Emerging arboviruses and implications for pediatric transplantation: A review
Abstract
Recent years have brought a rise in newly emergent viral infections, primarily in the form of previously known arthropod-transmitted viruses that have increased significantly in both incidence and geographical range. Of particular note are DENV, CHIKV, and ZIKV, which are transmitted mostly by Aedes species of mosquitoes that exhibit a wide and increasing global distribution. Being important pathogens for the general population, these viruses have the potential to be devastating in the international transplant community, with graft rejection and death as possible outcomes of infection. In this review, we discuss the current state of knowledge for these viruses as well as repercussions of infection in the solid organ and HSCT population, with a focus, when possible, on pediatric patients.
Abbreviations
-
- ADEM
-
- acute disseminated encephalomyelitis
-
- ARDS
-
- acute respiratory distress syndrome
-
- CHIKV
-
- chikungunya virus
-
- CYD-TDV
-
- CYD-tetravalent dengue vaccine
-
- DENV
-
- dengue virus
-
- DF
-
- dengue fever
-
- DHF
-
- dengue hemorrhagic fever
-
- DHS
-
- dengue hemorrhagic shock
-
- DSS
-
- dengue shock syndrome
-
- EUA
-
- emergency use authorization
-
- HCV
-
- hepatitis C virus
-
- HSCT
-
- hematopoietic stem cell transplant
-
- IVIG
-
- Intravenous immunoglobulin
-
- MRSA
-
- methicillin-resistant Staphylococcus aureus
-
- POD
-
- post-operative day
-
- RT-PCR
-
- reverse transcription-polymerase chain reaction
-
- SOT
-
- solid organ transplant
-
- UNOS
-
- United Network for Organ Sharing
-
- VLP
-
- viral-like particle
-
- VSV
-
- vesicular stomatitis virus
-
- ZIKV
-
- Zika virus
1 INTRODUCTION
Arboviruses, such as West Nile virus, have previously proven problematic in the transplant community, with donor-derived and mosquito-transmitted infections causing severe neuroinvasive disease, high morbidity, and mortality.1-5 Although many arboviruses are reemerging, DENV, CHIKV, and ZIKV pose the highest new risk to transplant recipients as they have been recently associated with large epidemics of disease and are becoming endemic in a wider geographical range than ever before. Circulation of these viruses has expanded due to multiple factors, including population growth, travel, climate change, and insufficient vector control.6-8 These viruses are unique among arboviruses in that they generate high titer viremia in humans sufficient to maintain a human-mosquito-human transmission cycle, which enables large urban outbreaks of infection. Even in endemic areas with broad preexisting immunity, low infection rates and the presence of animal reservoirs allow for continued transmission of these viruses. Individuals infected with any of these arboviruses can be asymptomatic or present with a typically mild and self-limited febrile illness accompanied by malaise, rash, and myalgia or arthralgia. As these viruses continue to cause large-scale outbreaks and expand geographically, bringing awareness to the potential risks to immunocompromised transplant recipients is important for prevention, diagnosis, and treatment. In this review, we discuss the current state of knowledge for these viruses as well as repercussions of infection in the SOT and HSCT population, with a focus, when possible, on pediatric patients.
As with other pathogens, the transplant population is at increased risk of acquiring infection and experiencing severe disease caused by these arboviruses due to immunosuppression and, specifically, impaired T-cell function.9 Although each virus discussed in this review elicits specific immune responses that do not fully overlap with one another, intact immune responses are required for viral clearance.10, 11 The effects of immunosuppression on the risk of infection and disease severity are not well understood. However, disease severity for these viruses has been linked to the level of viremia.12 Failure to control viral replication due to immunosuppression may lead to increased viral load, prolonged viremia, enhanced disease severity, and possible graft failure. Therapies for these pathogens are limited and consist almost exclusively of supportive care and reduction in immunosuppressive agents. This comes at a cost, however, as decreased immunosuppression increases the risk of graft rejection. As a result of this challenge, prevention of viral infection through infection control and exposure reduction is critical.13
Aside from mosquito transmission, there is a risk of transmission through the donated organ in addition to any transfused blood products patients may require during or after transplantation. Laboratory-based screening for these pathogens is not universal, especially in areas where the virus is not endemic, and highly sensitive and specific diagnostic tools are not readily available in most hospitals. Strategies to improve diagnostics and screening of donors are essential to mitigate risk of patients receiving infected transplanted or transfused materials.
