ISPAD Clinical Practice Consensus Guidelines 2022: Exercise in children and adolescents with diabetes
1 WHAT IS NEW OR DIFFERENT
- Since the previous guideline, progress has been made in the field of diabetes management and physical activity (PA).1 An e-book that includes 10 articles on PA and type 1 diabetes (T1D) has been published2 and the epidemiological evidence and gaps in knowledge and research in this book have been recently reviewed (Section 7).3 Impact of age, sex, and physical fitness glucose responses to PA,4 and a structured approach to exercise consultation (Section 4)5 was presented. Finally, the benefits and limitations of technological advances in relation to PA were described in the same compilation.6 Of note, many of the new data were derived from adult, rather than pediatric populations.
- This guideline incorporates a new theme focused on strategies for glucose management for athletes living with T1D based in part on a randomized controlled trial (RCT)7 of the impact of acute hyperglycemia. General therapy recommendations for athletes8 were described, and later a review regarding competitive athletes with T1D was published (Sections 6 and 9).9
- Several, technological developments since the last guideline in 2018 have been incorporated into these new guidelines (Section 8). Specifically, an international group released a position statement providing practical approaches for glucose management before, during, and after exercise using real-time continuous glucose monitoring (CGM) and intermittently scanned CGM (isCGM) (Section 7).10 Closed-loop systems have also been evaluated in the context of PA and in RCTs illustrating the first steps towards optimal glycemia in relation to PA (Section 8).11-16
2 EXECUTIVE SUMMARY AND RECOMMENDATIONS
- Exercise is a cornerstone in the management and mitigation of cardiometabolic risk factors for children and adolescents with T1D and type 2 diabetes (T2D). Children and adolescents with T1D and T2D should be encouraged and supported to achieve the recommended 60 min of moderate to vigorous intensity PA every day. B
- Exercise is recommended to be regularly discussed as part of routine diabetes care for children and adolescents with T1D and T2D. E
- There is an increased risk of hypoglycemia during, shortly after, and up to 24 h after exercise due to increased insulin sensitivity. A
- A history of severe hypoglycemia in the preceding 24 h is generally a contraindication to exercise. A
- During all forms of physical exercise high glycemic index carbohydrates should be available to prevent and treat hypoglycemia. E
- Self-monitoring of blood glucose (SMBG), isCGM or CGM are essential for optimizing time in range and preventing hypoglycemia during and after exercise in all children and adolescents with diabetes. A
- Use of CGM during exercise is strongly recommended for children and adolescents with T1D, with CGM as the preferred modality to assist both user and guardian as symptoms of hypoglycemia and hyperglycemia may be difficult to detect. A
- CGM lags during prolonged aerobic exercise. It is recommended that glucose levels are confirmed by capillary fingerstick measurements if recent antecedent or present hypoglycemia is noted. A
- A wide range of insulin adjustment and nutrition strategies can be combined to keep the glucose level in the exercise range of 5.0–15.0 mmol/L or 90–270 mg/dl and prevent exercise induced-hypoglycemia. A
- Ketone levels, ideally measured using blood rather than urine, are generally recommended prior to exercise for children and adolescents with T1D if glucose values indicate possible insulin deficiency because elevated ketone levels before exercise pose a potential risk. D
- Exercise in children and adolescents with T1D and T2D is contraindicated in the presence of blood ketones ≥1.5 mmol/L or urine ketones: 2+ or 4.0 mmol/L. If blood ketone levels are between 0.6 and 1.4 mmol/L, exercise should be postponed until the cause of elevated ketone levels has been evaluated and an insulin bolus dose is given equal to half the usual individual correction dose (or 0.05 U/kg). B
- The type and amount of carbohydrates used in relation to exercise should be tailored to the specific activity. B
- Moderate intensity aerobic activity, such as walking and cycling for 15–45 min between meals, safely lower glucose levels >10.5 mmol/L (190 mg/dl). B
- Alcohol should be avoided before and during exercise as it may increase hypoglycemia risk, including nocturnal hypoglycemia after exercise, and impair performance. A
- Insulin should be administered in areas not actively engaged in muscle contraction. B
- Insulin dose adjustments are mostly required for aerobic exercise, and less likely required for very high intensity or anaerobic exercise which is more commonly associated with elevated glucose levels. A post-exercise insulin correction for hyperglycemia may be considered in such circumstances. B
- Recent technology advancements, including insulin pumps with hybrid closed loop (HCL) automated insulin delivery, provide benefits in relation to exercise for children and adolescents with T1D. Optimal use during exercise remains uncertain, and new systems will require individualized approaches, but the benefits of reduced hypo- and hyperglycemia after PA and specifically at night are clear. B
- Children and adolescents with T1D and T2D with significantly unstable diabetes, frequent severe diabetic complications (severe hypoglycemia, recurrent ketoacidosis) or advanced chronic complications of the disease should reduce or stop participating in vigorous exercise until metabolic control has improved and a specific exercise management plan has been made. High intensity exercise is generally contraindicated in those with more advanced or proliferative retinopathy. C
- An episode of severe hypoglycemia or recurrent antecedent hypoglycemia within the previous 24 h is a temporary contraindication to PA, C as is hyperglycemia ≥15.0 mmol/L (≥ 270 mg/dl) with concomitant ketonemia/ketonuria due to insulin deficiency, D acute injury or infection. C
Of note, many of the recommendations in this guideline are based on data derived from studies in adults with T1D. Therefore, practitioners and caregivers of children and adolescents should apply the evidence and adapt them where necessary based on local context. Furthermore, many of the studies have been conducted predominantly in male participants, and evidence cannot therefore be universally applied to females. Moreover, these recommendations are general, and it should be clarified that the physiological responses to exercise are individual, and thus optimal management might differ from individual to individual and context to context within the same person. These uncertainties are reflected in the grading above.
3 INTRODUCTION
Regular PA is one of the cornerstones of diabetes management.17, 18 Despite this, over the years, PA levels in children have decreased in many countries with <10% of the global population of youth meeting the current 24-Hour Movement Guidelines.19 In addition to reduced PA, an increase in body mass index (BMI) and declining oxygen uptake capacity (an indicator of physical fitness) have been reported in youth with T1D and T2D, leading to increased cardiovascular disease risk.20-24 Consequently, these results require some form of action as the level of PA is often passed on from childhood into adulthood.25, 26
- Children and adolescents should do at least 60 min per day of moderate to vigorous-intensity, primarily aerobic, PA across the week.
- Vigorous intensity aerobic activities and activities that strengthen muscle and bone should be incorporated at least 3 days a week.
- Children and adolescents should limit the amount of time spent being sedentary, particularly the amount of recreational screen time.
It is not surprising that the benefits of PA have also been documented in children with chronic diseases.
- Lower HbA1c by approximately 0.3%–0.5% depending on baseline HbA1c level and the amount of PA, specifically in children and adolescents
- Lower risk of premature all-cause and cardiovascular mortality
- Increased cardiovascular and cardiorespiratory fitness
- Enhanced muscle mass and strength
- Reduced adiposity
- Increased bone mineral density
- Improved insulin sensitivity
- Improved cardiovascular risk profile
- Improved sense of overall well-being
- May extend remission time in children with new onset diabetes mellitus
Despite these benefits, very few individuals with or without diabetes meet the recommendations for PA. Children with T1D, younger than 7 years, engage in less daily PA than children without T1D of the same age.36 Many adolescents with T1D,37 and especially T2D,38 have high rates of sedentary behavior and engage in less moderate to vigorous PA than youth without diabetes.39 Thus, children and adolescents with diabetes may in general be less physically active than their peers.39, 40 In the general population, the reasons are multifactorial: lack of time, low motivation, access to facilities,41, 42 or disability.43 The barriers for young people with diabetes are similar, but there are also many disease-specific barriers to manage. These include recurrent hypoglycemia and fear of hypoglycemia, elevated HbA1c and/or elevated glycemic variability, issues around body image, the planning required, parental hesitancy, social determinants of health, and general lack of knowledge in the field of exercise and diabetes.44, 45
Incorporating regular exercise and PA into the lives of children and adolescents with diabetes is challenging as there is not a “one size fits all” approach. Health care professionals must feel confident in motivating and advising children and adolescents with diabetes and their caregivers to adopt and sustain a new behavior, have the necessary resources, and empower young people to incorporate PA and exercise into their daily lives and self-management plans. There are still many gaps in knowledge related to PA and pediatric diabetes. These include a lack of RCTs and large prospective cohort studies using adequate serial measurements, in individuals of different ages and sexes, that can elucidate appropriate “doses” of PA on diabetes-specific and general health-related outcomes. As new technologies become available, studies are also required to understand the impact of incorporating them into regular exercise and PA behaviors on cardiometabolic endpoints and psychological outcomes. Finally, in the current era of person-centered care and person-oriented research it is will be essential to involve individuals with diabetes, their partners, and caregivers when studies regarding PA and diabetes are planned and carried out.3
These guidelines cover many broad aspects of exercise and diabetes for children and adolescents with T1D and T2D. The recommendations are designed to serve as a starting point for health care professionals and allow progression to more detailed personalization of exercise management for specific exercise scenarios and diabetes management regimens.
4 APPROACH TO CONSULTATION AND ASSISTANCE
The structured approach to the clinical consultation and planning of exercise for youth with diabetes requires a logical stepwise process. First, the dialogue starts with an exploration of personalized PA goals and a discussion about exercise physiology and expected glycemic excursions. The next step is to develop a methodical framework that encompasses glucose monitoring, insulin dosing strategy and fueling plan, to ensure safety and prevent hypoglycemia for youth with T1D.5 For children and adolescents with T2D, exploring barriers and stage of change for increasing regular PA can help with co-designing individualized plans for behavior change.46 Children and adolescents with T2D requiring insulin will need to discuss safely incorporating exercise into their dosing strategies. These templates may then be stratified to account for planned vs. unplanned exercise. The latter is associated with reduced flexibility to adjust insulin dose before exercise, thus necessarily emphasizing nutritional intake and vigilant glucose monitoring. The detailed evidence supporting specific insulin adjustments, nutrition/fueling, and glucose monitoring to guide exercise are discussed in the relevant sections below.
As many children with diabetes are sedentary, thoughtful planning is required to get started safely and sustain an active lifestyle in such situations. The following approach may be used for both habitually active and sedentary youth. The recommendation is to work outwards from the center of the “dartboard” in discussion with the young person with diabetes to develop an individualized plan (Figure 1).5
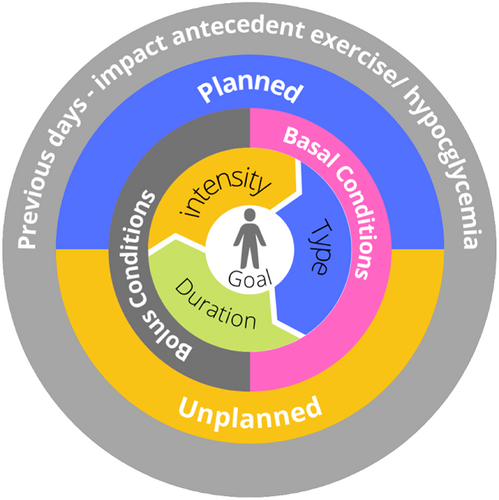
4.1 Step 1: Setting and adjusting person-centered activity goals
Any clinical discussion must begin with a person-centered approach to exercise goals and motivation; clinicians may guide this discussion with individual-specific factors explored. These may include a desire for increased fitness, improved body composition, social inclusion such as peer activities or team sports, better glycemia, sports-specific high level or elite performance, and/or overall enjoyment.
Youth with T1D tend to be overweight37, 38 and most youth with T2D are overweight or obese.47-49 Where improvements in body composition are sought, a strategy built around insulin dose reduction will reduce the need to prevent or treat hypoglycemia with extra carbohydrates. Additional attention should be paid in the initial consultation to known general barriers to exercise,44, 50-52 especially in adolescents, including personal barriers (self-motivation, motor skills, body image), social, environmental, and time factors.53 In addition, psychosocial assessment and dietary advice should be included. Importantly, baseline fitness should be considered; lower baseline fitness is associated with greater glycemic variability in youth with T1D.54 Youth with lower fitness will preferentially utilize muscle and liver glycogen stores (as a greater proportion of total energy expenditure) over fat oxidation Additionally, for the same amount of work performed, those less fit will necessarily be exercising at higher intensity, which is associated with risks of post-exercise hypoglycemia.55 For athletes, education must also include planning for management during both training and competition. An athlete with newly diagnosed diabetes requires support to return to routine exercise as soon as possible. The information should then also be provided to the coach/trainer.
For children and adolescents with T1D participating in competitive sports, where optimal exercise performance is the goal, increased fueling for work performed together with an overall increase in both carbohydrate and protein intake across the day is likely required. Thus, insulin doses may need minimal adjustment or even need to be increased,56 depending on the balance between the increase in nutritional intake and the improved insulin sensitivity from the higher overall intensity or volume of work performed. Dietitians should be closely involved in planning nutrition and the insulin doses required around an exercise training plan for children and adolescent athletes with T1D.
