Clinical presentation and long-term outcome of patients with KCNJ11/ABCC8 variants: Neonatal diabetes or MODY in the DPV registry from Germany and Austria
Funding information: EU-IMI2-projects SOPHIA and INNODIA; German Diabetes Association (DDG); Robert Koch Institute; German Ministry of Education and Research (BMBF) within the German Center for Diabetes Research (DZD)
Abstract
Objective
To describe clinical presentation/longterm outcomes of patients with ABCC8/KCNJ11 variants in a large cohort of patients with diabetes.
Research Design and Methods
We analyzed patients in the Diabetes Prospective Follow-up (DPV) registry with diabetes and pathogenic variants in the ABCC8/KCNJ11 genes. For patients with available data at three specific time-points—classification as K+-channel variant, 2-year follow-up and most recent visit—the longitudinal course was evaluated in addition to the cross-sectional examination.
Results
We identified 93 cases with ABCC8 (n = 54)/KCNJ11 (n = 39) variants, 63 of them with neonatal diabetes. For 22 patients, follow-up data were available. Of these, 19 were treated with insulin at diagnosis, and the majority of patients was switched to sulfonylurea thereafter. However, insulin was still administered in six patients at the most recent visit. Patients were in good metabolic control with a median (IQR) A1c level of 6.0% (5.5–6.7), that is, 42.1 (36.6–49.7) mmol/mol after 2 years and 6.7% (6.0–8.0), that is, 49.7 (42.1–63.9) mmol/mol at the most recent visit. Five patients were temporarily without medication for a median (IQR) time of 4.0 (3.5–4.4) years, while two other patients continue to be off medication at the last follow-up.
Conclusions
ABCC8/KCNJ11 variants should be suspected in children diagnosed with diabetes below the age of 6 months, as a high percentage can be switched from insulin to oral antidiabetic drugs. Thirty patients with diabetes due to pathogenic variants of ABCC8 or KCNJ11 were diagnosed beyond the neonatal period. Patients maintain good metabolic control even after a diabetes duration of up to 11 years.
1 INTRODUCTION
Pathogenic variants in the ABCC8 and KCNJ11 genes are a common cause of neonatal diabetes, particularly in families without consanguinity.1 However, they can also cause diabetes onset later in life, classified as Mature Onset Diabetes of the Young (MODY) 12 in patients with ABCC8 variants and MODY 13 in patients with KCNJ11 variants. Activating variants of the genes ABCC8 and KCNJ11 encoding for the subunit Kir6.2 and the sulfonylurea receptor SUR1 of the ATP-dependent potassium channel of the beta-cell lead to transient or persistent neonatal diabetes. Affected patients can often be successfully switched to sulfonylurea therapy.2-6 In patients with ABCC8/KCNJ11 variants, neurological sequelae such as DEND (developmental delay, epilepsy, and neonatal diabetes mellitus) syndrome may occur,7 thus neurological development must be observed. In particular with the ABCC8 variant, transient diabetes courses are possible, which then often relapse after a few years.
From the “Diabetes Prospective Follow-up” (DPV) registry, a report on neonatal diabetes was published 12 years ago,8 with the description of 90 cases with neonatal diabetes. Of these patients, an ABCC8 variant was reported in 7 patients and a KCNJ11 variant in 10 patients. With a steady improvement of molecular genetic diagnostics and increasing knowledge about monogenic diabetes, the number of cases documented in DPV has increased significantly, and extensive follow-up data are now available.
ABCC8 and KCNJ11 variants as causes of diabetes beyond the neonatal period, classified as “MODY 12” and “MODY 13”, are hardly reported in the literature.
The aim of this paper was to better characterize the clinical presentation and outcome in a cohort of children and adults diagnosed with KCNJ11/ABCC8 related diabetes identified through the large DPV registry. We focused on differences between patients with neonatal and later onset, and between the two genes, in addition to long-term follow-up, comorbidities and therapy.