2 DENGUE VIRUS
2.1 Virus and epidemiology
DENV is a positive-sense, single-stranded RNA virus in the Flaviviridae family with five serotypes that cause disease in humans. DENV is the most prevalent mosquito-borne viral illness worldwide, causing an estimated 100-400 million cases per year.7, 62 DENV is endemic in the tropical areas of Asia, Latin America, and the Pacific and circulates across Africa. Recent travel-imported and local outbreaks in the United States and Europe have also been reported.15, 16
2.2 Clinical manifestations—adult
Up to 75% of DENV infections are asymptomatic in all age-groups,17 but the symptomatic individual can present within a range of disease severity (Table 1). Patients with DF present with a self-limited illness characterized by high fever, myalgia, rash, petechiae, and retro-orbital pain. More severe clinical presentations such as DHF and DSS occur in about 1% of patients but carry significantly higher mortality rates (~10.9%).10 Symptoms include thrombocytopenia, hemorrhage, liver damage, leukopenia, vascular leak, hypovolemic shock, and multisystem organ failure.14 The infecting DENV serotype and an individual's previous exposure to other DENV serotypes influence disease severity due to antibody-dependent enhancement, where previous exposure to heterologous serotypes and adaptive immune response to DENV challenge contribute to the more severe subsequent presentation.18 Rapid identification of severe DENV is crucial to successful management and patient recovery.
2.3 Clinical manifestations—pediatric
The common pediatric manifestations of DENV include fever, irritability, and feeding refusal in infants (Table 1). In older children, fever and vomiting are common, though arthralgia and myalgia are less common than in adults. Cough is prevalent in patients with pleural effusion or ARDS. Although rare in the pediatric population, bleeding of skin and mucosal surfaces is common in children with more severe disease.19 Infants aged 4-9 months and children aged 5-7 years are at higher risk of severe disease, and as with adults, secondary DENV infection is a risk factor for severity.20 Symptoms such as shock, plasma leakage, and marked thrombocytopenia are more severe and frequent in children than in adults.21
| Feature | DENV | CHIKV | ZIKV | |||
|---|---|---|---|---|---|---|
| Children | Adult | Children | Adult | Children | Adult | |
| Fever | 97%19 | 90%12 | >38.9C, 1-8 d59 | 90%12 | 55%-93%75, 106 | 65%-80%12 |
| Skin | Rash (22%)19 | Rash (50%),12 petechiae |
Rash (33%-60%)59 Pigment changes (42%)59 Bullous rash (38%-48% in infants)59 |
Rash (35%-50%)59 | Rash (82%-100%)75, 106 | Rash (91%)12 |
| HEENT | Not reported |
Retro-orbital pain (43%) Conjunctivitis (73%)12 |
Oral ulcers (rare)59 |
Retro-orbital pain (38%)12 Conjunctivitis (77%)12 |
Conjunctivitis (29%)75 URI symptoms (14%)75 |
Retro-orbital pain (48%)12 Conjunctivitis (82%)12 |
| CV | Hypotension (19%)19 | Vascular leak (26.5%) 107 | Shock (infants)108 | Not reported | Not reported | Not reported |
| GI |
Vomiting (63%)19 Abdominal pain (53%)19 Hepatomegaly (34%)19 Ascites (41%)19 |
Nausea (44%)12 Vomiting (14%)12 Abdominal pain (38%)12 |
Not reported |
Nausea (41%)12 Vomiting (10%)12 Abdominal pain (18%)12 |
Not reported |
Nausea (27%)12 Abdominal pain (12%)12 |
| Mucocutaneous |
Epistaxis (20%)19 GI bleed (20%)19 |
Epistaxis, oral bleeding (12%)109 | Oral ulcers (rare)59 | Oral ulcers (16%)59 | Not reported | Not reported |
| MSK | Not reported |
Myalgia (58%)12 Arthralgia (67%)12 |
Myalgia (30%-50%)59 Arthralgia (30%-50%)59 |
Myalgia (60%-93%)59 Arthralgia (69%)12 |
Myalgia (21%)75 |
Myalgia (65%)12 Arthralgia (70%)12 |
| Chronic arthralgia | Not reported | Not reported | 5%-11% at 2 y59 |
1 y: 57%59 3-5 y: 12%59 |
Not reported | Not reported |
| Heme |
Hemorrhage (1%)19 Skin bleeds (55%)19 hematuria (5%)19 |
Thrombocytopenia, Leukopenia, Hemorrhage (10%)12 | Purpura, ecchymoses (10%)59 | Hemorrhage (4%)12 | Not reported | Hemorrhage (6%)12 |
| Neurologic | Irritability, feeding refusal in infants19 | Headache (69%)12 |
Headache (15%)59 Acute encephalopathy (14%-32%)59 Seizures (40%-50%)59 |
Headache (40%-81%)59 Acute encephalopathy (<0.1%)59 |
Headache (21%)75 |
Headache (79%)12 GBS (rare)110 ADEM (rare)74 |
| Perinatal infection |
Fever, thrombocytopenia, intracerebral hemorrhage, miscarriage 111, 112 low birth weight, preterm delivery113 |