For many youth, the most uncomplicated goal is to foster participation and enjoyment of an active lifestyle. Hypoglycemia is well known to be associated with reduced exercise capacity. The impact of hyperglycemia remains less clear; the balance of evidence does not support a powerfully detrimental performance as a result of mild–moderate hyperglycemia.7 Thus, hypoglycemia prevention and general safety should take precedence as the primary aim of the management plan. Where improved fitness also exists as a goal for a child or adolescent with T1D participating in competitive sport, the person, parent, and provider should discuss the anticipated improvements in insulin sensitivity that will likely occur over weeks and thus potential reductions in total daily insulin dose that may be needed, regardless of insulin regimen.
4.2 Step 2: Discussion of exercise type
The type and duration of exercise will impact the expected acute glycemic excursions for children and adolescents with T1D, as discussed elsewhere in this chapter.57 Predictable falls in blood glucose levels (BGLs) should be incorporated into a plan based around general aerobic activity, with commensurate reductions in pre-exercise insulin dose and basal insulin exposure (where possible and with enough time for adjustments to be effective) together with a strategy to fuel appropriately. The risk of hypoglycemia also increases with exercise duration. Even at low intensity, prolonged exercise will inevitably require some adjustment of both insulin and fueling, which may be additive and progressive as activity extends.58 Conversely, acute hyperglycemia may be seen with very high-intensity exercise, especially in fasted states. However, the glycemic response to bolus insulin and ingested carbohydrates is much less predictable. Persons with diabetes should be educated accordingly to anticipate this. Such acute hyperglycemia can be managed with either conservative correction doses59 or components of low-intensity aerobic activity which increase glucose disposal without increasing the rate of glucose appearance, or cool-downs that lower serum lactate60 and catecholamine levels. These acute excursions in BGLs are less likely to occur for adolescents with T2D.
4.3 Step 3: Discussion of exercise timing and insulin action
In youth with T1D, and for some with T2D, exercise or general PA frequently occurs with some residual active insulin from a recent bolus (“insulin on board”). Examples include school sports, lunch breaks with playtime, after-school team practice, or generally spontaneous play. Thus, discussing insulin action time with youth and parents and how this impacts glycemic responses to exercise is crucial. Rapid-acting analogs generally attain peak action 60–100 min after injection, with a total duration of up to 5 h. It is ideal to manage glucose levels around exercise when minimal or no active rapid insulin is in the circulation. However, this is an uncommon scenario in youth who eat frequently and are unlikely to exercise before their first dose of prandial insulin of the day or several hours after their last meal or snack.
When exercise is planned to occur within 2–3 h of a meal, appropriate adjustment to the corresponding dose of pre-exercise insulin should be considered. General suggestions are delineated below based on clinical trial evidence in Tables A and B. Still, they will depend on whether the activity is predicted to cause a fall in BGL (see above, step 2) and the planned duration if known. Aggressive reductions of prandial insulin more than 90 min before exercise may reduce the risk of hypoglycemia during or immediately after exercise but may also be associated with hyperglycemia before exercise commences. Accordingly, these possible outcomes must be balanced and prioritized according to the personalized goals as set out and settled upon with the person with diabetes, as above in Step 1.
As fueling to maintain target glycemia during exercise is necessarily a function of the prevailing insulinemia, carbohydrate intake (as detailed later in this chapter) can be adjusted; less carbohydrate (in the range of 0.3–0.5 g/kg/h) is generally required when only basal insulin is active. In contrast, in adults double these amounts (or more) may be required when exercise coincides with peaking rapid-acting analog insulin.57 It is important to discuss with the person that 0.3–0.5 g/kg/h may avoid hypoglycemia. Still, where optimal performance or maximal work is the desired goal, higher fuel intake is optimal. The approach is discussed in detail with specific recommendations below, and additional informative data are provided by glucose concentrations to fine-tune the fuel required.
When formulating a plan with youth and families, these same principles should be discussed by the diabetes team for planned activity. The time of day can then be discussed in detail, with clear evidence from several studies showing afternoon exercise of both low and high intensity is associated with more significant risks of delayed nocturnal hypoglycemia, frequently 7–11 h later.61 This discussion can then be used to formulate the plan for any adjustments to the evening insulin dose, such as basal rate adjustments overnight62 or the setting of predictive glucose suspension modes in those on pump therapy, or an adjustment to the evening basal analog in persons with diabetes on insulin injections, possibly by splitting the basal dose into two doses per day, where a reduction of the basal dose at night does not affect a whole day. At this point, individuals and their caregivers should be reminded that high intensity afternoon exercise that causes acute hyperglycemia is nonetheless associated with a risk of delayed nocturnal hypoglycemia. Therefore, exercise early in the day can be a strategy to reduce the risk of nocturnal hypoglycemia. There is a lack of evidence on best practice insulin advice for youth with T2D undertaking afternoon activity.
4.4 Step 4: Contextualizing risks of hypoglycemia and safety considerations
Recent hypoglycemia prior to exercise is associated with an increased risk of further hypoglycemia (shown in adults)63 due to attenuated counter-regulatory responses and glycogen depletion. A history of severe hypoglycemia in the preceding 24 h is generally a contraindication to exercise, while a background of hypoglycemia unawareness needs to be explored and included in a final action plan, as this may further increase the risk of hypoglycemia after exercise. In these individuals, extra fuel or greater insulin reductions should be discussed. This risk may be especially pertinent overnight during sleep, which is associated with impaired counter-regulation in youth with T1D.64
These discussions can logically lead to a discussion of glucose monitoring which is core to the optimal management of glucose levels during and after the event. CGM can provide data, including alerts, to inform incremental management, especially any need for carbohydrate intake to maintain optimal glucose levels, as discussed in detail below. In those not using CGM, BGL measurement should be performed as often as required, with management recommendations in Table 4 below based on a fingerstick BGL every 30 min.
4.5 Step 5: Reviewing results and further adjustments to the plan
A follow-up consultation should be scheduled with persons living with diabetes and their families. This ideally provides an opportunity for further detailed sharing of information about insulin, fuel intake, and glucose levels before, during, and after exercise. Modern pump and CGM downloads make this rich information easily accessible to youth with diabetes and providers alike.
As acknowledged in the recommendations and tables below, any dose or fuel strategy should be considered as a starting point, as they are based on consensus and overall responses in clinical studies. Individual responses to exercise vary widely around these means,65 and thus health care providers and people with diabetes must be prepared to modify and review a plan based on practical experience, as goals change (see Step 1), as children grow, as physical fitness improves or as the insulin replacement modality changes. Therefore, a clinical review cycle incorporating all these factors should occur as required, in the clinic setting, or more frequently if necessary or desired.
5 PHYSIOLOGY
Exercise is considered a structured form of PA that can be classified as predominantly aerobic (oxidative metabolism) or anaerobic (non-oxidative metabolism) because of the major fuel systems used and how fuel is metabolized. With aerobic activities like walking, jogging, and cycling at a light to moderate intensity, heart rate and oxygen consumption increase from the resting state while lipids (i.e., free fatty acids and muscle triglycerides) and carbohydrates (blood glucose and muscle glycogen) are oxidized.66 With brief anaerobic activities like sprinting and weightlifting, the skeletal muscle generates energy from anaerobic glycolysis, phosphocreatine, and free adenosine triphosphate.66 Most forms of exercise, sport, play, and daily PA are a mix of aerobic and anaerobic metabolism. An understanding of the pathophysiology of exercise is valuable for the health care professional to be able to provide individualized advice to people living with diabetes, because of the complexity of exercise and diabetes.
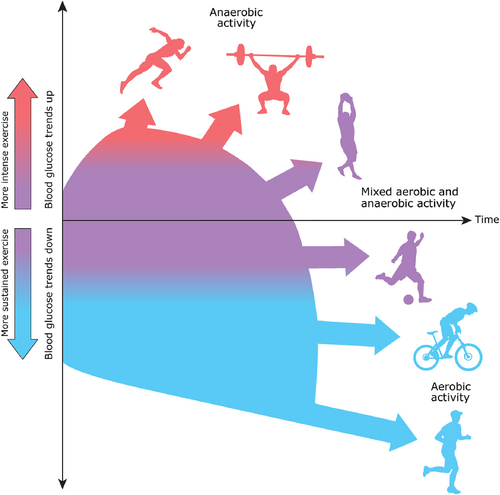
Aerobic exercise tends to cause circulating glucose levels to drop,65 while anaerobic or mixed forms of exercise are typically associated with an attenuated drop67, 68 or a rise in glycemia.69 In general, mixed activities tend to have a moderating effect. However, several factors are thought to influence these general tendencies (Figure 2 and Table 1). The acute effects of anaerobic exercise on glycemia for youth with T2D are unclear.
| Exercise type | Physiological characteristics | Effect on glucose level of person with type 1 diabetesa | Examples | |
|---|---|---|---|---|
| Aerobic | Continuous moderate-intensity exercise predominantly below lactate threshold where glucose uptake by the muscles is greater than glucose output from the liver65, 101, 106 | ↘ | ↓ | Running, walking, hiking, cycling, rowing, and swimming |
| Mixed with short intervals of anaerobic | Moderate-to-vigorous intensity (aerobic) activity interspersed with shorter (5–30 s) anaerobic bursts throughout68, 107 | ↘ | → | Basketball, football, soccer, cricket, handball, and martial arts |
| Mixed with long intervals of anaerobic | Low-to-moderate intensity (aerobic) activity interspersed with longer (10–180 s) anaerobic bursts throughout108 | ↗ | → | Resistance training, circuit training, gymnastics, sprint training (running, swimming, rowing, cycling, etc.) |
| Anaerobic | Maximum effort exercise to fatigue (5 s–10 min) at an intensity above lactate threshold when glucose output from the liver is greater than uptake by the muscles67, 69 | ↗ | ↑ | 500–2000 m row, 50–1500 m competition, 1–2 k cycle time trial, powerlifting |
| Competition day | Glucose output from the liver is likely to be exaggerated during competition leading to pronounced hyperglycemia compared to practice days | ↑ | Race, team, or individual game/match | |
- a These are general trends that are also influenced by several other factors such as insulin on board (IOB), macronutrient intake, pre-exercise glucose level, antecedent exposure to hypoglycemia, fitness level, time of day, intensity and duration of exercise, training status, environmental conditions. Adult male data.108 Adult male and female data.68, 69, 107 Pediatric male data.67 Pediatric male and female data.65 This table was created with the assumption of low to moderate circulating IOB.
5.1 Aerobic exercise
The main determinants of glucose concentration in diabetes are nutrient intake, the timing of the meal, insulin concentrations in the circulation, the rate of glucose production by the liver, and the rate of glucose utilization by the skeletal muscles and central nervous system.9 In the fasted state, circulating glucose is predominantly determined by the amount of glucose released by the liver and the rate of glucose uptake into skeletal muscle and the brain.70 The lower the circulating insulin concentrations and the higher the levels of glucose counter-regulatory hormones, the greater the glucose rate of appearance from the liver during aerobic exercise.70 The volume of skeletal muscle engaged in exercise primarily determines the rate of glucose disposal. While skeletal muscle contractile actions increase the rate of glucose disposal during exercise via contraction-mediated GLUT 4 translocation to the sarcolemma, elevated catecholamine levels limit the uptake of glucose from the circulation to help prevent the drop in glycemia and increase the muscles' reliance on its glycogen stores as fuel.66
The contraction-induced translocation of the GLUT 4 transporter protein allows skeletal muscle to take up and utilize blood glucose as fuel even when insulin concentrations are extremely low.71 However, low circulating insulin concentrations in T1D increases the rate of appearance of glucose from the liver72 and ketone production,73 which can be dangerous because this can cause severe hyperglycemia and dehydration ketoacidosis.
Because of the glucose-lowering action of aerobic exercise, exogenous insulin levels for children and adolescents with T1D should ideally be low to help prevent hypoglycemia.58 Unfortunately, lowering insulin concentrations quickly is not possible, even with an insulin pump, so more proactive measures need to be taken. These may include a reduction in prandial insulin at the meal before exercise and/or a reduction in basal insulin delivery on the insulin pump58 (see below for details). When insulin adjustments have not been made, increased carbohydrate consumption is the only option to prevent hypoglycemia58 (see below for details).
5.2 Very high intensity and anaerobic exercise
Anaerobic activities like sprinting, and weightlifting can cause glucose levels to rise, particularly if done early in the day with little to no prandial insulin in the circulation and if the activity is performed in isolation (i.e., without aerobic exercise), such as a 100-meter track event, a judo match, or a rowing sprint.74 In addition, increased circulating concentrations of stress hormones associated with competition and intensive anaerobic exercise may augment the increase in glucose level, even before the event occurs. For example, Garry Hall Jr., who competed in the Sydney 2000 Olympic Games in sprint swimming (50-meter freestyle), raised his BGL to 300 mg/dl during his world record race that lasted over 21 s.
