Monitoring intra-abdominal pressure after liver transplantation in children
Abstract
IAH after LTX can impair perfusion and threaten graft viability. This study aimed to assess the feasibility of longitudinal IAP measurements as an IAH screening method in children after LTX. A cohort of 23 children with a mean age (range) 3.1 (3 months–14 years) who underwent LTX between May 2017 and February 2018 were evaluated retrospectively. Longitudinal IAP measurements were compared to bedside Doppler US monitoring data. In total, 425 IAP measurements and 257 US examinations were performed. The mean ± SD (range) time expenditure for IAP measurement was 1.9 ± 0.4 (0.5-3.2) minutes. The mean post-operative IAP was 7.9 ± 3.6 (1-25) mm Hg. IAH (IAP ≥ 10 mm Hg) was noted in 102 (24%) of 257 measurements. Agitation had a significant impact on IAP (estimate: 9.3 mm Hg, CI: 6.72-11.97, P < .01). In patients with TAC, IAP was increased (6.7 ± 2.1 vs 8.7 ± 3.1 mm Hg, P = .02) while peak portal venous velocities decreased (38 ± 27 vs 26 ± 22 cm/s, P = .03) after patch reduction. An abdominal compartment syndrome with severely impaired vascular flow was noted in one patient. Episodes of elevated IAP were noted in a large proportion of patients, underscoring the need for IAP monitoring in pediatric liver transplant recipients. The safety and low time expenditure associated with IAP measurement could be included easily into standard nursing procedures for these patients.
Abbreviations
-
- APP
-
- abdominal perfusion pressure
-
- CI
-
- confidence interval
-
- IAH
-
- intra-abdominal hypertension
-
- IAP
-
- intra-abdominal pressure
-
- LTX
-
- liver transplantation
-
- MAP
-
- mean arterial pressure
-
- PICU
-
- pediatric intensive care unit
-
- RI
-
- resistance index
-
- SD
-
- standard deviation
-
- TAC
-
- temporary abdominal closure
-
- US
-
- ultrasound
-
- WSACS
-
- World Society of Abdominal Compartment Syndrome
1 INTRODUCTION
Pediatric LTX is an established procedure for children with end-stage liver disease or congenital metabolic disorders. Because the majority of pediatric patients requiring LTX are younger than 1 year old and there are few pediatric donor organs available, partial adult organs are often used.1 A size mismatch between a small abdominal cavity and a relatively large split organ in small children can lead to traction and insufficient compliance of the abdominal wall,2-5 a circumstance that has the potential to produce high IAP. Impaired arterial and portal blood flow due to an elevated IAP can lead to graft dysfunction.6-11 Therefore, a TAC with a patch is often used to enlarge the abdominal cavity in small children receiving a liver transplant.3-5 Vascular flow assessment with Doppler US is the gold-standard method for monitoring graft perfusion though it does not detect IAP directly.12, 13
In 2013, the WSACS published consensus guidelines to standardize definitions and management of IAH.14 In children, IAH is defined as an IAP ≥ 10 mm Hg (grade I, 10-12 mm Hg; grade II, 13-15; grade III, 16-19 mm Hg; grade IV ≥ 20 mm Hg).14 IAH affects 15%-45% of PICU patients.15, 16 Excessively high IAP that interferes with vascular inflow and venous return and thereby endangers the viability of abdominal organs constitutes a condition known as abdominal compartment syndrome, which is diagnosed in 0.7%-4.7% of children treated in PICUs and associated with very high mortality (40%-80%).17-20
The clinical relevance of IAH and abdominal compartment syndrome in intensive care patients and their impacts on morbidity and mortality have come into focus in recent years.14-21 This study aimed to analyze longitudinal IAP measurements in children who underwent LTX. IAP values were compared to other standards of monitoring graft viability, including US, laboratory, and clinical parameters.