Not applicable |
Fever, rash, edema in 1st week of life. Intracerebral hemorrhage, status epilepticus, multi-organ failure, death.60 |
Not applicable | Miscarriage, Microcephaly, Ocular changes, limb anomalies114, 115 | Not applicable |
| Asymptomatic | 75%17 | 75%17 |
35%-40% in children Rare in infants59 |
16%-27%59 | Not reported | 80% |
2.4 Infections in transplant patients
Due to DENV global prevalence, more information on DENV in transplant patients is available than for CHIKV and ZIKV, with over 200 reported cases in transplant patients (HSCT, liver, and kidney), though mostly in adults22-27 (Tables 2 and 3). DENV can present atypically in transplant patients, such as acute colitis28 or encephalitis.29 The presentation of DENV in renal transplant patients is less characterized by fever, myalgia, arthralgia, and headache, while pleural effusion and ascites were more often observed.23 Severe DENV was significantly more common among transplant patients (16% vs 3.7%) and had a significantly higher mortality (8.9% vs 0.062%).24 Age and immunosuppressive agents have not yet been reported to affect disease severity, mortality, or graft outcome. However, transplant patients can have prolonged viremia, up to 80 days in one report, as opposed to median of 5 days in immunocompetent patients.22 Importantly, 25% of transplant patients with primary disease did not mount an IgG response after 15 weeks of illness, making this an unreliable diagnostic test for this population. Moreover, nearly 60% had graft dysfunction during the illness.23
| Virus | Country of origin | Age | Transplant type | Onset | Symptoms | Infection mechanism | Diagnosis | Outcome | Ref |
|---|---|---|---|---|---|---|---|---|---|
| DENV | Brazil | 15 y | Kidney | POD 3 | Fever, abdominal pain, ascites, ATN, graft site bleeding | Transplant | +RT-PCR and serology | Death | 38 |
| DENV | Brazil | 21 y | Kidney | POD 3 | Fever, anuria, pain at graft, pancytopenia | Transplant | +RT-PCR | Recovery | 38 |
| DENV | Thailand | 16 y | Kidney | POD 13, Tested +POD10 | Ascites, pleural effusion, pancytopenia, shock, perinephric hematoma | Unknown | + DENV NS1 Ag test, +IgM, +IgG, + RT-PCR | Graft failure | 39 |
| DENV | Thailand | 16 y | BMT | 5 mo | Fever, HA, myalgia, nausea | Mosquito | +DENV Ag test, +IgM, +IgG, +RT-PCR | Recovery | 40 |
- No known pediatric cases of CHIKV or ZIKV in transplant recipients.
| Virus | Country of origin | Age | Transplant type | Onset | Symptoms | Infection mechanism | Diagnosis | Outcome | Ref |
|---|---|---|---|---|---|---|---|---|---|
| DENV | >200 cases reported in transplant patients, organ, transfusion, and mosquito transmission reported | 22-35 | |||||||
| CHIKV | Singapore | 45 y | Liver | 7 y post | Fever, headache, thrombocytopenia, encephalitis | Mosquito | RT-PCR-, IgM+, IgG+ | Recovered | 61 |
| CHIKV | Italy (travel to Dominican Republic) | 48 y | Kidney | 6 y post | Fever, diarrhea, nausea, HA, myalgia, arthralgia | Mosquito | RT-PCR-, IgM+, IgG+, plaque reduction neutralization + | Recovered | 62 |
| CHIKV | Brazil | 41 y | Kidney | 8 y | Fever, HA, rash, conjunctivitis, polyarthralgia, poly arthritis | Mosquito | IgM+ | Recovered | 63 |
| CHIKV | Brazil | 54 y | Liver | 4 y | Fever, HA, polyarthralgia | Mosquito | IgM+ | Recovered | 63 |
| CHIKV | Brazil | 60 y | Liver | 5 y | Fever, polyarthralgia | Mosquito | IgM+ | Recovered | 63 |
| CHIKV | Brazil | 49 y | Liver | 8 y | Fever, HA, rash, polyarthralgia, polyarthritis | Mosquito | IgM+ | Recovered | 63 |
| CHIKV | Brazil | 40 y | Kidney | 2 y | Fever, polyarthralgia, polyarthritis | Mosquito | IgM+ | Recovered | 63 |
| CHIKV | Brazil | 51 y | Kidney | 2 y | Fever, HA, rash, polyarthralgia | Mosquito | IgM+ | Recovered | 63 |
| CHIKV | Brazil | 46 y | Kidney | 23 y | Fever, HA, polyarthralgia | Mosquito | IgM+ | Recovered | 63 |
| CHIKV | Brazil | 47 y | Kidney | 2 y | Fever, HA, conjunctivitis, polyarthralgia, polyarthritis | Mosquito | IgM+ | Recovered | 63 |
| CHIKV | Brazil | 49 y | Kidney | 8 y | Fever, HA, rash, conjunctivitis, polyarthralgia, polyarthritis | Mosquito | IgM+ | Recovered | 63 |
| CHIKV | Brazil | 48 y | Kidney | 2 y | HA, polyarthralgia, polyarthritis | Mosquito | IgM+ | Recovered | 63 |
| CHIKV | Brazil | 49 y | Liver | 1 y | Fever, HA, polyarthralgia, polyarthritis | Mosquito | IgM+ | Recovered | 63 |