Because of the potential for glucose levels to rise with some forms of anaerobic exercise, insulin dose reductions are often not recommended, and post-exercise insulin correction for hyperglycemia may be considered58 (see below for details).
5.3 Mixed exercise
Most forms of PA for many youth consist of spontaneous play and/or team and field sports. These settings are often characterized by repeated bouts of relatively intense activity interspersed with low to moderate-intensity activity or rest.
This type of “interval” or “mixed” activity has been shown to result in a lesser rate of fall glycemia in persons with T1D compared to continuous moderate-intensity exercise, both during and after the event.74 Mixed forms of exercise, therefore, may not require insulin dose adjustments.
5.4 Reasons for dysglycemia during exercise in youth with T1D
The reasons for dysglycemia with exercise in diabetes are complex and multifaceted. The main factors associated with greater decreases in glycemia during aerobic exercise are likely the levels of circulating insulin and the exercise intensity and duration of the activity.58 The levels of glucose counter-regulatory hormones (glucagon, catecholamines, cortisol, growth hormone) and the pre-exercise glucose level may also impact the change in glucose during aerobic exercise.58 Additional factors, including an individual's physical size, muscle mass, age, sex, fitness level, stress levels and genetics, may also impact the change in glucose; however, the magnitude of these effects are less clear.
Exercise may increase the rate of absorption of subcutaneously delivered insulin,75 which may increase insulin action soon after bolus administration. Insulin should be given in an area that is not actively engaged in muscle contraction. This may be difficult with some whole-body activities like swimming or when individuals have an insulin infusion set that is not easily moved for exercise. In addition, the impact of exercise on the absorption rate of ultra-long-acting basal insulin is unclear. However, one study in adults with T1D found that insulin detemir was associated with less hypoglycemia during and post-exercise.76
For youth with T2D, there is little evidence for the influence of duration, type, or intensity of exercise on acute glycemic excursions or glucose time-in-range. Cross-sectional studies suggest that more frequent bouts of structured PA,77 particularly vigorous intensity structured activity17 are associated with improved glycemia and cardiometabolic risk factors.
The unpredictable nature of activity in youth with T1D can make glycemic management challenging. Nonetheless, several strategies can be implemented to help limit dysglycemia associated with exercise (see below for details).
5.5 Antecedent hypoglycemia
Moderate or sustained levels of hypoglycemia in the 24–48 h prior to exercise appear to blunt the counter-regulatory responses to exercise and may increase the risk for exercise-induced hypoglycemia.78 Obesity and exercise in the cold may also blunt some of the counter-regulatory hormones (i.e., growth hormone, catecholamines)79, 80 which may increase hypoglycemia risk.
5.6 Glycemia, musculoskeletal health, and exercise performance
An acute episode of mild to moderate hyperglycemia does not appear to impact exercise or sport performance in T1D.7 However, even mild hypoglycemia negatively impacts reaction time and overall sport performance.81 On the other hand, sustained hyperglycemia (days and weeks) likely impacts several metabolic and circulatory processes that could, at least in theory, negatively impact exercise capacity, including an apparent loss of muscle mass and muscle mitochondrial content, reduced muscle capillarization, and general dehydration.82 In the long term, elevated HbA1c levels in youth with T1D may impact growth and development83 and likely adversely affect musculoskeletal health.84 For youth with diabetes, doing regular PA, prolonged periods of hyperglycemia caused by exercise, or the fear of developing hypoglycemia from exercise may negatively influence achieving overall glycemic management targets. Nonetheless, similar to youth with T2D,17, 77 days with increased PA may improve the likelihood of achieving glycemic targets in youth with T1D compared to days with inactivity.85 There are currently no data on exercise performance and glycemia in youth with T2D.
6 NUTRITION AND EXERCISE
6.1 Nutrition requirements and food quality
Advice on sports nutrition to maximize performance will include information about the type and amount of food and the intake timing. The amount of carbohydrates and protein required at meals will vary with age, sex, and activity levels. For youth undertaking daily activities associated with health (i.e., 60 min of moderate to vigorous PA daily), daily food intake should be sufficient to meet the demands of the activity, provided meals are distributed regularly across the day. Country-specific guidelines on energy and macronutrient intake exist in many parts of the world and, in general, increased activity levels are linked to increased energy requirements. Calculating increased energy and carbohydrate requirements may be necessary for very active youth, and youth specific PA compendium tables offer comprehensive lists to aid energy expenditure calculations.86 Advice on supplementary carbohydrates for hypoglycemia prevention should aim not to increase total energy intake above expenditure, and the use of snacks should not decrease dietary quality. The nutrition table (Table E) suggests the most effective carbohydrate choices for hypoglycemia prevention with the lowest total energy content. Adequate fluid intake is essential to reduce the risk of dehydration.87 In most situations, water or sugar-free fluids are most suitable for maintaining hydration. Detailed nutrition recommendations for health and exercise can be found in the ISPAD 2022 Consensus guidelines Chapter 10 for Nutritional Management in Children and Adolescents with Diabetes, along with further advice about nutritional supplements.
6.2 Nutritional and sports supplements
There is minimal evidence on using protein or other nutritional supplements to support athletic performance in adolescents. Protein supplements in adolescent athletes may not have additional benefits for exercise performance88 although there is some evidence they may reduce post-exercise inflammatory responses88 and have acute benefits on post-exercise muscle anabolism; however, demonstrable muscle damage and recovery changes have not been clearly shown.89 Therefore, protein supplementation should not be routinely recommended for youth partaking in regular PA.
Adolescent sports competitors often use sports supplements.90, 91 However, the International Society of Sports Nutrition's review of performance-enhancing supplements identified a dearth of efficacy data for their use in children under 18 years.92 Therefore, counseling on using food to maximize training adaptions should be prioritized. Advice on the risks of sports supplement use, which include contamination with banned performance-enhancing substances, should be provided with guidance on anti-doping according to the sport and level of competition. Some sports begin anti-doping procedures below the age of 18 years. Educational programs on anti-doping in sports are available through many national sporting organizations. Information about therapeutic use exemption for insulin is available on the world anti-doping authority website (https://www.wada-ama.org).
6.3 Alcohol
Adolescents and young adults need to understand the effects of alcohol on the response to exercise and falling BGLs. As some sports are associated with a “drinking” culture, alcohol safety advice should be provided without endorsing its consumption. Based on studies in adults with diabetes, alcohol impairs glucose counter-regulation by inhibiting hepatic gluconeogenesis (but not glycogenolysis) and increases the risk of hypoglycemia.93-96 Alcohol should be avoided before and during exercise as it may increase hypoglycemia risk, including nocturnal hypoglycemia after exercise, and impair performance. If alcohol is consumed after exercise, it may be necessary to advise more aggressive insulin reductions and higher supplementary carbohydrate amounts from the adjustment tables discussed later in this chapter (Tables A–E).
6.4 Low carbohydrate diets
No studies have specifically examined exercise performance of youth with diabetes using low carbohydrate diets. A recent systematic review of adult recreational exercisers without diabetes showed no impairment in aerobic performance or time to exhaustion after diet acclimatization on a low carbohydrate diet.97 The only difference was higher FFA utilization.97 However, a clinical trial has shown an impairment in exercise economy and performance when elite endurance athletes consumed a low carbohydrate diet.98 The elite-level performance deficit has recently been replicated, and the impairment was attributed to blunted carbohydrate oxidation rates.98
It is questionable how relevant this research is for children with T1D who are administering exogenous insulin. People with T1D have peripheral circulating insulin levels that are 2.5 times higher than people without diabetes.99 A high level of peripheral insulin alters hepatic and muscle metabolism.100 In the absence of clinical trials, it is advisable to counsel against this dietary approach, especially for optimal exercise performance. If a child or family insists on a low carbohydrate diet, it is essential to provide advice on exercising safely. Following the insulin adjustment strategies suggested in Tables 2 and 3 are sensible to start. However, the amount of supplementary carbohydrates required during exercise may be less than indicated in Tables 4 and 5. An individualized assessment and process of trial and error with an evolving plan will be required.
| Before exercise | After exercise | |||||
|---|---|---|---|---|---|---|
| Prandial insulin | Basal rate for non-fasting exercise | Post-exercise prandial insulin | Choose one or both options if exercise after 16:00 and exercise duration more than 30 min | |||
| Exercise type | Plan execution | If meal is consumed more than 2 h before exercise, administer regular prandial dose to prevent hyperglycemia108 If meal is consumed within 2 h of exercise, adjust prandial dose using these suggestions107, 109, 110 |
If exercise is more than 120 min since prandial insulin, basal reduction 90 min before112 |
Prandial insulin reduction |
Basal rate change |
If glucose level less than 10.0 mmol/L (180 mg/dl) low glycemic index carbs snack without bolus insulin before bed128 If glucose level less than 7.0 mmol/L (126 mg/dl) add an additional 15 g protein128 |
| Aerobic | >15.0 mmol/L (270 mg/dl) using starting plan |
−25%108, 109 | −25% | −25% | Regular dose | 0.2 g/kg/BW |
| Starting plan | −50%107-109 | −50%112 | −50%110 | −20% for 6 h62 | 0.4 g/kg/BW107, 110 | |
<5.0 mmol/L (90 mg/dl) using starting plan |
−75%108, 110 | −80%112 | −75% | −40% for 6 h |
0.6 g/kg/BW | |
| Mixed | >15.0 mmol/L (270 mg/dl) using starting plan |
−25%108 | Regular dose | Regular dose107, 108 | Regular dose | 0.2 g/kg/BW |
| Starting plan | −50%107, 108 | −25% | −25% | −20% for 6 h |
0.4 g/kg/BW107 | |
<5.0 mmol/L (90 mg/dl) using starting plan |
−75%108 | −50% | −50% | −40% for 6 h |
0.6 g/kg/BW | |
| Anaerobic | >15.0 mmol/L (270 mg/dl) using starting plan |
Regular dose | Regular dose and small bolus 15 min pre- exercise | Regular dose108 | Regular dose | 0.2 g/kg/BW |
| Starting plan | −25%108 | Regular dose | −25% | −20% for 6 h | 0.4 g/kg/BW | |
<5.0 mmol/L (90 mg/dl) using starting plan |
−50%108 | −25% | −50% | −40% for 6 h | 0.6 g/kg/BW | |
- Note: BW, body weight. If body mass index centile is ≥91st then use the IBW in kg = (BMI at the 50th centile for age × [height in meter]2),111 unless the high BMI centile is due to large muscle mass. Consider reducing carbohydrate suggestions for populations with less lean body mass than healthy male adults, such as females and sedentary males. Adult male data.108-110 Adult male and female data.107, 112, 128 Pediatric male and female data.62, 111 The table suggests a starting plan (first recommendation to be given) that can then be personally adapted based (evidence level D.) The table provides guidance on how to adapt plans (first recommendation given in gray) based on trialing the starting plan. Only the before or after strategy that results in hyper- or hypoglycemia requires adjustment, not the whole plan.
| Before exercise | After exercise | ||||
|---|---|---|---|---|---|
| Mealtime insulin | Post-exercise meal insulin | Choose one or both options if exercise after 16:00 and exercise duration more than 30 min | |||
| Exercise type | Plan execution | If meal is consumed more than 2 h before exercise, administer regular prandial dose to prevent hyperglycemia108 If meal is consumed within 2 h of exercise, adjust prandial dose using these suggestions109, 110 |
Meal insulin reduction | Evening basal insulin |
If glucose level less than 10.0 mmol/L (180 mg/dl) low glycemic index carbs snack without bolus insulin before bed128 If glucose level less than 7.0 mmol/L (126 mg/dl) add an additional 15 g protein128 |
| Aerobic | >15.0 mmol/L (270 mg/dl) using starting plan |
−25%109 | −25% | Regular dose110 | 0.2 g/kg/BW |
| Starting plan | −50%107-109 | −50%110 | −20%110 | 0.4 g/kg/BW107, 110 | |
<5.0 mmol/L (90 mg/dl) using starting plan |
−75%108, 110 | −75% | −40% | 0.6 g/kg/BW | |
| Mixed | >15.0 mmol/L (270 mg/dl) using starting plan |
−25%108 | Regular dose107, 108 | Regular dose | 0.2 g/kg/BW |
| Starting plan | −50%107, 108 | −25% | −20% | 0.4 g/kg/BW107 | |
<5.0 mmol/L (90 mg/dl) using starting plan |
−75%108 | −50% | −40% | 0.6 g/kg/BW | |
| Anaerobic | >15.0 mmol/L (270 mg/dl) using starting plan |
Regular dose | Regular dose108 | Regular dose | 0.2 g/kg/BW |
| Starting plan | −25%108 | −25% | −20% | 0.4 g/kg/BW | |
<5.0 mmol/L (90 mg/dl) using starting plan |
−50%108 | −50% | −40% | 0.6 g/kg/BW | |
- Note: BW, body weight. If body mass index centile is ≥91st then use IBW in kg = (BMI at the 50th percentile for age × [height in meter]2),111 unless the high BMI centile is due to large muscle mass. Consider reducing carbohydrate suggestions for populations with less lean body mass such as sedentary individuals. Adult male data.108-110 Adult male and female data.107, 128 Pediatric male and female data.111 The table suggests a starting plan (first recommendation to be given) based evidence level D. These guidelines serve as starting point that require personalized adaptation. The table provides guidance on how to adapt plans (first recommendation given in gray) based on trailing the starting plan. Only the before or after strategy that results in hyper- or hypoglycemia requires adjustment, not the whole plan.