2 PATIENTS AND METHODS
2.1 Subjects
This retrospective single-center study was legitimated by the local medical statue (details for blinding omitted) and written-informed consent was waived. All examinations were conducted according to the Declaration of Helsinki. All 23 pediatric patients who underwent LTX between the 26th of May in 2017 and the 5th of February in 2018 at our center were included in this study. Their demographics, clinical characteristics (including indications for LTX and donor liver characteristics), intra-operative variables (including primary vs temporary closure), and post-operative care variables are summarized in Table 1. Primary abdominal closure was performed when possible; TAC with a silicon patch (reinforced Silicon Sheeting, Invotec International) tailored to the needs was applied when donor grafts were too large for the recipient patient's abdominal cavity. The mean ± SD (range) number of patch reduction procedures performed before definitive abdominal closure per patient was 2.0 ± 1.0 (1-5). Data including patient demographics, peri-operative findings, complications, and outcomes, were extracted from the liver transplant database and surgery, anesthesia, and PICU records.
| Number of patients | 23 |
| Sex | |
| Male | 14 (61) |
| Female | 9 (49) |
| Age, y | 3.1 ± 4.0 (3 mo-14 y) |
| Bodyweight, kg | 14.3 ± 12.8 (5.0-60.0) |
| Length, cm | 86 ± 29 (58-163) |
| Body mass index | 16.7 ± 2.8 (11.8-22.6) |
| Organ | |
| Living donor | 6 (26) |
| Deceased donor | 17 (74) |
| Split | 21 (91) |
| Full | 2 (9) |
| Weight, g | 423 ± 365 (230-1570) |
| Graft-to-body weight ratio, % | 3.1 ± 1.2 (1.4-5.2) |
| Abdominal closure | |
| Primary | 5 (22) |
| Temporary | 18 (78) |
| No. patch reductions to definitive abdominal closure | |
| One | 5 (22) |
| Two | 6 (26) |
| Three | 8 (35) |
| Four | 2 (9) |
| Five | 2 (9) |
| Days between successive patch reduction operations | 4.7 ± 1.4 (2.8-7.6) |
| Post-operative days to definitive abdominal closure | 9.3 ± 6.9 (1-8) |
| Length of PICU stay after transplantation, d | 11.5 ± 10.7 (2-53) |
| Length of PICU stay, d | 14.7 ± 18.7 (3-96) |
| Diagnosis distribution | |
| Biliary atresia | 6 (26) |
| Metabolic disease | 7 (30) |
| Acute liver failure | 3 (13) |
| Idiopathic cirrhosis | 4 (17) |
| Progressive familial intra-hepatic cholestasis, type II | 2 (9) |
| Chronic cholestatic liver disease | 1 (4) |
- Note: Counts and percentages are written as n (% of all patients). Continuous variables are reported as means ± SDs (range).
2.2 IAP measurement
According to WSACS's recommendations,14 we measured IAP intra-vesically. We used a hydrostatic manometer, the UnoMeter™ AbdoPressure™ IAP Monitoring System (Unomedical, ConvaTec™) connected to a Foley catheter (Uromed™ Prosil, Kurt Drews KG). The IAP Monitoring System was already installed preoperatively during anesthesia and surgery preparation in 10 patients. In the remaining 13 patients, it was installed post-operatively in the PICU. The IAP Monitoring System was inserted between the Foley catheter and the drainage hose. The hose clip was closed to prevent flow in the direction of the patient. A 20-mL volume of sterile saline solution was then injected into the system through a needle-free KombiKon™ sample port under sterile conditions to prime the system once after installation in order to remove the air from the tubing system. The IAP measurements were performed in a completely supine position at end expiration. The zero reference of the IAP monitoring system was zeroed at the mid-axillary point. The Unometer AbdoPressure tubing system was then lifted above the patient's level, and the hose clip was opened to allow a passive backflow of the patient's urine into the bladder. When the water column settled into a steady state, IAP was read and documented in mm Hg. IAP measurements were performed in the PICU at least every 8 hours until the patient was discharged from PICU. To evaluate the influence of patch reduction on IAP, the last IAP value obtained during the 4 hours preceding and the first IAP value obtained during the 4 hours after patch reduction were analyzed.