| CHIKV | Brazil | 73 y | Kidney | 12 y | Rash, polyarthralgia | Mosquito | IgM+ | Recovered | 63 |
| CHIKV | Brazil | 54 y | Kidney | 24 y | Fever, headache, monoarthralgia, polyarthritis | Mosquito | IgM+ | Recovered | 63 |
| ZIKV | Brazil | 36 y | Heart | 8 mo | Fever, malaise, HA, sz, Meningoencephalitis | Mosquito | CSF RT-PCR+ | Death d/t rejection induced arrhythmia | 88 |
| ZIKV | Brazil | 52 y | Liver | 43 d | Fever, myalgia | Mosquito | RT-PCR+ | Recovered | 87 |
| ZIKV | Brazil | 68 y | Liver | 115 d | Myalgia, HA, cough, coryza | Mosquito | +serum RT-PCR | Recovered | 87 |
| ZIKV | Brazil | 53 y | Kidney | 590 d | Fever, malaise, weight loss, vomiting, diarrhea | Mosquito | +serum RT-PCR | Recovered | 87 |
| ZIKV | Brazil | 51 y | Kidney | 61 d | Myalgia, malaise, HA | Mosquito | +serum RT-PCR | Recovered | 87 |
| ZIKV | Brazil | 55 y | Liver | Tested on POD 4 | Asymptomatic | Transfusion during transplant | + serum RT-PCR | Recovered | 89 |
Multiple severe and fatal cases of dengue have been reported in renal transplant patients, as well as a fatality in a liver recipient.24, 30 In fatal cases, disease developed within the first month after transplant. Occurrence of DHF, especially in the early post-operative period, can be particularly dangerous, with two patients reported to have had thrombocytopenia, platelet dysfunction, and coagulopathy resulting in profuse bleeding around the renal graft and one case of graft loss.31 DENV acquisition has been described both from mosquito exposure and via graft transmission for both HSCT32, 33 and SOT.26, 34, 35 Transfusion-related DENV transmission has also been reported.36, 37 Due to the risk of disease severity, appropriate precautions to reduce infection as well as proper monitoring and care of transplant patients who contract DENV are necessary to mitigate the possibility of severe illness and death. Laboratory-based screening of donors is recommended in endemic areas to prevent this complication, though available screening tests are not standardized (discussed further below).
Even in countries where DENV is endemic, there are few reports of DENV infection in pediatric and young adult transplant recipients (Table 2). One Brazilian case study describes two cases of DENV in new kidney recipients in 2015. The initial diagnosis in each of these cases was hyperacute graft rejection; neither donor had a history of DENV.38 The first patient was 15 years old and had an uneventful transplant but developed fever, abdominal pain, and ascites on POD 3. Kidney biopsy demonstrated acute tubular necrosis, but no rejection. DENV type 2 was isolated from a blood sample on POD 4 and high DENV IgM was isolated on POD 6. Abdominal surgery on POD 26 demonstrated active bleeding at the graft site. Post-surgically, the patient demonstrated hemodynamic instability that was not controlled with pressors or additional blood products, and the patient ultimately died of cardiac arrest. The second patient was 21 years old and also had an uneventful transplant. He was initially given antibiotics post-operatively because the donor was receiving antibiotics. He developed fever, anuria, pain at the surgical site, and pancytopenia on POD 3. CT revealed fluid in the perirenal site, and he underwent three surgeries and several transfusions of blood products to achieve hemostasis. He was diagnosed with DENV on POD 6 via blood RT-PCR. He ultimately recovered and was discharged.
A 16-year-old kidney recipient in Thailand experienced a severe case of DENV. She developed ascites, bilateral pleural effusions, and thrombocytopenia without fever on day 13 after transplant. She also developed refractory pancytopenia and anemia resulting in hypovolemic shock and graft failure 28 days after transplant. She was found to have a large perirenal hematoma that required surgical evacuation, but gradually recovered and was discharged.39 Another Thai 16-year-old patient received an allogeneic peripheral blood stem cell transplant for acute myeloid leukemia. She acquired DHF 5 months after transplant and made a full recovery after 14 days of hospitalization.40 It is not known whether the patients in the first three of these case reports acquired DENV via the graft, but the timing of infection raises this concern. The HSCT patient likely was infected via the bite of an infected Aedes mosquito.
