated as prone position scores were generally difficult to score due to the effects of surgery. The median time of this assessment was 3.5 weeks post-LTX at which prone position was not recommended. Since we could not observe the prone items of the Bayley III-NL, items were scored negative. But this underestimation cannot explain the delayed gross motor development at 3 months post-LTX. Only eight of the possible 26 were seen at 3 months assessment. Of these children, five received pediatric physical therapy for delayed motor development. It could be that the motor development not assessed in our hospital was higher.
For long-term outcomes, a normal motor development appears to be important as studies in children with high compared to low motor scores suggested that children with low motor scores have low scores on physical fitness as well.21, 22 Therefore, the findings of our study suggest the importance to identify the level of motor development in young children and during follow-up as for long-term outcome normal motor development is necessary to prevent low physical fitness later in life, but also to be able to participate in physical activities. When children are unable to run, jump, catch, and throw etc, they have limited opportunities to participate in physical activities because they lack the necessary skills. It is of clinical importance to continue to monitor the motor development of these children in order to be able to refer the children to a pediatric physical therapist, because still little is known about long-term motor development in these children and therefore the possible limitations in participation in sports and physical activity for health benefits later in life. Despite the fact that many children received physical therapy, the gross motor development post-LTXs were low normal after 1 year. Children with physical therapy gradually improved over time, and the children who did not receive physical therapy declined in their gross motor development scores between 6 months and 1 year. However, these data should be interpreted with caution, because of missing gross motor skills physical therapy data. Not all children with a delayed gross motor development received physical therapy at time of the BSID III-NL assessment. It is up to the parents/caregivers to follow the advice of starting physical therapy in case of a delayed motor development. In addition, we only made an inventory of whether children had physical therapy at the time of the assessment of the BSID III-NL. Besides we did not systematically monitor the content and frequency of the pediatric physical therapy interventions and therefore no conclusions can be made about the effect of physical therapy on motor development in these children. Therefore, it is unknown what the direction of the effect of physical therapy is in these children. In general, in a systematic review, it was found that interventions with a task oriented framework is effective in increasing motor development in children with developmental coordination disorders or cerebral palsy.23 Future study of the interventions of pediatric physical therapy in stimulating gross motor outcome in children post-LTX is needed.
This study has some limitations. It was a small sample, but all but one available cases in the Netherlands were analyzed in this study. We were not able to assess Bayley III-NL at all the control visits for logistic reasons, and assessments were postponed to the next visit. The 3-month post-LTX evaluation was the most difficult regarding the assessment with the Bayley III-NL, because of recent discharge or check-up was done at a local hospital. Ideally, we would have performed statistical analysis of changes over time, but given the small cohort and missing values we showed trajectories over time for each child (Figure 3). Therefore, we were not able to analyze potential risk factors on gross motor development more specifically. As earlier mentioned prone position especially for the assessment around discharge was not recommended and therefore prone position items were scored as negative as we could not observe these items and therefore gross motor skills were underestimated. Growth scores were assessed by the different observers which might have caused measurement errors. As our cohort had a low incidence of growth failure pretransplantation and we found low normal gross motor development scores 1 year after LTX, our findings might be an underestimation of the risk of motor developmental delays in other cohorts with more growth failure.
In conclusion, both gross and fine motor skills Z-scores decline post-LTX and tend to recover after one year, gross motor skills to low normal and fine motor skills to normal levels. Monitoring of gross motor development and attention on stimulating gross motor development post-LTX remains important, to enable participation in physical activity and sport for health benefits later in life.
ACKNOWLEDGMENTS
The authors would like to thank Ronald de Jong and Anneke Hegeman, pediatric physical therapists of the University Medical Center Groningen of the Department of Rehabilitation Medicine for assessing gross and fine motor development and Tietie Dijkstra for collecting growth data.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
AUTHORS' CONTRIBUTIONS
GJF Joyce Bos, Otto THM Lelieveld, Rene Scheenstra, Pieter JJ Sauer, Jan HB Geertzen, and Pieter U. Dijkstra: Participated in research design; GJF Joyce Bos and Pieter U. Dijkstra: Analyzed data; GJF Joyce Bos, Carola Y. Timmer, Otto THM Lelieveld, Rene Scheenstra, Pieter JJ Sauer, Jan HB Geertzen, and Pieter U. Dijkstra: Wrote the paper; and Carola Y. Timmer: Collected data.
Appendix 1
Boxplots were constructed (Appendix Figure A1A-D) for possible pre-LTX risk factors for delayed gross motor development: (A) ascites, (B) hospital admission, (C) height Z-score < −1, and (D) MUAC Z-score <−1) are set against Z-score of gross motor skills over time. Both children with Pre-LTX ascites and children with Pre-LTX hospital admission had higher gross motor skills scores compared to children without Pre-LTX ascites or admission. Children with a height Z-score below −1 (n = 7) did not catch up on the gross motor development score and were delayed 1 year post-LTX. MUAC Z-score <−1 (n = 10) compared to children with higher MUAC scores showed no clear pattern. Plots were also constructed for central venous lines, weight for age and weight for height, but showed no new insights (available on request of the author).
In Appendix Figure A2A-D, the possible post-LTX risk factors are as follows: (A) hospital admission post-LTX >38 days (median admission duration), (B) weight for height Z-score < 1, (C) MUAC Z-score <−1, and (D) PELD score >17 at transplantation are set against Z-score of gross motor skills over time. Children with a admission duration post-LTX >38 days (n = 12) had lower gross motor skill scores compared to children with a shorter hospital admission duration. Children with a weight for height Z-score <−1 (n = 3) did not catch up on their gross motor skill score and were delayed in their gross motor development 1 year post-LTX. MUAC Z-score <−1 (n = 5) compared to children with higher MUAC scores showed no effect on gross motor development. Children with a higher PELD score >17 (n = 16) at LTX seem to have worse gross motor development scores post-LTX compared to children with lower PELD scores. Plots for intensive care admission, height for age were also constructed, but showed no new insights or consisted of a too small number of observations (available on request of the author).