The IAP monitoring device was removed in cases of severe Foley catheter leakage or oliguria for more than 6 hours. In all patients, urine cultures were obtained at least every 7 days. The patient's level of arousal/agitation was classified as calm, moderately agitated, and severely agitated. In a random sample of 10 patients, the time required for IAP measurements was taken. For each IAP value, we calculated the APP with the formula: APP = MAP − IAP.
2.3 Doppler US examination
Doppler US investigations were carried out by experienced radiologists using a commercial Scanner (GE Logiq 9 US system, GE Medical Systems) with a C1-5 MHz curved array transducer and an L9 MHz linear array transducer. All examinations were conducted in accordance with a standardized Doppler US protocol with the patient in a supine position. Post-operative US examinations were performed twice a day for 3 days, and until graft function was considered to be adequate. The US examination included measurements of flow velocities of the central and peripheral hepatic artery and of the central and peripheral hepatic portal vein. The RI of the central hepatic artery was calculated. The mean time between IAP measurement and Doppler US examination was 2.5 ± 2.3 (range, 0.02-11.5) hours, and the mean time between patch reduction and Doppler US examination was 4.0 ± 4.1 (range, 0.3-15.1) hours.
2.4 Statistical analysis
For qualitative data, counts and percentages were calculated. For quantitative data, means and SDs were calculated. We used a paired t test to compare data before vs after patch reduction. We used a multiple linear mixed model to investigate the potential impact of time after transplantation, diuresis, volume balance, creatinine, and agitation on IAP. To analyze the influence of IAP on US parameters, we calculated a linear mixed model. All analyses were performed in SPSS 23 for Mac (IBM SPSS Statistics, IBM Corporation), GraphPad Prism V6.0c (GraphPad Software), and R version 3.5.2 (R Core Team, Vienna, Austria). A P value <0.05 was considered statistically significant.
3 RESULTS
3.1 IAP
The mean number of IAP pressure measurements performed per patient during the post-operative period ± SD (range) for the 23 patients in the study was 18.5 ± 12.7 (5-54), constituting a total of 425 IAP measurements (Figure 1). Nurses spent a mean ± SD (range) of 1.9 ± 0.4 (0.5-3.2) minutes measuring and 0.3 ± 0.1 (0.2-0.6) minutes documenting IAP values. In 16 (3.6%) of 441 attempts, the measurements were unsuccessful, most often due to IAP monitoring device malfunction. We noted no complications of IAP measurements, and all urine cultures were negative.
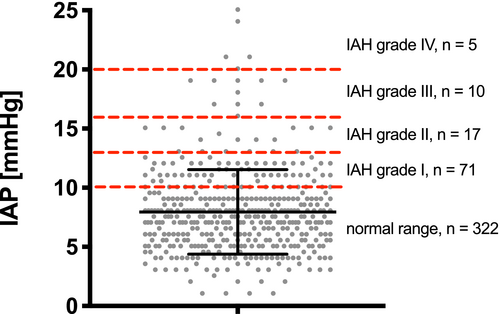
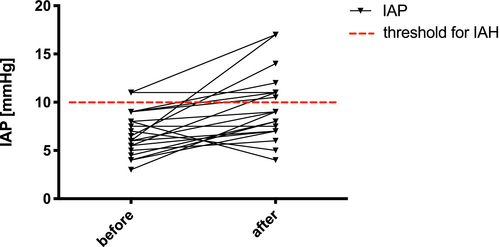
Post-operatively, the mean IAP ± SD (range) following LTX for all 23 patients was 7.9 ± 3.6 (1-25) mm Hg. The mean APP ± SD (range) in all patients was 68 ± 14 (33-125) mm Hg. Increased IAP fulfilling the criterion of abdominal hypertension (≥10 mm Hg)12 was found in 102 (24%) of 425 IAP measurements distributed among 20 (87%) of the 23 patients (Figure 1). APP was significantly lower in cases with intra-abdominal hypertension compared to cases with normal IAP (normal IAP 70 ± 14 mm Hg vs IAH 63 ± 14 mm Hg, P < .01). First post-operative IAP values after LTX did not differ significantly between cases with primary abdominal closure and TAC (Table 2). The first post-operative APP values were also similar in both groups. As shown in Figure 2, a slight increase in mean IAP values occurred after the patch reduction procedure in patients with a TAC (before 6.7 ± 2.1 mm Hg vs after 8.7 ± 3.1 mm Hg, P = .02). The mean APP values remained similar after patch reduction compared to the values before patch reduction (before 70 ± 13 mm Hg vs after 68 ± 13 mm Hg, P = .32).