2.5 Screening and diagnosis
FDA-approved diagnostics for DENV include tests for IgM detection and a RT-PCR test developed by the CDC.36 Viral detection with RT-PCR for DENV is highly sensitive, but expensive. Several commercial ELISA-based rapid diagnostics are available for DENV targeting a specific DENV protein, but they have a wide range of sensitivity depending on the DENV serotype, manufacturer, and whether primary or secondary infection. DENV IgM measurement can be helpful, but cross-reactivity with other flaviviruses is a concern for antibody-based assays.14
2.6 Advances in prevention and treatment
There is a DENV vaccine, CYD-TDV, or Dengvaxia, which is licenced in 20 countries for individuals between 9 and 45 years of age.41 Dengvaxia is a recombinant, live-attenuated vaccine that uses a yellow fever virus backbone to express structural genes from four of the five DENV serotypes. Initial findings from two large phase 3 clinical trials have demonstrated incomplete protection, though fewer hospitalizations and less severe disease were noted in vaccinated patients.42, 43 The vaccine has varied performance in patients who have been previously infected by DENV vs those without prior DENV infection. Of concern, vaccination of seronegative individuals, often children, increases the risk of severe DENV and death, leading to recommendations by WHO Strategic Advisory Group of Experts to perform pre-vaccination screening in countries considering introduction of this vaccine and not to vaccinate seronegative patients.44, 45 Other vaccines for DENV are in various phases of clinical trial. Vaccination could be considered as part of the series of recommended pre-transplant vaccines for seropositive patients, but the use of live vaccines is contraindicated in patients post-transplant. The risk of severe DENV after vaccination of seronegative patients has not been studied in the immunosuppressed population.
As for recent advances in DENV-targeted therapy, there has been some promise in mouse models using a combination of sunitinib, a VEGF receptor tyrosine kinase inhibitor, and TNF inhibitors for the reduction in vascular leak and protection from lethal disease.46 TNF inhibition has also been useful in limiting DENV-induced encephalitis in mouse models.47 IVIG has been successfully used to halt capillary leak and hemodynamic instability in a renal transplant patient.27 Quinine sulfate inhibits in vitro replication of all four DENV serotypes.48 Targeted monoclonal antibodies are also under investigation for the capacity to prevent or reduce infection or disease.49 At the mosquito transmission level, Wolbachia (a bacteria that infects mosquitoes) is being developed to prevent DENV transmission and could prevent other mosquito-borne infections as well.50 Unfortunately, drug trials with balapiravir,51 chloroquine,52 lovastatin,53 and celgosivir54 have not demonstrated these agents to be clinically effective against DENV.
3 CHIKUNGUNYA VIRUS
3.1 Virus and epidemiology
CHIKV is a positive-sense, single-stranded RNA virus from the Togaviridae family. CHIKV outbreaks may have occurred since the 18th century, but became more notable between the 1950s and 2000s, when CHIKV was responsible for several outbreaks in Africa and Asia.55 It reemerged in 2004 and spread from Africa to islands of the Indian Ocean, India, and South-East Asia, as well as at least three small outbreaks in Europe, and has since caused millions of cases worldwide. Beginning in late 2013, CHIKV caused an explosive epidemic in the Western Hemisphere, with ~3 million estimated cases in the Caribbean and Central, South, and North America.56 As with DENV and ZIKV, travelers infected with CHIKV returning from endemic or epidemic areas to regions with immunologically naïve populations have been the main cause of the rapid geographical expansion of the virus. In addition, a mutation in the E1 gene of CHIKV is thought to have increased the viral fitness for transmission through the Aedes albopictus vector,57 thereby expanding the geographical range of the virus.
3.2 Clinical manifestations—adult
After the bite of an infected mosquito, viral replication in different cell types within the skin takes place, followed by the viremic phase, which disseminates virus into the bloodstream. Secondary sites of infections include muscles, joints, liver, spleen, and brain. A hallmark of CHIKV infection is chronic polyarthralgia, with joint pain lasting months to years after infection in a significant proportion (~43%) of infected individuals (Table 1).58 It is thought that chronic CHIKV disease is due to host immune responses, though persistent infection cannot yet be ruled out.