2 RESEARCH DESIGN AND METHODS
2.1 Study design and participants
This observational study was based on data from the DPV Diabetes Registry. In the DPV registry, anonymous, standardized, prospective data from routine diabetes care are transmitted by diabetes centers from Germany, Austria, Switzerland, and Luxembourg for central validation and analysis twice a year. For optimal data validity, inconsistent data are reported back to participating centers, corrected if necessary, and re-entered into the database as previously described.9 For this analysis, the data set of September 2021was used. Fifty six centers contributed data to this analysis. Analysis of anonymized data within the DPV initiative was approved by the Ethics Committee of the Medical Faculty of the University of Ulm, Germany, and the institutional review boards at the participating centers.
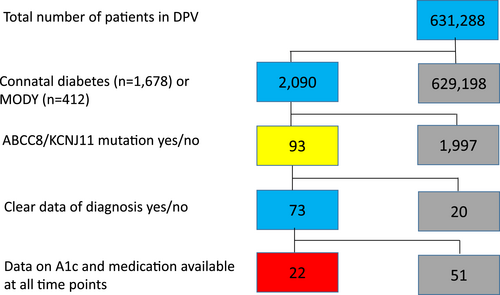
We included only patients with ABCC8/KCNJ11 variants. For longitudinal analyses, a specific date of diagnosis of diabetes and data on medication and hemoglobin A1c (A1c) values for the classification as ABCC8/KCNJ11 variant, a follow-up after 2 years (visit in the period from 1.5 until 2.5 years after classification as ABCC8/KCNJ11 variant) and the most recent visit (without overlap to the first follow-up period) were required. Twenty two patients fulfilled the criterion of complete documentation at the three given time points and were included in the analysis of follow-up.
2.2 Patient data
A1c was used as an indicator of glycemic control. Levels were mathematically standardized to the Diabetes Control and Complications Trial (DCCT) reference range of 4.05%–6.05% with the MOM (multiple of mean) transformation to correct for different laboratory methods used by study centers.10 Anthropometric measurements were performed in the local centers according to inhouse protocols and analyzed using contemporary national reference data for height and weight.11 The BMI (body mass index: weight in kilograms/[height in meters]2), is an accepted measure of overweight and obesity in children, adolescents and young adults. BMI-SDS (standard deviation score) values were generated using the LMS method.12, 13 Sulfonylurea dosage was defined as maximum dosage within the last documented year.
2.3 Statistical analysis
Data were analyzed using SAS 9.4 (TS1M7, SAS Institute, Cary, NC). If data for a specific variable were not available in individual cases, the case was not considered for the analysis of that variable. Descriptive analyses with median and interquartile ranges (IQR) for continuous variables and numbers (proportions) for binary variables are presented for age, current anthropometric characteristics, A1C and treatment (insulin dosage, oral antidiabetic drugs). The number of patients with neonatal diabetes and ABCC8/KCNJ11 variants/100,000 births was calculated using the rate of live births per year since 2010 in Germany and Austria.14, 15 Data were summarized for both countries and for 2 years, respectively, from 2010 to June 2021. All documented patients in the DPV registry with neonatal diabetes and ABCC8/KCNJ11 variants were also summarized for 2 years regarding their year of birth (not the year in which the ABCC8/KCNJ11 variants were diagnosed or documented). Afterwards, for all 2-year-intervals, the respective number of patients with neonatal diabetes (and with ABCC8/KCNJ11 variants) was divided through the number of live births and multiplied by 100,000.