| Sensor or blood glucose level | Expected glucose response during exercise based on the type of exercise, insulin on board and bolus adjustments, basal adjustments, and previous glucose control | |
|---|---|---|
| Expected to fall during exercise | Expected to stay stable or rise during exercise | |
Higher than 15.0 mmol/L (270 mg/dl) and ketones more than 0.6 mmol/L |
Ketones >1.5 mmol/L: Follow usual ketone advice and avoid exercise Ketones 1.1–1.4 mmol/L: Give ½ correction dose by pen and wait 60 min to reassess Ketones 0.6–1.0 mmol/L: Give ½ correction dose by pen and wait 15 min to exercise |
|
Higher than 15.0 mmol/L (270 mg/dl) and ketones less than 0.6 mmol/L |
Consider ½ of usual bolus insulin correction | |
10.1–15.0 mmol/L (181–270 mg/dl) |
No carbohydrate | |
| Carbohydrate requirements (g/kg/BW/30 min do not exceed 60 kg)b | ||
Exercise targeta 7.0–10.0 mmol/L (126–180 mg/dl) |
0.2–0.5117 | 0 |
5.0–6.9 mmol/L (90–125 mg/dl) |
0.5101 | 0.2116 |
Delay or stop exercise for 20 min 4.0–4.9 mmol/L (70–89 mg/dl) |
0.3190 | 0.3190 |
3.0–3.9 mmol/L (54–70 mg/dl) |
Treat hypoglycemia and delay exercise until greater than 4.9 mmol/L (89 mg/dl) | |
Less than 3.0 mmol/L (54 mg/dl) |
Treat hypoglycemia and do not start exercise due to impaired counter-regulatory hormone response | |
- a If risk of hypoglycemia or hypoglycemia unawareness is medium or high, increase exercise target level to 8.0–11.0 mmol/L (145–198 mg/dl) or 9.0–12.0 mmol/L (162–216 mg/dl) respectively.
- b Do not exceed 60 kg when calculating carbohydrate amounts to prevent suggestions greater than the peak exogenous carbohydrate utilization of 1.0–1.2 g/min.102-104, 191 Also, if body mass index (BMI) percentile is ≥91st then use the body weight (BW) in kg = (BMI at the 50th percentile for age × [height in meter]2),111 unless the high BMI percentile is due to large muscle mass. Adult male data.102-104, 191 Adult male and female data.116, 117 Pediatric male data.101 Pediatric male and female data.111, 190
| Sensor or blood glucose level | Trend arrow | Expected glucose response during exercise based on the type of exercise, insulin on board and bolus adjustments, basal adjustments, and previous glucose control (If checking frequency is more than 20 min, select the carbohydrate amount based on a stable trend arrow and adjust according to checking frequency) | |
|---|---|---|---|
| Expected to fall during exercise | Expected to stay stable or rise during exercise | ||
Higher than 15.0 mmol/L (270 mg/dl) and ketones more than 0.6 mmol/L |
All | Ketones >1.5 mmol/L: Follow usual ketone advice and avoid exercise Ketones 1.1–1.4 mmol/L: Give ½ correction dose by pen and wait 60 min to reassess Ketones 0.6–1.0 mmol/L: Give ½ correction dose by pen and wait 15 min to exercise |
|
Higher than 15.0 mmol/L (270 mg/dl) and ketones less than 0.6 mmol/L |
→ ↗↑ | Consider ½ of usual bolus insulin correction | |
| ↘↓ | No carbohydrate | ||
| Carbohydrate requirements (g/kg/BW/20 min do not exceed 60 kg)b | |||
10.1–15.0 mmol/L (181–270 mg/dl) |
↑ | 0 | 0 |
| ↗ | 0 | 0 | |
| → | 0 | 0 | |
| ↘ | 0.1 | 0 | |
| ↓ | 0.2 | 0 | |
Exercise targeta 7.0–10.0 mmol/L (126–180 mg/dl) |
↑ | 0 | 0 |
| ↗ | 0.1 | 0 | |
| → | 0.2 | 0 | |
| ↘ | 0.3 | 0.1 | |
| ↓ | 0.4 | 0.2 | |
5.0–6.9 mmol/L (90–125 mg/dl) |
↑ | 0.1 | 0 |
| ↗ | 0.2 | 0.1 | |
| → | 0.3 | 0.2 | |
| ↘ | 0.4 | 0.3 | |
| ↓c | 0.5 | 0.4 | |
4.0–4.9 mmol/L (70–89 mg/dl) |
↑ | 0.2 | 0.1 |
| ↗ | 0.3 | 0.2 | |
Delay or stop exercise 20 min 4.0–4.9 mmol/L (70–89 mg/dl) |
→ | 0.3 | 0.3 |
| ↘c | 0.4 | 0.4 | |
| ↓c | 0.5 | 0.5 | |
3.0–3.9 mmol/L (54–70 mg/dl) |
All Arrows | Treat hypoglycemia and delay exercise until greater than 4.9 mmol/L (89 mg/dl) | |
Less than 3.0 mmol/L (54 mg/dl) |
All Arrows | Treat hypoglycemia and do not start exercise due to impaired counter-regulatory hormone response | |
- a If risk of hypoglycemia or hypoglycemia unawareness is medium or high, increase exercise target level to 8.0–11.0 mmol/L (145–198 mg/dl) or 9.0–12.0 mmol/L (162–216 mg/dl) respectively.
- b Do not exceed 60 kg when calculating carbohydrate amounts to prevent suggestions greater than the peak exogenous carbohydrate utilization of 1.0–1.2 g per min.102-104, 191 Also, if body mass index (BMI) percentile is ≥91st then use the body weight (BW) in kg = (BMI at the 50th percentile for age × [height in meter]2),111 unless the high BMI percentile is due to large muscle mass.
- c Consider blood glucose test as CGM value maybe lagging. Adult male data.102-104, 191 Pediatric male and female data.111
6.5 Elite athletes and high performers
Specific recommendations regarding increased nutritional requirements and advanced insulin adjustment strategies required to support high-performing athletes with diabetes are beyond the scope of this chapter. Youth who participate in elite-level sports should be referred to a team with multidisciplinary expertise in exercise and T1D management.
The nutrition section discusses calculating energy, carbohydrate, and protein requirements based on the regular training and competition schedule. A recent review article discusses bespoke insulin adjustments strategies and how to plan for dynamic training protocols for different modalities and exercise duration.9, 101-105
7 INTEGRATING INSULIN AND NUTRITION STRATEGIES FOR ACUTE EXERCISE MANAGEMENT
Tables 2–6 are included to illustrate the recommendations below along with clarifications regarding age and gender of study participants.
| Before exercise | During exercise | Post-exercise | Before bed |
|---|---|---|---|
Aim for a meal at least 180 min prior to exercise to minimizing circulating insulin114 and maximize glycogen stores115 following the post-exercise meal content and examples If eating within 180 min of exercise, aim to eat within 60–90 min of exercise to reduce the risk of pre-exercise hyperglycemia109, 110 |
High glycemic index carbohydrate choices when testing frequently during exercise Medium glycemic index carbohydrate choices when testing infrequently or never during exercise |
Meal within 90 min of completing exercise Prioritize including a protein source |
Exercise post-16:00 and duration ≥30 min |
Meal content within 60–90 min of exercise: |
Carbohydrate amount: Carbohydrate requirement table C and D |
Meal content: Carb 1–4 g/kg/BW, Protein: ≥15 g, Fat: Moderate115 |
Snack content: Carb: 0.4 g/kg/BW low-medium glycemic index107, 110 Protein: 15 g |
Breakfast examples for meal within 60–90 mina: Fruit salad Toast/marmite or vegemite/fruit Breakfast cereal/milk Oat based muesli bar Pikelets Bagel/low fat cream cheese Pancakes |
Fluid optionsa: Glucose based (most effective) options: Isotonic sports drinks 6%–8% (6–8 g/100 ml) Glucose energy drinks 8%–10% (8–10 g/100 ml) Glucose shots 25% (25 g/100 ml) Glucose sports gels 60%–70% (60–70 g/100 ml) Sucrose (glucose/fructose) options: Fruit juice 11% (11 g/100 ml) Sweetened drinks 8%–10% (8–10 g/100 ml) |
Breakfast examplesa: Fruit salad/milk/nuts/yoghurt Toast/eggs/tomato/fruit Breakfast cereal/milk Rolled oats/milk/nuts/fruit Toast/Avocado/eggs Pancakes/bacon/mushrooms/tomato Omelette/cheese/salad/bread roll Crepes/chicken/pea salad |
Low-medium glycemic index carb optionsa: 200 g milk (10 g) 1 slice multigrain bread or toast (15 g) 50 g cooked chickpeas (15 g) 1 large apple or medium banana (15 g) 200 g plain yoghurt (14 g) 50 g cooked rice (15 g) 30 g wholegrain breakfast cereal (15-20 g) 50 g cooked noodles or pasta (15 g) |
Lunch examples for meal within 60–90 mina: Sandwich or bread roll/salad Rice cakes/vegemite or marmite Wrap/lean meat/salad Wheat biscuits/fruit Rice/stir-fry vegetables Toast/marmite or vegemite/fruit |
Solid optionsa: Glucose based (most effective) options: Dextrose tablets (3 g each) Glucose tablets (4 g each) Sucrose (glucose/fructose) options: Candy/sweets 75%–90% (75–90 g/100 g) |
Lunch examplesa: Sandwich or bread roll/lean meat or cheese/salad Wholegrain toast/peanut butter/banana Wrap/chicken/salad/baked beans Wheat biscuits/low fat cottage cheese/fruit Cous Cous/hummus/vegetables/fruit Pasta/avocado/chicken/vegetables/pesto Quesadillas/vegetables/cheese |
Protein optionsa: 50 g mixed chopped nuts (8 g) 2 eggs (14 g) 70 g canned fish (15 g) 150 g low fat cheese (15 g) 200 ml milk (7 g) 200 g plain yoghurt (7 g) 50 g hard cheese (12 g) 50 g cooked chickpeas (3 g) |
Dinner examples for meal within 60–90 mina: Rice/vegetables/tomato-based sauce Vegetable soup/bread roll Tortilla/vegetables/salsa/guacamole/beans Jacket potato/baked beans Noodles/stir-fry vegetables |
If unable to monitor glucose level frequently or at all during exercisea: Before or during exercise include: Banana (22 g/100 g) Breakfast bar (67 g/100 g) Muesli bar (53 g/100 g) Rice cakes (83 g/100 g) Up and Go (10 g/100 ml) Low fat natural yoghurt (7 g/100 g) |
Dinner examplesa: Pasta/tomato-based sauce/mincemeat/vegetables Rice/fish/vegetables/tomato-based sauce Pad Thai/meat or fish/salad Jacket potato/tuna/mayonnaise/salad Lasagna/garlic break/vegetables Nut or lentil-based curry/chapattis/salad Vegetable stew with beans/baked potato Mashed potato/lean sausages/vegetables |
- a The examples are estimates that will vary by country, therefore, the reader must review the nutrition labels of individual products and adapt based on the carbohydrate per 100 ml or 100 g. BW, body weight. If BMI percentile is ≥91st then use BW in kg = (BMI at the 50th percentile for age × [height in meter]2),111 unless the high BMI percentile is due to large muscle mass, and use the lower end of carbohydrate ranges for sedentary individuals.
- b Target glucose levels may be individualized. Adult male data.109, 110, 114 Adult male and female data.107, 115, 128 Pediatric male and female data.111
7.1 Planned exercise
Planned exercise lasting at least 30 min requires therapy management strategies before, during, after, and then overnight. A wide range of insulin adjustment and nutrition strategies can be combined to keep the glucose level during activity in an exercise range of 5.0–15.0 mmol/L or 90–270 mg/dl and prevent exercise induced-hypoglycemia. It is paramount that the health care professional ensures the individual with diabetes, and if required, their family is aware that trial and error might be required and that plans must be adapted based on observed results. The insulin pump or continuous subcutaneous insulin infusion (CSII - Table 2) and multiple daily injections (MDI - Table 3) adjustment tables offer starting plans and adjustment protocols. Tables 4 and 5 offer guidance on how to calculate carbohydrates to prevent hypoglycemia just before and every 30 and 20 min during exercise, for people using SMBG and CGM, respectively. Ideas for meals, snacks, and carbohydrates during exercise can be found in Table 6.