| Parameter | All patients (N = 23) | Primary closure (N = 5) | TAC (N = 18) | P value |
|---|---|---|---|---|
| Mean IAP ± SD (range), mm Hg | 8.8 ± 4.8 (2-19) | 6.0 ± 3.5 (3-12) | 9.6 ± 4.9 (2-9) | .15 |
| Incidence of IAHa | 102/425 (24.0%) | 13/58 (22.4%) | 89/367 (24.3%) | .75 |
| Mean APP ± SD (range), mm Hg | 64 ± 18 (33-109) | 62 ± 16 (38-83) | 66 ± 19 (33-109) | .66 |
- a IAP ≥ 10 mm Hg.14
Multiple mixed linear model analysis showed a significant effect of the level of agitation on the IAP (estimate: 9.3 mm Hg, CI: 6.72-11.97, P < .01), whereas diuresis, fluid balance, and creatinine did not affect the IAP. The mean IAP ± SD in both calm children (7.5 ± 3.1 mm Hg) and moderately agitated children (7.9 ± 2.3 mm Hg) was significantly lower than in severely agitated children (18.0 ± 4.5 mm Hg; both comparisons, P < .01).
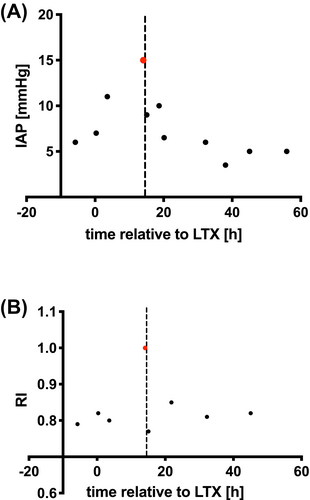
Of the 23 patients in this study, only 1 (4.3%) fulfilled the criteria for abdominal compartment syndrome (the combination of IAP > 10 mm Hg and a new organ dysfunction that can be attributed to elevated IAP): a 20-month-old baby with primary abdominal closure 14 hours after split LTX with an IAP of 15.0 mm Hg (grade II IAH). US examination showed severely impaired arterial inflow through the arteria hepatica, a sign of graft hypoperfusion (RI = 1.0) and impending liver dysfunction, in this patient. A protruding, firm, and painful abdomen was observed clinically. Immediate decompression with patch insertion was performed, resulting in a reduction of the IAP to 6.5 mm Hg and a normalization of the hepatic artery RI value to 0.8 (Figure 3).
3.2 US
The mean number of Doppler US examinations performed per patient ± SD (range) during the post-operative period was 11.2 ± 6.5 (3-25), resulting in a total of 257 measurements in 23 patients. Peak portal vein velocity was significantly reduced after patch reduction (26 ± 22 [−10 to 94] cm/s) compared to before patch reduction (38 ± 27 cm/s [12-112]; P = .03; Table 3). Overall, the mixed linear model analysis showed that patients with TAC compared to patients with primary abdominal closure had similar US parameters. Furthermore, we observed that high IAP values tended to lead to lower portal venous flows, although this effect did not reach statistical significance (estimate: −0.55 cm/s, CI: −1-13 to 0.04, P = .07). In patients experiencing episodes of IAH, we observed significantly elevated hepatic artery flow velocities relative to patients with normal post-operative IAP. Meanwhile, peak portal venous velocities and resistance indices between these two groups were similar (Table 4).