3 RESULTS
We identified 93 patients with a pathogenic variant in the genes ABCC8 (n = 54) or KCNJ11 (n = 39). For 22 of them, the prespecified set of follow-up data were available (Figure 1). For 71 patients, long-term follow up data were not available in the prespecified form and they were analyzed in the cross-sectional analysis, but not in the longitudinal analysis. There were no relevant significant differences between the patient groups with and without follow-up data, except for age and time of classification as K+-channel variant. Due to our criteria for the follow-up study, follow-up data were naturally more available for older patients with diabetes onset in earlier years and therefore with longer documentation time. Sixty three patients of our cohort were classified as “neonatal” diabetes with diabetes onset below the age of 6 months and 30 as “MODY” diabetes with later diabetes onset. Because, in principle, patients with diabetes onset between 6 and 12 months of age could also be classified as having neonatal onset, we specifically looked at this group of patients as well. However, there were no patients with ABCC8/KCNJ11 variant and diabetes onset between 6 and 12 months of age in the DPV registry. Clinical, anthropometric and laboratory characteristics, stratified according to neonatal or later onset, are summarized in Table 1. Based on our data, we estimate a prevalence of one child with neonatal diabetes per 64,261 live births and one child with KCNJ11/ABCC8 variant per 339,032 live births, regarding the time period from 2010 to 2021 (Figure 2).
| n | Whole cohort (n = 93) | n | neonatal diabetes (n = 63) | n | MODY-diabetes (n = 30) | |
|---|---|---|---|---|---|---|
| Age at diabetes onset | 93 | 0.18 (0.02–9.13) | 63 | 0.08 (0–0.19) | 30 | 12.27 (9.44–14.46) |
| Current age | 93 | 9.94 (3.2–16.77) | 63 | 5.79 (0.67–15.48) | 30 | 15.80 (12.02–18.09) |
| Age at classification as K+-channel variant | 62 | 2.65 (0.34–12.5) | 41 | 0.48 (0.25–3.25) | 21 | 13.96 (11.63–16.90) |
| Time between diabetes onset and genetic classification (years) | 73 | 1.22 (0.14–7.75) | 50 | 1.14 (0.09–12.34) | 23 | 1.22 (0.23–2.16) |
| Sex (% male) | 93 | 55.9 | 63 | 57.1 | 30 | 53.3 |
| Migration background (%) | 93 | 23.7 | 63 | 17.5 | 30 | 36.7 |
| ABCC8 variant (%) | 93 | 58.1 | 63 | 47.6 | 30 | 80.0 |
| Weighta SDS at diabetes onset | 48 | 0.16 (−0.77–1.61) | 31 | 0.14 (−0.78–1.14) | 17 | 1.11 (−0.75–1.75) |
| Current weight SDSa | 85 | 0.03 (−1.18–0.99) | 59 | −0.38 (−1.37–0.30) | 26 | 1.45 (−0.31–2.08) |
| BMI SDSa at diabetes onset | 43 | 0.51 (−0.38–1.33) | 27 | 0.4 (−0.78–1.11) | 16 | 0.89 (−0.27–1.97) |
| Current BMI SDSa | 81 | −0.15 (−0.84–1.08) | 55 | −0.36 (−0.90–0.28) | 26 | 1.43 (−0.15–2.26) |
| A1c (%) at diabetes onset | 36 | 8.55 (6.02–10.77) | 20 | 7.23 (4.58–10.09) | 16 | 8.94 (7.99–11.94) |
| A1c (mmol/mol) at onset | 36 | 69.96 (42.31–94.18) | 20 | 55.51 (26.54–86.83) | 16 | 74.20 (63.84–106.96) |
Current A1c (%) A1c (mmol/mol) at onset |
86 | 6.15 (5.61–7.31) | 58 | 6.01 (5.53–7.17) | 28 | 6.50 (5.97–7.35) |
| Current A1c (mmol/mol) | 86 | 43.75 (37.83–56.35) | 58 | 42.22 (36.90–54.87) | 28 | 47.50 (41.74–56.78) |