Recommendations in Tables 2–6 are based on studies with small numbers of mainly healthy adults performed on treadmills or cycle ergometers and do not mimic real-world exercise for youths. Therefore, extrapolating to populations with lower lean body mass, such as youths who are sedentary, overweight, or obese, may be problematic. Specific considerations for these populations are discussed in the relevant sections and in the tables. Finally, using the tables will not produce consistent results across a population due to the considerable inter and intra-individual variation in glucose responses to the same exercise. The recipients of plans devised from the tables must be informed of their limitations and that they are merely a starting point requiring adaptation from trial and error.
7.2 Prior to planned exercise: Insulin adjustments and nutrition strategies
Exercise following an unadjusted mealtime insulin bolus may lead to hypoglycemia in youth with T1D65, 101 even when provided 15 g carbohydrate during exercise.106 Reductions of pre-exercise prandial insulin by 25%–75% have proven successful for adults in preventing hypoglycemia for aerobic,107-109 mixed,108 and anaerobic108 exercise. For adult males, prandial insulin reductions made 1–2 h before exercise109, 110 limit pre-exercise hyperglycemia when compared to reductions made 2–4 h before exercise.108, 110 When extrapolating the male adult data to youths, it seems important to ascertain the time gap between the meal and activity and counsel to aim to keep it ideally within 90 min when reducing bolus insulin before exercise. To prevent gastro-intestinal distress in adult males, a low-fat carbohydrate rich meal of 1.0–1.5 g/kg/BW has proven effective and is tolerated when eaten within 2 h of starting exercise.109, 110 If the young person has a body mass index (BMI) centile ≥91st, use their ideal body weight (IBW), unless the high BMI centile is due to large muscle mass. The BMI method for calculating IBW in kg (BMI at the 50th centile for age × [height in meters]2) has been validated in pediatrics.111
When exercise is planned to begin more than 2 h after the meal, it is advisable to administer the regular meal insulin dose to prevent excessive hyperglycemia, which has been observed when reductions are made 2–4 h before exercise in adult males.108 Insulin pump basal rate reductions of 50% and 80%, reduced the risk of hypoglycemia during aerobic exercise in the absence of prandial insulin when the reductions were activated 90 min before exercise.112 However, disconnecting an insulin pump at the start of exercise generally does not prevent hypoglycemia during exercise112, 113 If the pre-exercise meal is to be consumed 2–3 h before exercise, keeping meal carbohydrate content to a maximum of 2 g/kg/BW, will prevent excessive circulating insulin at the start of exercise. Creating a gap of at least 3 h between mealtime and exercise is preferable to minimize circulating bolus insulin114 and provide ample time for carbohydrates to be digested and assimilated for use during exercise.115 If the gap is more than 3 h, a meal containing 1–3 g/kg/body weight (BW) of carbohydrate that is moderate to low in fat is recommended to improve liver and muscle glycogen stores.115 Endurance athletes with high training loads may need 4 g/kg/BW.
7.3 During the planned activity: Insulin adjustments and nutrition strategies
The mainstay of glucose management during activity is the consumption of extra carbohydrates. Research shows 0.5–1.0 g/kg/h is required in the presence of high circulating bolus insulin,101 but only 0.3–0.5 g/kg/h if more than 2 h have passed since the last prandial insulin.116, 117 The carbohydrate requirement table for people using SMBG offers starting suggestions for carbohydrates before and every 30 min during exercise (Table 4 and Appendix [Table A1] for weight banded suggestions). The suggestions are based on the exerciser's glucose level and weight and if the glucose level is expected to fall or remain steady or rise during exercise. The expectation of glucose change during exercise should be based on exercise type, bolus insulin on board, changes to basal insulin, and previous exercise experience.
In individuals with diabetes using CGM systems, the glucose trends (direction of arrows) should be considered. The BGL should be measured if sensor glucose is borderline since sensor accuracy deteriorates with exercise. CGM can permit adjustment of carbohydrate amounts based on real-time glucose levels and trend arrows. Providing smaller amounts of supplementary carbohydrates every 10–20 min based on glucose level has been shown to eliminate clinically important hypoglycemia (<3.0 mmol/L or < 54 mg/dl). Table 5 (Appendix [Table A2] for weight banded suggestions) offers starting suggestions for carbohydrates to be consumed before exercise and then every 20 min based on glucose value and trend arrows in the recent ISPAD/EASD consensus statement.10 For adequate interpretation of trend arrows in different CGM devices, it is important to understand their meaning (Table 7). To gain a deeper insight into CGM accuracy during exercise and how to mitigate issues, the reader is referred to the EASD/ISPAD consensus statement from which the summary of considerations is presented in Table 8.10
| Device | Trend arrow | Interpretation within 15 min | Conforms with generic trend arrow as used in the position statement |
|---|---|---|---|
Abbott devices Senseonics devices |
↑ | Increase >30 mg/dl (1.7 mmol/L) |
↑ |
| ↗ | Increase 15–30 mg/dl (0.8–1.7 mmol/L) |
↗ | |
| → | Increase/decrease <15 mg/dl (0.8 mmol/L) | → | |
| ↘ | Decrease 15–30 mg/dl (0.8–1.7 mmol/L) |
↘ | |
| ↓ | Decrease >30 mg/dl (1.7 mmol/L) |
↓ | |
| Dexcom devices | ↑↑ | Increase >45 mg/dl (2.5 mmol/L) |
↑ |
| ↑ | Increase 30–45 mg/dl (1.7–2.5 mmol/L) |
||
| ↗ | Increase 15–30 mg/dl (0.8–1.7 mmol/L) |
↗ | |
| → | Increase/decrease <15 mg/dl (0.8 mmol/L) | → | |
| ↘ | Decrease 15–30 mg/dl (0.8–1.7 mmol/L) |
↘ | |
| ↓ | Decrease 30–45 mg/dl (1.7–2.5 mmol/L) |
↓ | |
| ↓↓ | Decrease >45 mg/dl (2.5 mmol/L) |
||
| Medtronic devicesa | ↑↑↑ | Increase >45 mg/dl (2.5 mmol/L) |
↑ |
| ↑↑ | Increase 30–45 mg/dl (1.7–2.5 mmol/L) |
||
| ↑ | Increase 15–30 mg/dl (0.8–1.7 mmol/L) |
↗ | |
| Increase/decrease <15 mg/dl (0.8 mmol/L) | → | ||
| ↓ | Decrease 15–30 mg/dl (0.8–1.7 mmol/L) |
↘ | |
| ↓↓ | Decrease 30–45 mg/dl (1.7–2.5 mmol/L) |
↓ | |
| ↓↓↓ | Decrease >45 mg/dl (2.5 mmol/L) |
- a If Medtronic CGM system displays no trend arrow, this means that sensor glucose is stable as detailed below.
| Accuracy |
|---|
|
| Safety |
|---|
|
CGM lags by about 12 ± 11 min during prolonged aerobic exercise.118 Therefore, it is recommended that individuals confirm glucose levels by capillary glucose measurements if impending or present hypoglycemia is noted.118 Clinical trials of the benefits of CGM technology on the SMBG and exercise behaviors for adolescents with T2D are needed.
The upper limit of gastrointestinal absorption of glucose is around 1.0 g/min in adult males.102 By applying the male adult literature to youths, the carbohydrate calculations used for Tables 4 and 5 (appendices) were limited at 60 kg to prevent suggesting more glucose than can be absorbed to prevent delayed hyperglycemia. Rapidly absorbed high glycemic index products such as dextrose tablets, glucose drinks, and glucose gels will be the most effective when testing every 20 min (Table 5). Sports drinks with 8%–10% carbohydrates are effective during exercise in adolescents with T1D.119 More slowly absorbed carbohydrates such as fruit, biscuits/cookies, chocolate, and sweets will likely increase the risk of hypoglycemia during exercise and hyperglycemia afterwards if consumed every 20 min. However, if testing is less frequent, more slowly absorbed carbohydrates such as fruit, cereal bars or low-fat biscuits may prevent initial hyperglycemia. Practical nutrition recommendations with meal suggestions for before, during and after exercise can be found in Table 6. Hyperglycemia can be rectified by administering half the usual correction dose if the glucose level is above 15.0 mmol/L (270 mg/dl) with ketones less than 1.5 mmol/L.59
7.4 Immediately after planned activity: Insulin adjustments and nutrition strategies
Reductions of 50% in post-exercise prandial insulin have proven effective in preventing hypoglycemia in adult males after aerobic exercise.110 However, glucose level post-exercise remains higher after mixed exercise when compared to aerobic,107 suggesting smaller bolus reductions are needed after mixed and anaerobic exercise. In addition, in the 2 h after exercise muscle and liver glycogen replenishment and muscle protein synthesis rates are at their highest in adult males.120 Therefore, extrapolating to youth, it seems prudent to take advantage of this anabolic window by recommending balanced meals after exercise with 1–4 g/kg/BW of carbohydrates and 15–20 g of protein.107 Only endurance athletes will require 3 g/kg/BW or more of carbohydrates and IBW should be used if BMI centile ≥91st.
Completing short sprints just after the exercise finishes may help prevent hypoglycemia 120 min after exercise.67 However, the practicality of completing all-out sprints may prove challenging after exercise. Therefore, this strategy may best be reserved for when not eating in the post-exercise window, where bolus reductions will prevent hypoglycemia.
The glucose level can rise sharply immediately after exercise and there are several potential reasons why this may occur.59, 121, 122 Firstly, males undertaking exercise with many anaerobic components will build up both lactate and adrenaline in the bloodstream.108 Lactate not cleared within exercising muscles is shuttled to the liver to be converted into glucose by the Cori cycle and returned to circulation. A high level of circulating adrenaline causes insulin resistance and the liver to release stored glycogen.123, 124 Completing a cool-down for 10–15 min may lower serum lactate levels and delivering a 50% reduced correction dose of insulin is a common suggestion.59 However, cool-downs have not been tested experimentally and delivering 100% and 150% of correction insulin post-high intensity interval training were more effective than 50% and did not significantly increase rates of hypoglycemia.125 If the exerciser disconnects the insulin pump for the activity, there will be inadequate circulating insulin once the exercise stops, leading to hyperglycemia.126 One option is to bolus 50% of the missed basal rate before or during the activity. Finally, suppose the carbohydrate consumed during exercise exceeds 1.0 g/min and/or is a more slowly absorbed carbohydrates such as biscuits or chocolate. In that case, there will be a backlog of carbohydrates to be digested immediately after the exercise finishes without insulin present to cover. Consuming high glycemic options such as dextrose tablets, sports drinks, and gels in smaller amounts more frequently is the easiest way to avoid this cause of post-exercise hyperglycemia. Practical suggestions may be found in Table 6.
7.5 Overnight following planned activity: Insulin adjustments and nutrition strategies
Following exercise lasting 45 min the risk of hypoglycemia lasts for 7–11 h, which increases the risk of overnight hypoglycemia for activity performed after 4 p.m.61 Reducing background insulin by 20% for adults using MDI regimens has proven effective110 and reducing basal rates for insulin pump users by 20% for 6 h overnight mitigates hypoglycemia in youth with T1D.62 The efficacy of a 20% reduction has been corroborated in a closed-loop study where basal insulin was reduced on average 20% overnight following an exercise session.127 If reducing insulin is not desired or practical, consuming a bedtime snack of 0.4 g/kg/BW of low to medium GI carbohydrate without bolus insulin has prevented hypoglycemia in adult males.110
Additionally, a bedtime snack is only needed if the glucose level before bed is less than 10.0 mmol/L (180 mg/dl) and including 15 g of protein provided extra protection if the glucose is less than 7.0 mmol/L (126 mg/dl) in adult males.128 Smaller snacks will almost certainly be needed for younger children, especially those with overweight or obesity. These before bed snack targets should be individualized based on glucose response and habitual activity levels.
Exercise for 45 min performed at midday does not have the same hypoglycemia-inducing effect overnight and therefore does not require the same adjustments.129 This is important for school-aged children as it suggests that basal insulin dose adjustment is not required following daytime sports classes or lunchtime activities. The nutrition suggestions in Table 6 offer practical snack suggestions before bed.
7.6 Twice-daily insulin regimens
For those using twice-daily insulin regimens that combine long- and short-acting insulin, adjusting mixed doses for exercise can be problematic, and the more straightforward strategy is to consume additional carbohydrates to prevent hypoglycemia. However, twice-daily insulin regimen is not recommended. Tables 4 and 5 offer supplementary carbohydrate suggestions for before and during exercise. Preventing hypoglycemia overnight after exercise lasting 30 min or more performed after 4 pm can be achieved by consuming an additional snack before bed based on the glucose level, (Tables 3 and 6).
7.7 Unplanned exercise
Most activities for young children are unplanned, as they are sporadic in nature and usually last less than a minute.130 These activities are managed as part of the usual daily routine. Unplanned opportunistic activities such as jumping on a trampoline or playing at school break (recess) time usually last less than 15 min and rarely cause hypoglycemia. However, if these activities last longer than 15 min, rapidly absorbed carbohydrates will likely be required. Confirming this, one study of 50 young people walking on a treadmill for four intervals of 15 min found a minimal glucose drop after 15 min. However, between 15 to 30 min half of the participants experienced a drop of more than 2 mmol/L (36 mg/dl).106 Therefore, following the carbohydrate suggestions in Tables 4 and 5 for unplanned exercise lasting 20 min is recommended. These tables could also be used to manage gym lessons at school and activity camps. The suggestions should serve as a starting point that can be adapted based on experience.