3.3 Clinical manifestations—pediatric
Children infected with CHIKV are likely to present with fever and rash, with febrile seizures often described, but they less commonly exhibit arthralgia, myalgia, or headache59 (Table 1). Children are also more likely to have bleeding symptoms, including purpura, ecchymosis, and severe bleeding from mucosal surfaces. Perinatal infection was first described in 200560 and approaches 50% when mothers are viremic in the week preceding delivery, likely due to exposure during or soon after delivery. Neonates present with fever, rash, and edema within the first week of life, and complications can be devastating, including intracerebral hemorrhage, status epilepticus, multi-organ failure, and death. Early clearance of CHIKV in pediatric cases is associated with a strong innate immune response.10
3.4 Infections in transplant patients
Few cases of CHIKV have been reported among organ transplant recipients (Table 3). An adult liver recipient on azathioprine and prednisolone developed CHIKV disease 7 years post-transplant, complicated by encephalitis, though he had no joint symptoms and recovered completely.61 A woman with HIV and kidney transplantation developed high fever, diarrhea, and nausea for 2 days, and persistent headache, fatigue, myalgia, and severe, bilateral, symmetrical arthralgia after CHIKV infection, with the development of persistent arthralgia in follow-up.62 A study of 13 SOT recipients (nine kidney, four liver) infected with CHIKV exhibited a similar clinical presentation to immunocompetent hosts, including chronic joint symptoms in 46% of patients 3 months after infection.63 However, there were no incidents of complications or death and these transplant patients experienced no apparent damage to the graft. A recent case report revealed successful live-donor kidney transplant 3 months after the donor was infected with CHIKV. No signs of CHIKV infection in the recipient occurred, likely because the donor was no longer viremic at the time of donation.64 Additionally, infectious CHIKV can be isolated from corneal grafts from both symptomatic and asymptomatic donors, though no corneal transplant transmitted cases have been reported to date.65 Thus, in contrast to DENV, it is not yet clear that CHIKV infection is more severe in transplant recipients. There are currently no reports of CHIKV infection of pediatric transplant recipients.
3.5 Screening and diagnosis
Laboratory confirmation of CHIKV requires virological or serological tests. RT-PCR can detect virus in the blood during the first 5 days of infection. After that, anti-CHIKV IgM and IgG antibodies may be present. IgM antibodies peak at 3-5 weeks and decline at 2 months post-infection, though they may persist for years.59 The natural kinetics of anti-CHIKV antibodies in transplant patients are unknown. Commercially available serological tests often lack sensitivity and specificity, and improvement of these assays is essential for proper diagnosis.66, 67
3.6 Advances in prevention and treatment
There are currently no licenced vaccines to protect from infection with CHIKV. Phase 2 trials are currently underway for MV-CHIK, a vaccine utilizing a measles vector, both in the United States and in Europe. Another vaccine candidate based on a VLP platform (VRC-CHKVLP059-00-VP) is also being investigated.68 A recombinant VSV vaccine expressing the CHIKV envelope polyprotein and the ZIKV membrane-envelope glycoproteins induced neutralizing antibodies and protection in mouse models against both CHIKV and ZIKV.69
Chloroquine exhibited in vitro activity against CHIKV, but when used in humans during an outbreak, it paradoxically led to worse disease, higher viremia, and slower viral clearance.70 While several small compounds and existing antivirals prevent CHIKV replication in vitro, studies in animal models have not yet been published. Monoclonal antibodies against the E1 and E2 proteins of CHIKV have been used in animal models for both prophylaxis and treatment with reduction in lethality and symptoms.71
4 ZIKA VIRUS
4.1 Virus and epidemiology
ZIKV is a positive-sense, single-stranded RNA virus and member of the Flaviviridae family. It was initially identified in the 1940s in Africa and caused mild cases of illness in Africa and Asia.72 Beginning in 2007, a significant outbreak of ZIKV in the Islands of Yap led to the resurgence and international spread of ZIKV infection, culminating in an explosive outbreak in Brazil beginning in May 2015, with ~1.5 million cases worldwide and ~1.3 million cases in Brazil alone.73 Lack of preexisting immunity in these new areas of global distribution likely contributed to the vast infection rate.
4.2 Clinical manifestations—adult and pediatric
Up to 80% of ZIKV infections are asymptomatic, while the remaining cases usually have mild, nonspecific symptoms such as fever, pruritic maculopapular rash, arthralgia, myalgia, conjunctivitis, and headache. Symptoms in children are thought to be similar to those seen in adults (Table 1).74, 75 ZIKV infection can be particularly devastating for pregnant women, as it has been linked to fetal loss and congenital Zika syndrome, leading the World Health Organization to declare the ZIKV epidemic as a global public health emergency.76 Congenital Zika syndrome has been recently defined to include severe microcephaly, thin cerebral cortices with subcortical calcifications, macular scarring and focal pigmentary retinal mottling, congenital contractures, and marked early hypertonia and symptoms of extrapyramidal involvement; however, the full neurological sequelae of fetuses affected by ZIKV are yet to be described longitudinally.77, 78 Infection with ZIKV can also cause neurological complications such as Guillain-Barre syndrome and potentially trigger ADEM.74 Little is known about ZIKV infections in immunocompromised patients.