| Insulin dosage (IE/kg/day) at diagnosis of diabetes | 32 | 0.82 (0.56–1.31) | 21 | 0.86 (0.73–1.36 | 11 | 0.67 (0.39–1.26) |
| Current insulin dosage (IE/kg/day) | 27 | 0.61 (0.36–0.88) | 16 | 0.61 (0.37–0.83) | 11 | 0.61 (0.26–1.09) |
| % with diabetic retinopathy | 38 | 0 | 28 | 0 | 10 | 0 |
| % with microalbuminuria | 48 | 20.8 | 31 | 19.4 | 17 | 23.5 |
| % with DEND syndrome | 93 | 12.9 | 63 | 15.9 | 30 | 6.7 |
| % with ADHD | 93 | 3.2 | 63 | 1.6 | 30 | 6.7 |
| % with autism | 93 | 1.1 | 63 | 1.6 | 30 | 0 |
| % with selective mutism | 93 | 1.1 | 63 | 0 | 30 | 3.3 |
- Note: Values are expressed as medians with interquartile ranges [25th–75th percentile] or as percentage of cases. SDS, standard deviation score; BMI, body mass index; A1c, hemoglobin A1c; DEND, developmental delay, epilepsy, and neonatal diabetes mellitus; ADHD, attention deficit/hyperactivity disorder;
- a SDS calculated using national reference data (AGA).11

3.1 Differences by subgroup
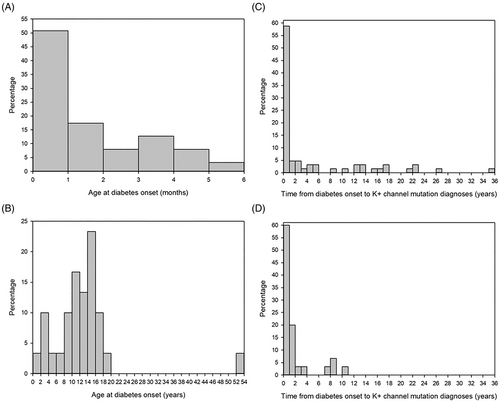
The median (IQR) time between diabetes onset and classification as potassium channel variant was 1.14 (0.09–12.34) years in patients with neonatal onset and 1.22 (0.23–2.16) years in patients with MODY. The time between diabetes onset and classification as potassium channel variant was different between the individual patients and not distributed normally. Diabetes was diagnosed within the first month of life in 50% of patients with neonatal diabetes (Figure 3 A), while the majority of patients with MODY12/13 developed the disease between 10 and 20 years of age with a peak between 12 and 14 years (>50% of patients, Figure 3B). The oldest MODY patient of this study cohort developed diabetes in his early 50s. While the classification as K+-channel variant was made within 1 year in more than 50% of the patients with neonatal onset (Figure 3C), this was the case for 40% of MODY patients (Figure 3D).
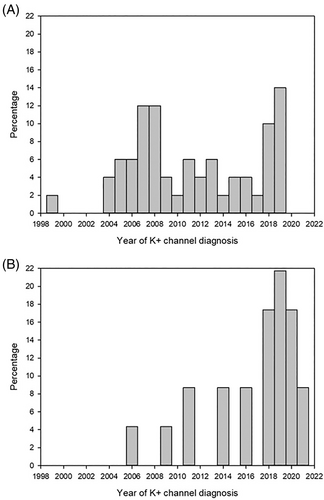
The diagnosis of a potassium channel variant as the cause of neonatal diabetes or MODY has been made with increasing frequency over the last 10–15 years. Sixty percent of patients with MODY due to a pathogenic variant in the KCNJ11 or ABCC8 genes were classified within the last 5 years, Figure 4.
Compared to MODY patients, patients with neonatal diabetes had lower BMI SDS values at the time of classification as K+-channel variant. In addition, presence of a ABCC8 variant was associated with the MODY-phenotype (the ABCC8 variant was present in 47.6% of cases with neonatal diabetes vs. in 80% of patients with MODY diabetes, p < 0.05). The majority of children with neurological complications were documented as having DEND syndrome (12 of 17 patients with neurological complications, i.e., 70.6%), while a small proportion were documented as having ADHD (attention deficit/hyperactivity disorder), autism or selective mutism, Table 1. A comparison between patients with ABCC8 and KCNJ11 variants in the whole cohort showed a higher frequency of “DEND syndrome” in patients with KCNJ11 variants (25.6% of cases with available data; n = 10) vs. ABCC8 variants (3.7% of cases with available data; n = 2) p < 0.05. No other significant differences were found between these two groups.