The glucose-lowering effect of moderate-intensity exercise after eating has been established in a report combining four data sets (n = 120) that showed a mean glucose decrease of 4.2 mmol/L (76 mg/dl) after 45 min.65 The most powerful predictor of glucose decrease was pre-exercise glucose level: subjects with a starting glucose level higher than 10.5 mmol/L (190 mg/dl) had a median (quartiles) drop of 6.1 mmol/L (4.3, 8.9) or 110 mg/dl (78, 160) with very few episodes of hypoglycemia.65 This suggests using moderate activity to quickly treat hyperglycemia between meals may be a novel strategy worth exploring in clinical trials. In addition, for 100 youths, the implementation of the mnemonic “GAME,” Glucose time in range desired, Alert on high set accordingly, Mode of moderate-intensity activity, Exercise on high alert between meals if possible for 10–40 min depending on glucose value and trend arrow, was the strongest predictor of time in range (3.9–10.0 mmol/L or 70–180 mg/dl) 6 months after attending structured education focused on pro-active CGM management.131 A strategy like this may offer parents and children another option to improve time in range by quickly lowering between-meal hyperglycemia, provided the blood ketone level is not elevated. Using exercise in this way requires further research but holds potential for activity to improve time in range.
8 HYBRID CLOSED LOOP STRATEGIES
8.1 Single hormone (insulin-only) hybrid closed loop technology
Commercially available HCL availability varies worldwide. Each of the commercially available HCL systems has the option of activating an exercise or activity glucose target in anticipation of exercise or PA. The purpose of an “exercise target” is to increase glucose levels and maintain a higher BGL target during exercise by adjusting the insulin-delivery algorithm. Table 9 outlines some of the differences between commercially available device systems, including the various names used to describe an activity target (e.g., Temp target, Exercise activity, Ease-off) and the various glucose targets during exercise by device type.
| Device system | Sensor and pump technology | Standard glucose target | Exercise glucose target | Exercise TARGET Terminology | Additional information |
|---|---|---|---|---|---|
| MiniMed 670G/770G (Medtronic) | Guardian sensor 3 and 670G or 770G pump | 6.7 mmol/L (120 mg/dl) |
8.3 mmol/L (150 mg/dl) |
Temp target | Program for duration of time, will automatically deactivate at end |
| MiniMed 780G (Medtronic) | Guardian sensor 3 and 780G pump | 5.5 mmol/L (100 mg/dl) 6.1 mmol/L (110 mg/dl) 6.7 mmol/L (120 mg/dl) |
8.3 mmol/L (150 mg/dl) |
Temp target | Program for duration of time, will automatically deactivate at end |
| Control-IQ (Tandem) | Dexcom G6 sensor and Tandem t-slim X2 pump | 6.2–8.9 mmol/L (112–160 mg/dl) |
7.8–8.9 mmol/L (140–160 mg/dl) |
Exercise activity Up to six personal profiles can be created with personalized basal doses, I:C, and ISF ratios for use with exercise mode |
Manual start/stop – Cannot program a duration of time Exercise mode suspends insulin delivery at a higher predicted glucose than the standard mode. Overrides programmed sleep mode unless exercise mode switched off |
CamAPS FX (CamDiab)a |
Dexcom G6 sensor and Dana RS and Dana-i pump | 5.8 mmol/L (105 mg/dl) (Customizable glucose target) |
No set glucose value (Customizable) | Ease-off or Planned Ease-off | Program for duration of time, will automatically deactivate at end |
Omnipod 5 (Insulet)b |
Dexcom G6 sensor and Omnipod 5 Pod | 6.1, 6.7, 7.2, 7.8, and 8.3 mmol/L (110, 120, 130, 140, 150 mg/dl) (Customizable glucose target) |
8.3 mmol/L (150 mg/dl) |
Activity feature | Enable for 1–24 h, will automatically deactivate at end |
| DIY APS (OpenAPS, AndroidAPS, Loop) | Variety of systems | Customizable | Set target as desired (Customizable) | Temporary target, profile switch, overrides, or activity mode |
Program for duration of time or scheduled for specific time, will automatically deactivate at end |
- Abbreviations: APS, artificial pancreas system; DIY, do it yourself; I:C, insulin to carbohydrate ratio; ISF, insulin sensitivity factor.
- a CamAPS has CE-marked approval in the European Union and United Kingdom and is currently only commercially available in Europe.
- b Omnipod 5 received FDA approval and is only commercially available in the United States.
8.2 Exercise targets and pump suspension using hybrid closed loop technology
Longer duration (30+ minutes), low-to-moderate intensity aerobic exercise typically causes glucose levels to fall and increases the risk of hypoglycemia.58 The following sections describe strategies to help reduce the risk of exercise-associated hypoglycemia for youth using HCL technology.
Irrespective of the HCL system being used, exercise targets optimally should be set well in advance of aerobic exercise. Similarly, studies have shown that using a HCL system, setting an exercise target 90–120 min before aerobic exercise (40+ min) also reduces the risk of hypoglycemia.16, 132 In situations where pre-planning for exercise is not possible, there is still value in setting an exercise target closer to the activity, even if the 90- to 120-min window is missed because setting an exercise target will stop the auto-correction bolus delivery (e.g., 770G/780G) and will increase the target glucose range so less basal insulin will be delivered during the activity.
For activities that may not cause drastic decreases in glycemia, (e.g., shorter duration activities [<30 min] and/or some high-intensity anaerobic exercise), and fasted exercise, it may not be necessary to set an exercise target. However, Morrison et al.132 recently showed that using the MiniMed® HCL system, setting an exercise target (i.e., temp target) 120 min before high-intensity exercise was effective in maintaining glucose time-in-range. For exercise of longer duration in youth the Tandem Control-IQ® system was compared with a remote monitored sensor-augmented pump system during a winter ski camp, showing improved percent time within range with the HCL system.13 Further research is warranted to understand whether an exercise target is needed for various exercise intensities and durations.
Alternatively, some HCL users may choose to suspend insulin delivery (i.e., pump suspension) rather than set an exercise target to reduce the risk of hypoglycemia during aerobic exercise. For high-impact activities and certain contact sports (e.g., wrestling, martial arts, football, handball, etc.), pump suspension and/or pump disconnect may be preferred or even required. This may be a more effective strategy for shorter duration PA.133 However, it is essential to turn off the HCL system, otherwise the algorithm will consider insulin delivered. Pump suspensions longer than 90 min should be avoided if not replaced by insulin administered for example every hour by connecting the pump or using an insulin pen for this purpose.
8.3 Bolus adjustment strategies before and after exercise using hybrid closed loop technology
8.3.1 Pre-exercise
Although there is limited research assessing the timing and specific bolus insulin adjustment strategies with HCL technology around exercise, this section was developed based on the existing, published literature15 and expert opinion. Even with HCL technology, manual reductions in bolus insulin at the meal before exercise may be needed because meal bolus insulin action time may extend into the exercise session when the session is within 1–3 h of a meal. As is done for open loop CSII systems, persons using HCL systems should consider using a 25–75% bolus reduction for the meal preceding exercise. Using HCL technology, a recent study in adults by Tagougui et al.15 found that the combination of an exercise target set just before exercise, along with a 33% reduction in mealtime bolus insulin, led to less hypoglycemia range (2.0 ± 6.2% time <3.9 mmol/L) as compared to an exercise target alone (7.0 ± 12.6%) or no announced exercise target with full bolus (13.0 ± 19.0%). Therefore, for aerobic and mixed exercise soon after a meal, we recommend a starting plan of 25% bolus reduction with the meal prior to exercise (Table 2). An important consideration is that not all commercially available systems have a specific function to allow for a bolus reduction. As such, one strategy is to enter fewer carbohydrates than what is being consumed into the HCL system. Some HCL systems (e.g., Tandem Control-IQ) allow multiple/additional profiles to be added to the pump. Using this approach, individuals may consider adding another “activity” profile with a higher insulin sensitivity factor (ISF) and less aggressive carbohydrate ratio (ICR). In turn, this will allow the HCL system to suggest a lower bolus insulin amount. However, there are currently no studies assessing these specific strategies and, therefore, they should be discussed with healthcare professionals, individualized, reviewed, and used with caution.
For higher-intensity anaerobic exercise or competition settings, a starting plan may include no bolus reduction (i.e., usual bolus dose) with the meal prior to exercise. It should also be noted that if the meal before exercise is high in carbohydrate content, a bolus insulin reduction may cause glycemia to rise before the onset of exercise, which will increase automatic basal insulin delivery on most HCL systems or even prompt automatic correction boluses right before exercise with the attendant increased hypoglycemia risk. This risk can be minimized by choosing a lower carbohydrate meal, where possible, and by setting the exercise target soon after the meal so that basal insulin delivery is curtailed to some degree.
8.3.2 Post-exercise
Recommendations around bolus reductions with the meal post-exercise to reduce the risk of exercise-associated hypoglycemia are justified. As the guidance around bolus reductions post-exercise with HCL systems has not been well researched to date, thus suggestions in this section are based on expert opinion. The starting plan (see Table 2) for post-exercise meal insulin is a 25% bolus reduction, irrespective of the type of exercise.
8.4 Carbohydrate needs before and during exercise using hybrid closed loop technology
There are a few important differences to guidance for carbohydrate intake for exercise for those on HCL systems. First, the timing of pre-exercise carbohydrate intake needs to be considered. Carbohydrate intake well before exercise (i.e., 20 min or more) tends to promote a rise in glycemia and subsequent increased insulin delivery by the HCL system. This may cause hypoglycemia during the activity. Second, the amount of carbohydrate consumed may need to be less than typical in settings where exercise mode has been activated well in advance of the activity and/or a pre-exercise bolus reduction has been made. The use of CGM systems informs decisions about carbohydrate intake to limit hypoglycemia during various forms of exercise based on the glucose concentration and directional trend arrows of the CGM.10
8.4.1 Pre-exercise
Although consuming uncovered snacks 30 min before exercise can reduce hypoglycemia for males on MDI,134 for HCL technology, the rise in sensor glucose levels associated with the uncovered snack will likely lead to a subsequent rise in automated insulin delivery and therefore increase the risk of hypoglycemia during the activity. The current consensus is that pre-exercise carbohydrate intake should be limited to within 5–10 min before the onset of exercise or if the individual develops hypoglycemia prior to the exercise session. In situations when carbohydrate intake is necessary in the 1–2 h before exercise, an insulin bolus reduced by approximately 25% should be given (see above) and then the HCLsystem should be placed into “activity mode”.
8.4.2 During exercise
Individuals should use their CGM glucose and trend arrows (where applicable) to make decisions about carbohydrate intake needs to prevent hypoglycemia during exercise10 (Table 5). During exercise, ingesting carbohydrates in smaller amounts may also reduce the likelihood of rebound hyperglycemia post-exercise. Additional strategies to reduce hypoglycemia include exercising with little-to-no bolus insulin in the circulation, if possible, or consider delaying exercise until the post-absorptive state (i.e., three or more hours after a meal with bolus insulin) to allow for prandial insulin levels to drop before exercise by placing the closed loop system into exercise mode. If hypoglycemia develops during exercise, individuals on closed loop systems may require less carbohydrate intake as a treatment (e.g., 10 g); however, this is also highly individualized based on the size of the individual and the amount of circulating insulin and counter-regulatory hormones.
8.5 Post-exercise hyperglycemia
In most cases, HCL systems appear to manage mild post-exercise hyperglycemia well, particularly if the system is placed back into the standard (i.e., not activity) closed loop automated mode. In some cases, a small corrective insulin bolus (e.g., 50% of the usual correction dose) may be required in settings of extreme post-exercise hyperglycemia (i.e., >15.0 mmol/L, 270 mg/dl).
8.6 Planned versus unplanned activity
Healthcare professionals should discuss various options of using HCL systems to prepare for exercise or PA based on the person's lifestyle and goals. For example, some youth may find pre-planning for exercise preferable whereas others may find pre-planning difficult and, therefore, choose alternate options for exercise. In the following section, we discuss the various HCL options for planned versus unplanned exercise to reduce the risk of exercise-associated dysglycemia.