In addition to transmission through mosquito vectors and vertical transmission, ZIKV has been shown to be persistent in semen for at least 188 days after infection and can be transmitted sexually from men or women to their partners.79-81 Persistence in semen exists despite negative blood-based screening assays.82 Persistence in other organ systems is presently unknown. Breast milk, urine, and saliva have high viral nucleic acids when tested,73, 83, 84 and one potential breast milk-associated transmission has occurred.85
4.3 Infections in transplant patients
Infection causes viremia, even in the 80% of infected individuals who are asymptomatic, creating a scenario for high likelihood of transmission via organ or blood donation if donor symptom screening alone is used without specific testing for ZIKV.86 Several SOT recipients are thought to have contracted ZIKV via their transplanted organ.87 One heart transplant patient in Brazil contracted ZIKV meningoencephalitis 8 months after transplantation. Tacrolimus and mycophenolate mofetil were stopped to allow immune reconstitution, but he ultimately died of arrhythmia caused by organ rejection.88 A case series in Brazil during the recent ZIKV epidemic described ZIKV infection in four transplant recipients (two kidney, two liver) with positive blood RT-PCR, with all patients developing symptoms. Symptoms included fever, myalgia, headache, cough, weight loss, vomiting, and diarrhea. Three patients had anemia, all had thrombocytopenia and graft dysfunction, and three developed superimposed bacterial infection (biliary abscess, cholangitis, MRSA bacteremia, and pneumonia). One patient required re-transplantation of liver due to hepatic artery thrombosis associated with infection. Timing of infections ranged from 1.5 months to 1.6 years post-transplantation, making mosquito exposure the most likely source of infection.87
ZIKV has also been transmitted via blood donation from an asymptomatic donor who developed symptoms consistent with ZIKV infection 5 days after donation. Two platelet units from this infected donor were transfused into other immunocompetent patients. RT-PCR testing of the donor and the two recipients was positive for identical viral RNA, though both recipients remained asymptomatic.86 ZIKV transmission was reported in Brazil from an asymptomatic blood donor, who later called the blood bank with symptoms, to a newly transplanted liver recipient who received those platelets during transplantation; the recipient did not develop symptoms.89 In addition, ZIKV has been detected in the vitreous of an asymptomatic cornea donor, though the transmission risk through corneal transplant is unknown.90 No cases of ZIKV infection of pediatric transplant recipients have been reported.
4.4 Screening and diagnosis
Symptomatic individuals have ZIKV in blood for only 3-5 days after symptom onset, when RT-PCR testing could be positive. IgM testing is often used, but is challenging due to cross-reactivity with other Flaviviruses, DENV in particular.91 Virus can also be detected in saliva, urine, semen, and amniotic fluid, with the latter three often remaining positive for virus after serum tests become negative.73 Despite these challenges, blood collection centers in Puerto Rico began screening for ZIKV in 2016 via a nucleic acid test,73 which demonstrated a weekly incidence rate of 1.1% of donations. The US FDA endorses universal testing of blood donations for ZIKV due to the number of asymptomatic infections and the increasing number of cases in the United States.82 Recently, a multiplex real-time RT-PCR assay (Trioplex) was developed to facilitate detection of DENV, CHIKV, and ZIKV with high sensitivity in various clinical specimens. The FDA issued an EUA for the Trioplex assay in March 2016.92 Validated and sensitive assays such as the Trioplex assay can be used as a rapid, discriminatory, and cost-effective tool for both diagnosis and surveillance in regions with endemic DENV, CHIKV, and ZIKV.93
4.5 Advances in prevention and treatment
No licenced vaccine currently exists for ZIKV. There are currently >10 vaccine candidates in clinical trials.94 As mentioned above, a combination CHIKV and ZIKV vaccine has been protective in mouse models.69 Another vaccine based on the strategy for Ebola vaccination with a VSV vector, VSV-ZIKV, was highly protective against ZIKV infection in mice as soon as 3 days after vaccination.95
As for specific therapies, a study in rhesus monkeys suggested that a DENV-specific antibody against the E-dimer epitope cross-neutralized ZIKV and was effective as a prophylactic and a therapeutic agent.96 Chloroquine administered to ZIKA-infected pregnant mice significantly attenuated vertical transmission and reduced the ZIKV load in the fetal brain.97 Research on small molecule inhibitors is ongoing, but no candidates are in clinical trials. An additional challenge is the need for safety and effectiveness of medications for pregnant mothers, a group often excluded from clinical trials.