Patients with neonatal diabetes classified as ABCC8 or KCNJ11 variants within 1 year of diabetes diagnosis tended to have lower A1c at the last follow-up (median 5.7%, IQR 5.2–6.3, i.e., 38.9 mmol/mol, IQR 33.2–45.8) compared with patients with a later classification as K+-channel variants (6.7%, IQR 6.0–8.0, i.e., 50.3, IQR 42.1–63.7), but this difference just missed significance. This trend was not only seen for patients with neonatal diabetes, but also if patients with neonatal diabetes and MODY were combined.
3.2 Patients with long-term follow-up
For further analysis, we included 22 patients (13 male) of this cohort with available data at all three relevant time-points (classification as ABCC8/KCNJ11 variant, 2-year follow-up, most recent visit) in the DPV registry (Figure 1), 11 each with ABCC8 and KCNJ11 variants. Nineteen of them were diagnosed with neonatal diabetes and three with later onset (MODY; all ABCC8). At the 2-year follow-up, the median (IQR) diabetes duration was 2.5 (2.2–4.5) years, while it was 10.9 (7.5–17.6) years at the most recent visit. Microalbuminuria was documented in 35.3% (six of 17 with available data) of patients with long-term-follow-up, while no one was affected by retinopathy. Patients were in good metabolic control with a median (IQR) A1c level of 6.0% (5.5–6.7), that is, 42.1 (36.6–49.7) mmol/mol after 2 years and 6.7% (6.0–8.0), that is, 49.7 (42.1–63.9) mmol/mol at the most recent visit. We observed a decrease in height SDS during follow-up, and also a mildly decreasing weight SDS, while BMI SDS remained stable over time. Detailed information about the study group at the three relevant time points is displayed in Table 2, and stratified by variants in the ABCC8 and KCNJ11 genes in Table 3.
| Patients with ABCC8 or KCNJ11 variant (n = 22) | ||||||
|---|---|---|---|---|---|---|
| n | At classification as K+-channel variant | n | 2-year follow-up | n | Most recent visit | |
| Current age | 22 | 1.2 (0.3–9.9) | 22 | 3.3 (2.4–12.2) | 22 | 14.8 (9.0–18.1) |
| Diabetes duration | 22 | 0.5 (0.2–2.5) | 22 | 2.5 (2.2–4.5) | 22 | 10.9 (7.5–17.6) |
| Height SDSa | 21 | −0.2 (−0.7–0.7) | 21 | −0.6 (−1.2–0.6) | 21 | −0.6 (−1.5–0.3) |
| Weight SDSa | 22 | −0.2 (−0.5–0.5) | 22 | −0.3 (−0.6–0.5) | 19 | −0.3 (−1.4–0.8) |
| BMI SDSa | 21 | −0.2 (−0.5–0.3) | 21 | −0.11 (−0.4–0.6) | 18 | −0.2 (−0.8–0.7) |
| A1c (%) | 22 | 5.8 (5.4–6.8) | 22 | 6.0 (5.5–6.7) | 22 | 6.7 (6.0–8.0) |
| A1c (mmol/mol) | 22 | 39.9 (35.5–50.8) | 22 | 42.1 (36.6–49.7) | 22 | 49.7 (42.1–63.9) |
- Note: Values are expressed as medians with interquartile ranges (25th–75th percentile) or as percentage of cases.
- Abbreviations: A1c, hemoglobin A1c; BMI, body mass index; SDS, standard deviation score.