| US parameter | Before patch reduction (n = 24) | After patch reduction (n = 24) | P value |
|---|---|---|---|
| Hepatic artery peak systolic velocity, cm/s | 61 ± 29 (27-141) | 68 ± 31 (26 to 134) | .30 |
| Hepatic artery RI | 0.74 ± 0.09 (0.61-1.0) | 0.73 ± 0.09 (0.52 to 0.85) | .61 |
| Portal vein peak velocity, cm/s | 38 ± 27 (12-112) | 26 ± 22 (−10 to 94) | .029 |
| US parameter | Estimate | CI | P value |
|---|---|---|---|
| Hepatic artery peak systolic velocity, cm/s | 10.8 | 3.8 to 17.7 | .003 |
| Hepatic artery RI | 0.02 | −0.01 to 0.04 | .18 |
| Portal vein peak velocity, cm/s | −3.2 | −7.9 to 1.5 | .18 |
- a IAP ≥ 10 mg Hg.
4 DISCUSSION
We report on longitudinal measurements of IAP in children following LTX as a tool to screen for abdominal hypertension. To our knowledge, this is the first study to focus on IAP measurements in pediatric LTX post-operative care.
Avoiding elevated IAP is considered to be a critical element in the successful surgical management of pediatric liver transplant recipient care. Additionally, graft volume should be well-matched to the recipient's abdomen size to prevent impairment of blood supply and graft dysfunction associated with abdominal compartment syndrome.3, 4, 10, 11 Surgeons perform TAC with patch enlargement of the abdominal cavity3-5 when split adult-liver grafts are too large to fit within the abdominal cavity of small patients without leading to pressurization of the organ.1, 22 TAC was applied in 78% of children with a mean time until final abdominal closure (Table 1) consistent with times in previously published case series.5, 22-24 The decision on the use and size of a TAC patch was based on surgeons' clinical experience and repetitive US measurements during abdominal wall closure.
Side effects of intra-vesical IAP measurement were not observed in any of our 23 patients, and the time expended by the nursing staff to perform the additional measurements and documentation was low (Table 1). First post-operative IAP measurements were within normal limits and similar in patients with TAC and patients with primary abdominal closure and TAC was not a risk factor for high IAP (Table 2), indicating an effective IAP avoidance strategy. However, subsequent post-operative IAP data showed that IAP control could remain difficult even with TAC. Between day 1 and day 53, IAP values above 10 mm Hg were recorded in 24% (102/425) of the measurements distributed among 87% of our patients. Also, children with TAC showed slight but significant increases in IAP after patch reduction procedures, with 35% of such measurements reaching or exceeding the IAH threshold (Figure 2).
Although we observed a relatively high prevalence of IAH episodes in our cohort, a higher prevalence was reported in adult patients after LTX.25 In contrast to that adult series, we did not detect a significant effect of IAH on diuresis, fluid balance, or kidney function, perhaps due to the substantially higher percentage of patients with IAH in the adult study. A direct effect of elevated IAP on graft viability cannot be excluded, given that microvascular perfusion was not tested in this study.11 In response to patch reduction, patients with TAC in our study had significantly reduced portal venous inflow in association with a slight increase in IAP (Table 3). Our observations of significantly increased hepatic artery flow velocities during IAH episodes, compared to periods with normal IAP, suggest that high IAP may impact graft perfusion, even in a heterogeneous population of children.
We identified the level of agitation as a significant risk factor for high IAP. Severely agitated children had significantly higher IAP values than both calm and moderately agitated children, a finding that can be explained by the effects of crying and pressing. A multiple mixed model analysis showed that severe agitation increased the IAP by 9.3 mm Hg, which is a very relevant effect given that the threshold for IAH is 10 mm Hg. High IAP due to traction after abdominal closure itself may cause further pain and distress. Our results suggest that severe agitation (eg, due to pain) in children recovering from LTX may lead to critically high IAP values and thus may threaten graft viability. Therefore, we recommend close monitoring of these patients for signs of pain with effective pain treatment in accordance with WSASC's guidelines.14 To exclude the effects of agitation on IAP measurements, the WSASC recommends that IAP be measured when children are calm.26 In the context of LTX in children, however, this may not be possible at times. We suggest conducting IAP measurements on a regular, scheduled basis in as calm a clinical situation as possible while also documenting the level of agitation. The data may then be used to verify and pilot effective pain treatment in this population.