8.7 Planned exercise with hybrid closed loop technology
Based on limited clinical research on HCL strategies around exercise and expert consensus, the following options should be considered in situations where individuals have time to prepare for exercise:
| Bolus reduction before exercise |
|
| Exercise target before exercise |
|
| Bolus reduction and exercise target before exercise |
|
| Lower IOB before exercise onset |
|
| Pump suspension or disconnect |
|
8.8 Unplanned exercise with hybrid closed loop technology
For situations where individuals do not have time to prepare for exercise, the following options may be considered:
| Carbohydrate feeding before exercise |
|
| Carbohydrate feeding during exercise |
|
| Bolus reduction after exercise |
|
| Lower IOB before exercise onset |
|
| Pump suspension or disconnect |
|
8.9 Special considerations
In this section, particularly in situations where the above recommendations do not seem appropriate or effective, we highlight some special considerations and tricks around exercise. In addition, this section also aims to address some unique differences between HCL systems around exercise.
| Switch to manual mode or open loop CSII to prepare for exercise |
|
| Pump suspension or disconnect |
|
| Tandem Control-IQ tricks for exercise |
|
| CamAPS tricks for exercise |
|
9 SPECIFICS FOR YOUTH WITH TYPE 1 DIABETES
9.1 Glycemia and exercise performance
Among young people, it has been shown that only a few carry out planned adaptations before and during PA, which calls for the need for training and motivational talks.135 Exercise-related acute hypoglycemia avoidance is an important goal for safety in youth with T1D; additionally, hypoglycemia impairs performance and may increase rate of perceived exertion. It remains uncertain, however, whether and to what degree acute hyperglycemia impairs exercise capacity. A recent study7 of recreationally active adolescents and young adults with T1D comparing euglycemia with hyperglycemia in both normal and hypoinsulinemic states found that VO2peak was only marginally lower when participants were clamped at 17.0 mmol/L (306 mg/dl) and peak sprint cycling power was, in fact, slightly higher. Reaction time was marginally impacted by hyperglycemia in the hypo-insulinemic state, but no other differences were found. Fuel utilization, VO2 kinetics and other markers were not evaluated in this study. In adults,136 with T1D, mild hyperglycemia (12.4 mmol/L; 223 mg/dl) did not impact exercise capacity or perceived exertion or carbohydrate oxidation.
Elevated HbA1c level is associated with impaired exercise capacity in adults with T1D,137 but tight glycemia is associated with exercise capacity on par with those without T1D. Pulmonary, cardiac, and vascular responses to exercise are impaired in people with sub-optimally controlled T1D, and chronic hyperglycemia in animal models attenuates beneficial effects of exercise training138 with impaired aerobic remodeling of skeletal muscle. Thus, achieving long term target glycemic control is likely required for optimal cardiovascular fitness and exercise performance.
9.2 Competition day
Acute hyperglycemia is commonly reported by youth with T1D around exercise or activities associated with competition, even when usually associated with euglycemia or hypoglycemia under training or low stress non-competitive conditions. An elevated adrenergic state likely contributes to increased hepatic glucose output and, possibly, insulin resistance. Given the paucity of clinical trials addressing this situation, a practical approach is favored emphasizing increased time to prepare for the planned competition, early glucose monitoring to detect emerging stress hyperglycemia and reducing the possibility of over-fueling prior to competition.
For those on insulin pump therapy, a temporary increase in basal insulin delivery can be set at the predicted (or observed) onset of hyperglycemia; however, it is important to reduce the rate back to baseline or below shortly before competition onset to avoid hypoglycemia resulting from resolution of the adrenergic state during or shortly after activity competition. For those using a HCL system, delaying the use of the exercise mode may reduce the risk of stress-related hyperglycemia, by allowing for increased basal insulin delivery and/or continuation of automatic correction doses.
Practicing a pre-match or pre-race routine may be beneficial for those who frequently experience competition-associated hyperglycemia. This may include performing a low intensity aerobic warmup (walk or light jog) to reduce counter-regulatory hormones and facilitate glucose uptake, or other mental preparedness strategies. Data are scarce on the effectiveness of these strategies. Acute excitement or stress-mediated hyperglycemia will likely settle quickly with the activity itself. The risk of delayed, or post-exercise hypoglycemia likely increases with aggressive correction of pre-competition excitement or nervousness-related hyperglycemia.
9.3 Prolonged pump disconnection
Prolonged pump disconnection is sometimes desirable. Sports performed in water (swimming, diving) or on water (sailing) are reasons to disconnect some devices. Likewise, devices should be disconnected for some contact sports (e.g., wrestling, handball, ice hockey, American/Australian football). Sometimes the rationale for disconnecting the pump is to reduce the risk of hypoglycemia. For youth with T1D using insulin pump therapy, stopping the basal insulin infusion (i.e., pump suspension/disconnection) at the start of moderate aerobic exercise (around 60 min duration) in the late afternoon may reduce the risk of hypoglycemia during the exercise period.139 However, pump suspension may not be as effective as reducing basal insulin112 (or setting a higher exercise target) 90–120 min in advance of exercise. Although generally uncommon,140 some concerns around prolonged pump suspension (>120 min) especially in younger children (4–9 years of age),141 include the potential increase in blood ketone levels and the possibility of forgetting to resume insulin delivery post-exercise. If disconnection is used for more than 90 min, different strategies can be used to avoid insulin deficiency: reattach pump every 60 min and administer a bolus corresponding to approximately 50% of the standard insulin administration per hour or use a hybrid regimen of injected insulin described below.
9.4 Environmental considerations: open water swimming/surfing/sailing, ambient temperature, high altitude, and scuba diving
9.4.1 Open water swimming/surfing/sailing
Open water swimming, surfing, and sailing expose the body to both cold temperature (see below) and water. Prolonged pump disconnection may be required (see above) and/or insulin pump treatment combined with insulin pen treatment and selected insulin type to adapt to the length of time the pump is disconnected. A hybrid regimen of injected insulin degludec and insulin pump therapy (disconnected during exercise) has been shown to be safe as well as effective in adults.142 The same approach with a combination of insulin pump treatment and injected insulin glargine in children also showed that this strategy is feasible and might reduce the risk of hyperglycemia and ketoacidosis during prolonged pump suspension.143
9.4.2 Ambient temperature
High ambient temperature tends to increase the insulin absorption rate and low ambient temperature has the opposite effect.144 The latter could have an impact during open water swimming (mentioned above), using a wetsuit can protect against the cold. High ambient temperature might also result in stress, resulting in greater energy expenditure, thus increasing the risk of rapidly decreasing glucose levels.
The accuracy of blood glucose meters can be affected by several factors, including temperature and altitude (see below), and it is recommended to acquire knowledge of which limit values apply to the meter of use. Moreover, high temperature might result in dehydration which also may affect the accuracy of CGM devices. Therefore, hydration is of utmost importance as severe dehydration may cause inaccurate sensor glucose readings.
Conversely, low temperatures also may reduce measurement accuracy or cause no glucose value to be obtained. This situation is quite typical for blood glucose monitors kept at temperatures below 0 degrees Celsius (32 degrees Fahrenheit). Thus, during PA in such circumstances CGM is a better option.145
9.4.3 High altitude
Downhill skiing or rock climbing are examples of exercises at high altitude. High altitude-induced anorexia and increased energy expenditure might cause dysglycemia and hypoxia may cause erroneous decisions. Exercise and stress during these conditions also affects the counter-regulatory hormonal response. Thus, optimal glycemia becomes essential. As blood glucose meters may be inaccurate at high altitude; therefore, CGM could be recommended for combined use. Additional information about exercise in high altitude conditions can be found in a review.146
9.4.4 Scuba diving
Formal guidelines on diving in people with insulin-treated diabetes was published in the early 1990 s. Subsequent consensus was then created following a workshop in 2005.147
Diving with concomitant insulin-treated diabetes is now approved with certain reservations in most countries around the world.148, 149 However, careful and periodic evaluation is still required to ensure that participation in diving activities is appropriate. In connection with diving, it is therefore important to have careful self-monitoring, well-thought-out adjustments to insulin doses and carbohydrate intake before each diving occasion.
Glucose levels should be checked at 60, 30, and 10 min before a dive and immediately after a dive. During this period, stable glycemia without falling values or trends is sought, and levels in the safe zone of 8.3 mmol/L (150 mg/dl) as a minimum before dive.150
Applicable to youth, programs are available to allow scuba diving at shallower depth limits, but in combination with diabetes other aspects must also be considered in addition to age. The individual who starts diving should generally be fit-to-dive but also have a suitable personality and well-controlled glycemia. Regarding youth, this also means that the individual must have the ability to make the right decision in urgent situations, including an ability to assess the consequences of decisions. With this as a basis, a Junior Open Water Diver Certificate can only be recommended in rare cases in youth with T1D, whereas the limiting factor in T2D in the same age group possibly is being fit-to-dive.
10 CONTRAINDICATIONS TO EXERCISE AND SPORTS
T1D should not be a contraindication to participation in physical education and sport participation at each level of education, in training, and in competitions. The optimal target range for BGL before exercise is between 90 and 270 mg/dl (5.0–15 mmol/L). In persons with diabetes using CGM systems, the glucose trends should be considered. The BGL should be measured if sensor glucose is borderline since sensor accuracy deteriorates with exercise. Persons with diabetes with BGL in the optimal range can usually proceed safely with exercise, carbohydrate intake, and insulin dosing adjustments.
10.1 Temporary contraindications to exercise
- Episode of severe hypoglycemia within the previous 24 h (hypoglycemia associated with severe cognitive impairment requiring external assistance for recovery). Antecedent severe hypoglycemia impairs the hormonal counter-regulatory response during exercise, thus increasing the risk for recurrent hypoglycemia.151
- Hyperglycemia ≥270 mg/dl (15.0 mmol/L) with concomitant ketonemia/ketonuria due to insulin deficiency and not carbohydrate excess. Ketonemia ≥1.5 mmol/L is an absolute contraindication to initiation and continuation of physical exercise. In the case of ketonemia 1.0–1.4 mmol/L (urine ketones ++) exercise should be postponed until ketone levels normalize after administration of an insulin correction bolus.
- Injury and acute infection. They may precipitate hyperglycemia in persons with diabetes because they tend to increase catecholamine and cortisol responses.
In addition to temporary contraindications to exercise, contraindications to competitive sport should be considered. Persons with diabetes with significantly unstable diabetes, frequent severe acute diabetic complications and advanced chronic complications of the disease should not participate in competitive sports until the disorder is stabilized.
11 SCHOOLS AND CAMPS
Schools frequently provide opportunities for physical activity for many youth. The school environment has the potential to encourage physical activity in youth through physical education lessons, extracurricular activities (structured physical activity), and recess or lunchtime (discretionary physical activity). Students with diabetes should fully participate in physical education classes and other physical activities at school provided they do have any contraindications to exercise.
Physical education lessons and other active parts of the school day may be associated with glycemic disturbances. Good communication and cooperation between the student, their health care provider, parents, school nurse, physical education instructor or team coach, and goal setting that includes a well-designed regimen of glucose measurements, insulin adjustments and nutrition during and after exercise are essential. Therefore, education about diabetes is essential and virtual courses in diabetes are available (e.g., in Australia T1D Learning Centre - Courses).
For physical education lessons, a diabetes care plan should be developed, including detailed instructions for the students and their teachers and coaches. The main goal is to avoid hypoglycemia during and after exercise. For most physical activities at school, the guidelines are similar to those presented above.
Dedicated camps for children with T1D provide an excellent opportunity to learn additional skills to manage physical activity. Counseling on nutrition and insulin adjustment for exercise can lower HbA1c levels.152 Children gain experience, which they can also share with others with diabetes. Furthermore, health care professionals can also benefit from such experiences.153
12 EXERCISE IN CHILDREN WITH DIABETES ON INSULIN LIVING IN LIMITED CARE SETTINGS
Although intensive insulin regimen (MDI and CSII) is strongly recommended for the treatment of youth with T1D, substantial numbers of youth with T1D still use conventional insulin regimens.154-156
In many low-income countries, glucose test strips are not covered by universal health coverage. Even optimal SMBG (at least four times/day), is not possible due to the costs.157 Even when blood ketone testing is available, the cost is high and not widely used by many persons with diabetes. For children using conventional insulin regimens together with limited SMBG, maintaining normoglycemia during exercise is challenging.
12.1 Conventional insulin regimen
In conventional regimens, a combination of NPH and regular insulin or rapid-acting insulin analog are administered at breakfast and dinner time, or premixed insulin is administered twice daily. However, this type of regimen is not recommended.
When exercise occurs after meal, the dose of premixed insulin should be reduced by approximately 20%–50%158 in order to reduce the risk of hypoglycemia during exercise, although hyperglycemia might occur later during the day because the amount of intermediate-acting insulin is decreased concomitantly.
If exercise occurs within 2–3 h after insulin injection and is planned, the dosage of rapid-acting insulin or regular insulin can be reduced. If the exercise will occur around the peak of NPH action (e.g., at noon) or exercise will last for hours, then the dose of NPH should be reduced. However, in many circumstances, even with the reduced dosage of insulin, individuals might still require extra carbohydrate intake during exercise. If exercise is unplanned, carbohydrate intake prior to and during exercise is recommended.
13 TYPE 2 DIABETES AND EXERCISE
Much of the above part of the guidelines applies also to T2D and this section gives a few additional considerations to care of youth with T2D. Comorbidities are described in ISPAD 2022 Consensus guidelines Chapter 3 on Type 2 diabetes in children and adolescents.