4.6 Challenges in arbovirus screening and diagnosis
Given that many manifestations of these diseases are thought to be primarily driven by immune responses to infection, the symptoms of these emerging viral infections in immunocompromised patients may be nonspecific and unrecognizable, leading to missed or delayed diagnosis. The common symptoms of malaise and fever seen with these infections could suggest anything from expected post-operative course to rejection to a separate and serious infection, demonstrating the need for appropriate and standardized diagnostic testing when clinical suspicion arises, even if outside the expected window and especially in endemic areas.98
As demonstrated with HIV, hepatitis B, and HCV, increased screening can lead to decreased risk of donor-derived infections from asymptomatic infected individuals donating organs and blood products.86 However, blood-based screening and diagnosis present several challenges, including the lack of standardized testing and cross-reactivity among serologic tests for these viruses.99 As with the case of traditional blood-borne pathogens (eg, HIV, HCV), assessment of donor epidemiological information, including residence in endemic areas, the presence of signs or symptoms of infection, and travel history, may help prevent donor-derived infections. However, the use of these questionnaires can be problematic in blood or organ donor screening, as symptoms are often nonspecific or absent and as questionnaires are not instructive in endemic areas. Moreover, it can be costly and time-consuming to change questionnaire-based screening practices, let alone adding additional laboratory testing.100
In addition to screening for these pathogens, other methods of protecting blood products have been investigated, such as a combined treatment with Amotosalen, a nucleic acid crosslinker, and UV light, a system that inactivates the RNA genome of many pathogens. This method was demonstrated to be effective against ZIKV.101
4.7 Reducing exposure risk
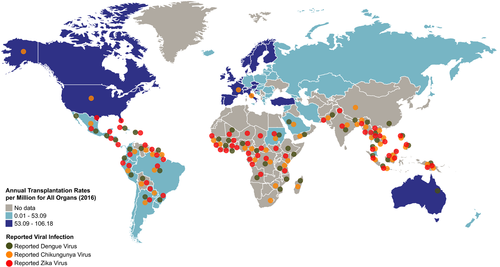
Exposure risk is greatest for those in endemic regions, which continue to expand with the increased distribution of the appropriate mosquito vectors, viral adaptation, and global spread of these arboviruses (Figure 1). The prevalence of these infections in transplant recipients is likely under-estimated due to the wide range of clinical manifestations, including asymptomatic infections. Transplant recipients in regions endemic for any of these arboviruses should be advised to reduce exposure to vectors (by wearing suitable clothing, using insect repellents, timing activities for low-risk time of day, etc.), especially in the 6 months following transplantation. Additionally, transplant recipients should avoid travel to areas with ongoing outbreaks. Travel of potential donors also poses an infection risk. Pre-operative vaccination of donor or transplant recipients who reside in or travel to endemic areas would be an important risk mitigation strategy, if and when vaccines become available and safe in this cohort. However, vaccines are not often assessed for efficacy or risk in immunocompromised populations. There is a licenced DENV vaccine, but there are no vaccines available for CHIKV or ZIKV.
Organ transplantation is already a medical practice comprised of heavily weighing the risk-benefit analysis of the patient's current condition and the patient's future transplant-related hazards. As with other potential challenges in the immunocompromised host, the risk of donor-derived infections should be balanced with the benefits of transplant on a case-by-case basis.102 Strategies to enhance donor supply, such as pooled regions of organ distribution used in the United States, also pose an enhanced risk. While a few cases of ZIKV transmission have been reported in southern states in the United States, Puerto Rico has a higher ZIKV incidence and organs from this area are included in a UNOS-designated region. UNOS has published a guidance statement on ZIKV, encouraging procurement organizations to focus on recent travel history, epidemiologic factors, and recent donor symptoms in each case. Due to the high prevalence of asymptomatic infections and the limitations of diagnostic tests, potential living donors should avoid mosquito bites and practice safe sex to decrease transmission risk until donation.103 If a potential donor contracts ZIKV, it is recommended that they defer donation for 120 days. Thirty-day donor deferral is recommended for CHIKV, and no deferral is yet recommended for DENV; however, living donors are encouraged to report any symptom development in the first week after donation.104 Although there is great variability in the screening policies and reporting of transmission events of donor-derived arbovirus infections from procurement agencies within and among nations, advances in donor screening, standards of investigation, and improvements in reporting and data sharing will allow for enhanced transplant safety.105
5 CONCLUSIONS
The quickly changing epidemiology of the viruses highlighted in this review is multifactorial and related to international travel, increased vector range due to climate change, trade, inadequate vector control, viral adaptation, and increasing human populations. Broader circulation of these arboviruses leads to increased exposure risk to naïve populations, which can cause large outbreaks in new regions. Infection with DENV, CHIKV, or ZIKV is a global health concern and is of particular concern in the transplant community, especially with pediatric patients, since less is understood about infection in children and the immunocompromised. Most importantly, symptoms of these infections are nonspecific and can be confused with other post-transplant diagnoses such as acute rejection. In endemic regions in particular, infections with DENV, CHIKV, and ZIKV should be on the differential diagnosis for evaluation of the transplant patient. Although this review focuses on only three arboviruses, a large number of other mosquito- and tick-borne pathogens are currently circulating throughout the world and may also pose risks to transplant recipients. As more attention is drawn to these important pathogens in the transplant community, additional research and strategies to mitigate risk will alleviate the potential effects of these emerging arboviruses on transplant recipients.
ACKNOWLEDGMENTS
The authors would like to thank Alexander Culler for his assistance with graphic development and FreeVectorMaps.com (https://freevectormaps.com) for the use of the world map outline.
AUTHORS’ CONTRIBUTIONS
Dr. Freeman: Drafted the initial manuscript; and Dr. Freeman and Drs. Coyne, Green, Williams, and Silva: Reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.