By measuring IAP routinely in our PICU, we identified one patient with abdominal compartment syndrome shortly after primary closure of the abdomen. High IAP was associated with severely impaired hepatic vascular flow through the arteria hepatica on Doppler US in this patient. Surgical decompression of the abdomen with a silicon patch led to the resolution of this complication and normalized perfusion (Figure 3). The case indicates that IAP monitoring can help identify the patients in whom traction and insufficient abdominal wall compliance are impairing graft perfusion, as opposed to other causes of hypoperfusion.
Some authors have proposed targeting an APP > 60 mm as a resuscitation end-point more predictive of outcome than IAP,27 but this is not universally accepted, and treatment strategies should probably focus on mitigating IAH rather than driving up MAP. However, we found that in cases with intra-abdominal hypertension, the mean APP was 63 mm Hg and was thus significantly lower than in cases with normal IAP, where a mean of 70 mm Hg APP was determined. The critical lower APP threshold for adults is considered to be 60 mm Hg. There is no such threshold for the pediatric population. Nevertheless, the observation that APP is significantly lower during episodes of IAH in children after LTX is new and requires further research.
IAP monitoring may help to clarify the optimal timing of patch reduction and enable surgeons to adapt abdominal wall traction intra-operatively with the goal of reducing the total time with a patch in place. Future studies should examine whether the use of IAP-guided abdominal closure can reduce the time to definitive abdominal closure and the time to PICU discharge.
4.1 Limitations
- The study design was retrospective and therefore, subject to potential selection bias. Data were acquired prospectively and extracted retrospectively.
- The exact timing of IAP measurements and Color Doppler examinations in patients varied relative to surgical revision (ie, patch reduction) because they had to be performed sequentially. Both examinations were completed during comparable levels of agitation with a defined 4-hour time frame, to minimize the effects of different clinical situations on the results.
- IAP measurement and color Doppler US were performed by different investigators. To reduce measurement variability, both followed strict protocols.
5 CONCLUSION
Retrospective analysis of longitudinal IAP measurements demonstrated that IAP measurement is a safe, feasible supplemental assessment that can be performed easily and quickly in children recovering from LTX. IAP data may provide essential additional information useful for detecting IAH episodes and abdominal compartment syndrome. In our cohort, agitation was strongly associated with elevated IAP and impaired vascular flow. Therefore, we recommend regular evaluation of children after LTX for signs of pain and agitation with a prompt, rigorous treatment of symptoms. More studies are needed to determine whether IAP monitoring can improve the quality of post-operative management in children after LTX and reduce the length of time to patch reduction and final closure.
ACKNOWLEDGMENT
The authors thank the entire PICU team for supporting the study and the anesthesia team for their intra-operative support.
CONFLICT OF INTEREST
There is no conflict of interest.
AUTHORS' CONTRIBUTIONS
Philipp Deindl developed the idea for this study and designed the study. He supervised data collection and drafted the manuscript for the publication. Jula Wagner performed intra-abdominal pressure measurements and extracted all patient data from the liver transplant database and surgery, anesthesia, and PICU records. Uta Herden transplanted some of the patients, advised on the surgical aspects of the study, and critically revised the manuscript. Sebastian Schulz-Jürgensen helped to discuss the data and critically revised the manuscript. Raphael Schild advised when planning the study and revised the manuscript. Eik Vettorazzi calculated all statistics. Marlies Bergers supported the study by instructing the nursing team to perform intra-abdominal pressure measurements in PICU patients. Maike Keck helped with the design of the study and discussed the data. Dominique Singer helped conceptualize the study and interpret the data. Lutz Fischer transplanted some of the patients and advised on the surgical aspects of the study. Jochen Herrmann helped with the study design, critically revised the manuscript draft and helped with the interpretation of the US examinations from the Radiology Database.