13.1 Physical activity improves cardiovascular health for adolescents with T2D
Daily PA is a cornerstone for preventing cardiometabolic complications associated with T2D and a clinical target in national and international guidelines for diabetes care.1, 159, 160 Systematic reviews reveal robust dose–response associations between PA and several cardiometabolic health outcomes in healthy weight and obese youth.161-163 These associations were replicated in experimental studies among adolescents living with obesity.164-167 Importantly, the cardiometabolic health benefits associated with regular moderate to vigorous PA are evident in adolescents living with various forms of chronic disease.168, 169 There is a significantly smaller body of research on the role of PA for cardiometabolic health for adolescents with T2D.
Only three studies to date have examined the association between PA17, 77, 170 and cardiometabolic health outcomes in adolescents with T2D, and all of them are cross-sectional. The largest study (n = 588) relied on surveys delivered during clinic visits and found that adolescents with T2D who report being active on three or more days per week display lower HbA1c levels and higher high density lipoprotein cholesterol, compared to less active adolescents.77 A recent observational study from Canada found that that physically active adolescents with T2D were 40% less likely to have albuminuria (aOR: 0.60; 95% CI: 0.19, 0.84) and 50% less likely to have HbA1clevels above 8.0% (>60 mmol/mol; aOR: 0.50; 95% CI: 0.26, 0.98).17 Adolescents with T2D who engaged in regular vigorous intensity activity also observed a trend towards lower odds of nocturnal hypertension (aOR: 0.54; 95% CI: 0.27, 1.07). Collectively, these observations provide some evidence that regular PA is associated with better cardiometabolic health in adolescents with T2D. RCTs, however, are needed to confirm these observations.
13.2 Psychosocial factors are common and impede behavior change among adolescents with T2D
For many adolescents with T2D, implementing healthy lifestyle behaviors, including daily PA, is challenging.171, 172 This is due, in part, to exposure to psychosocial factors including adverse childhood experiences, poverty173, 174 and mental health disorders.175-180 Mental health disorders are common among adolescents with T2D,181, 182 reducing quality of life and readiness to adopt regular daily PA.46 For example, the odds of being ready to adopt new health behaviors (including daily PA) are ~14% lower for every unit increase in anxiety, depression, and emotional distress among adolescents with T2D.46 In contrast, adolescents with T2D who reported having more resilient characteristics, particularly a connection to others and a sense of mastery over their lives, were 5%–10% more likely to be in the action and maintenance stage of change.46 There is an urgent need to develop behavioral lifestyle interventions that specifically address these stressors and support adolescents with T2D to increase their daily PA.
13.3 Conventional approaches to behavior change are ineffective for adolescents living with T2D
Changing behaviors among adolescents at risk for or living with T2D is challenging, and the optimal approach for increasing PA among adolescents with T2D remains uncertain. Recent systematic reviews183-185 suggest that the efficacy of conventional behavioral lifestyle interventions for adolescents living with obesity is modest and rarely sustained. The modest effects may be related to the observation that ~80% of the RCTs of behavioral lifestyle interventions offered only 30 min of support weekly, and only 2 of 35 interventions addressed psychosocial factors.186, 187 The Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study was the only therapeutic trial that compared a behavioral lifestyle intervention that included increasing daily PA to standard of care for adolescents living with T2D.188 This 2-year long intensive lifestyle intervention was grounded in the tenets of cognitive behavioral therapy (CBT) and provided extensive support for adolescents with T2D to lose weight and increase PA.188 Despite rigorous efforts by the behavioral team, the intensive lifestyle intervention was not successful in maintaining target HbA1c levels (<8% [<60 mmol/mol])48 or lifestyle behaviors.189 Failure to address psychosocial factors was identified as a possible explanation for the poor efficacy of this approach.189 RCTs are needed to determine the optimal approach to support adoption and maintenance of regular daily PA for adolescents living with T2D.
ACKNOWLEDGEMENTS
We are grateful for excellent revision including valuable comments from Jane Yardley, and guidance provided by Leena Priyambada.
CONFLICT OF INTEREST
Peter Adolfsson has received speaker's honoraria from Dexcom, Eli Lilly, Insulet, Novo Nordisk, Sanofi, and Tandem in the last 24 months. He has had consulting fees and/or advisory positions with Dexcom, Eli Lilly, Medtronic, Novo Nordisk, and Roche. Craig E. Taplin has received honoraria from Medtronic Diabetes Australia, Insulet Australia and Eli Lilly Australia. Dessi P. Zaharieva has received speaker's honoraria from Medtronic Diabetes, Ascensia Diabetes, and Insulet Canada; and research support from the Helmsley Charitable Trust and ISPAD-JDRF Research Fellowship. She is also on the Dexcom Advisory board. John Pemberton has worked for Medtronic from 2011 to 2016 and has attended two SIGMA CGM educational events sponsored by Dexcom in 2017 and 2019. Elizabeth A. Davis has received honoraria from Eli Lilly Australia in the last 24 months. Michael C. Riddell has received speaker's honoraria from Novo Nordisk, Eli Lilly, Dexcom and Roche in the last 24 months. He has had consulting fees and/or advisory positions with Zealand Pharm, Zucara Therapeutics, Eli Lilly, and Indigo Diabetes. Jonathan McGavock: none. Othmar Moser has received speaker's honoraria from Medtronic, Sanofi, Novo Nordisk and TAD Pharma. Research funding/support: Novo Nordisk, Sanofi, Abbott, Medtronic, Dexcom, Maisels, Horizon 2020, EFSD. Agnieszka Szadkowska has received speaker's honoraria from Medtronic Diabetes, Ascensia Diabetes, Abbott, Dexcom, Roche Diabetes, NovoNordisk, Eli Lilly, Sanofi, has been a member of advisory board for Medtronic Diabetes, Ascensia Diabetes, Abbott, Dexcom, Roche Diabetes, NovoNordisk, Eli Lilly, and has received a research funding from Roche Diabetes. Prudence Lopez: none. Jeerunda Santiprabhob has received speaker's honoraria from Sanofi, Novo Nordisk, and Ferring and has been a member of Thailand advisory board of Liraglutide and Norditropin (Novo Nordisk). Elena Frattolin: none. Gavin Griffiths: none.
APPENDIX A
| Sensor or blood glucose level | Expected glucose response during exercise based on the type of exercise, insulin on board and bolus adjustments, basal adjustments, and previous response to exercise (Carbohydrate per 30 min by body weight in kilogramsa) | |||||
|---|---|---|---|---|---|---|
| Expected to fall during exercise | Expected to stay stable or rise during exercise | |||||
Higher than 15.0 mmol/L (270 mg/dl) and ketones more than 0.6 mmol/L |
Ketones >1.5 mmol/L: Follow usual ketone advice and avoid exercise Ketones 1.1–1.4 mmol/L: Give ½ correction dose by pen and wait 60 min to reassess Ketones 0.6–1.0 mmol/L: Give ½ correction dose by pen and wait 15 min to exercise |
|||||
Higher than 15.0 mmol/L (270 mg/dl) and ketones less than 0.6 mmol/L |
Consider ½ of usual bolus insulin correction | |||||
10.1–15.0 mmol/L (181–270 mg/dl) |
No carbohydrate | |||||
| Weight (kg)b | 10–30 kg | 30–50 kg | >50 kg | 10–30 kg | 30–50 kg | >50 kg |
Exercise targeta 7.0–10.0 mmol/L (126–180 mg/dl) |
2–12 g117 | 6–25 g117 | 12–24 g117 | 0 g | 0 g | 0 g |
5.0–6.9 mmol/L (90–125 mg/dl) |
5–15 g101 | 15–25 g101 | 30 g101 | 2–6 g116 | 6–10 g116 | 12 g116 |
Delay or stop exercise for 20 min 4.0–4.9 mmol/L (70–89 mg/dl) |
3–9 g190 | 9–15 g190 | 18 g190 | 3–9 g190 | 9–18 g190 | 18 g190 |
3.0–3.9 mmol/L (54–70 mg/dl) |
Treat hypoglycemia and delay exercise until greater than 4.9 mmol/L (89 mg/dl) | |||||
Less than 3.0 mmol/L (54 mg/dl) |
Treat hypoglycemia and do not start exercise due to impaired counter-regulatory hormone response | |||||
- a If risk hypoglycemia or hypoglycemia unawareness is medium or high, increase exercise target level to 8.0–11.0 mmol/L (145–198 mg/dl) or 9.0–12.0 mmol/L (162–216 mg/dl), respectively.
- b If body mass index (BMI) percentile is ≥91st then use body weight (BW) in kg = (BMI at the 50th percentile for age × [height in meter]2),111 unless the high BMI percentile is due to large muscle mass. Adult male data.102-104, 191 Adult male and female data.116, 117 Pediatric male data.101 Pediatric male and female data.111, 190
| Sensor or blood glucose level | Trend arrow | Expected glucose response during exercise based on the type of exercise, insulin on board and bolus adjustments, basal adjustments, and previous glucose control (If checking frequency more than 20 min, select the carbohydrate amount based on a stable trend arrow and adjust according to checking frequency) | |||||
|---|---|---|---|---|---|---|---|
| Expected to fall during exercise | Expected to stay stable or rise during exercise | ||||||
Higher than 15.0 mmol/L (270 mg/dl) and ketones more than 0.6 mmol/L |
All | Ketones >1.5 mmol/L: Follow usual ketone advice and avoid exercise Ketones 1.1–1.4 mmol/L: Give ½ correction dose by pen and wait 60 min to reassess Ketones 0.6–1.0 mmol/L: Give ½ correction dose by pen and wait 15 min to exercise |
|||||
Higher than 15.0 mmol/L (270 mg/dl) and ketones less than 0.6 mmol/L |
→↗↑ | Consider ½ of usual bolus insulin correction | |||||
| ↘↓ | No carbohydrate | ||||||
| Weight (kg)b | 10–30 kg | 30–50 kg | >50 kg | 10–30 kg | 30–50 kg | >50 kg | |
10.1–15.0 mmol/L (181–270 mg/dl) |
↑ | ||||||
| ↗ | |||||||
| → | |||||||
| ↘ | 1–3 g | 3–5 g | 6 g | ||||
| ↓ | 2–6 g | 6–10 g | 12 g | ||||
Exercise targeta 7.0–10.0 mmol/L (126–180 mg/dl) |
↑ | ||||||
| ↗ | 1–3 g | 3–5 g | 6 g | ||||
| → | 2–6 g | 6–10 g | 12 g | ||||
| ↘ | 3–9 g | 9–15 g | 18 g | 2–6 g | 6–10 g | 12 g | |
| ↓ | 4–12 g | 12–20 g | 24 g | 3–9 g | 9–15 g | 18 g | |
5.0–6.9 mmol/L (90–125 mg/dl) |
↑ | 1–3 g | 3–5 g | 6 g | |||
| ↗ | 2–6 g | 6–10 g | 12 g | 1–3 g | 3–5 g | 6 g | |
| → | 3–9 g | 9–15 g | 18 g | 2–6 g | 6–10 g | 18 g | |
| ↘ | 4–12 g | 12–20 g | 24 g | 3–9 g | 12–20 g | 18 g | |
| ↓c | 5–15 g | 15–25 g | 30 g | 4–12 g | 12–20 g | 24 g | |
4.0–4.9 mmol/L (70–89 mg/dl) |
↑ |
2–6 g | 6–10 g | 12 g | 1–3 g |
3–5 g |
6 g |
| ↗ | 3–9 g | 9–15 g | 18 g | 2–6 g | 6–10 g | 18 g | |
Delay or stop exercise 20 min 4.0–4.9 mmol/L (70–89 mg/dl) |
→ |
3–9 g | 9–15 g | 18 g | 3–9 g | 9–15 g | 18 g |
↘c |
4–12 g | 12–20 g | 24 g | 4–12 g | 12–20 g | 24 g | |
| ↓c | 5–15 g | 15–25 g | 30 g | 5–15 g | 15–25 g | 30 g | |
3.0–3.9 mmol/L (54–70 mg/dl) |
All Arrows | Treat hypoglycemia and delay exercise until greater than 4.9 mmol/L (89 mg/dl) | |||||
Less than 3.0 mmol/L (54 mg/dl) |
All Arrows | Treat hypoglycemia and do not start exercise due to impaired counter-regulatory hormone response | |||||
- a If risk hypoglycemia or hypoglycemia unawareness is medium or high, increase exercise target level to 8.0–11.0 mmol/L (145–198 mg/dl) or 9.0–12.0 mmol/L (162–216 mg/dl) respectively.
- b If body mass index (BMI) percentile is ≥91st then use the body weight (BW) in kg = (BMI at the 50th percentile for age × [height in meter]2),111 unless the high BMI percentile is due to large muscle mass.
- c Consider blood glucose test as CGM value maybe lagging. Pediatric male and female data.111
Open Research
DATA AVAILABILITY STATEMENT
All authors conceived the guideline and discussed the content and the structure of the manuscript. All authors contributed to the manuscript and critically read and revised the manuscript including tables and figures.