- a SDS calculated using national reference data (AGA).11
| Patients with ABCC8 variant (n = 11) | Patients with KCNJ11 variant (n = 11) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | At classification as K+ channel variant | n | 2-year follow-up | n | Most recent visit | n | At classification as K+ channel variant | n | 2-year follow-up | n | Most recent visit | |
| Current age | 11 | 0.5 (0.3–12.3) | 11 | 2.7 (2.3–14.1) | 10 | 16.4 (7.6–17.7) | 11 | 1.5 (0.3–6.2) | 11 | 3.5 (2.5–8.1) | 11 | 14.1 (9.0–18.5) |
| Diabetes duration | 11 | 0.4 (0.2–0.6) | 11 | 2.4 (2.0–2.6) | 11 | 9.0 (4.9–16.7) | 11 | 1.4 (0.3–5.9) | 11 | 3.4 (2.2–7.8) | 11 | 14.1 (8.8–18.5) |
| Height SDSa | 11 | −0.2 (−0.7–0.3) | 11 | −0.6 (−1.2–0.2) | 11 | −0.9 (−1.6–0) | 10 | −0.1 (−0.7–0.7) | 10 | −0.6 (−1.3–0.8) | 10 | −0.3 (−1.4–0.6) |
| Weight SDSa | 11 | −0.3 (−0.8–1.4) | 11 | −0.2 (−0.4–1.0) | 9 | −0.4 (−1.1–0.8) | 11 | −0.2 (−0.5–0.3) | 11 | −0.4 (−0.7–0.5) | 10 | −0.1 (−1.6–0.6) |
| BMI SDSa | 11 | −0.4 (−0.6–1.0) | 11 | −0.1 (−0.4–1.3) | 9 | −0.3 (−0.8–0.2) | 10 | −0.1 (−0.3–0.3) | 10 | 0.0 (−0.4–0.5) | 10 | 0.1 (−0.7–0.7) |
| A1c (%) | 11 | 5.8 (5.4–7.3) | 11 | 6.0 (5.4–6.3) | 11 | 6.5 (5.8–8.0) | 11 | 5.9 (5.1–6.8) | 11 | 6.1 (5.5–7.2) | 11 | 6.9 (6.0–9.6) |
| A1c (mmol/mol) | 11 | 39.9 (35.5–56.3) | 10 | 42.1 (34.3–45.4) | 11 | 47.5 (39.9–63.9) | 11 | 41.0 (32.2–50.8) | 11 | 43.2 (36.6–55.2) | 11 | 51.9 (42.1–81.4) |
- Note: Values are expressed as medians with interquartile ranges (25th–75th percentile) or as percentage of cases.
- Abbreviations: A1c, hemoglobin A1c; BMI, body mass index; SDS, standard deviation score.
- a SDS calculated using national reference data (AGA).11
3.3 Treatment
At the time of classification as KCNJ11/ABCC8 variant, 19 of 22 patients were treated with insulin, while 3 received OAD (oral antidiabetic drugs). 14 were on OAD at the most recent visit, while 6 were still on insulin (5 patients only insulin, 1 patient insulin + OAD) and 2 were without medication. If OAD were used, sulfonylurea were used in almost all cases. Only one patient was switched from sulfonylureas to glinides. Detailed information about the first medication, the current medication and a change of therapy is displayed in Table 4.
| All patients (n = 22) | ABCC8 (n = 11) | KCNJ11 (n = 11) | |
|---|---|---|---|
| First medication: Insulin | 19 | 10 | 9 |
| First medication: OAD | 3 | 1 | 2 |
| Current medication: Insulin | 5 | 2 | 3 |
| Current medication: OAD only | 14 | 6 | 8 |
| Current medication: OAD + Insulin | 1 | 1 | 0 |
| Current medication: none | 2 | 2 | 0 |
| (Temporarily) without therapy | 7 | 5 | 2 |
| All the time on insulin | 3 | 2 | 1 |
| All the time on OAD | 3 | 1 | 2 |
| Change from insulin to OAD | 11 | 5 | 6 |
| Change from insulin to no medication | 2 | 2 | 0 |
| Change from insulin to OAD to insulin + OAD | 1 | 1 | 0 |
| Change from insulin to OAD + insulin to insulin | 2 | 0 | 2 |
- Abbreviation: OAD, oral antidiabetic drugs.
The median (IQR) duration of insulin treatment was 1.3 (0.3–5.2) years in patients switched to sulfonylureas (data about the duration of treatment available for n = 8) and 1.3 (0.3–10.1) years in patients who did not require medication at least some of the time (n = 6). Patients with available follow-up data and information on the duration of insulin therapy (n = 8) who are currently treated with sulfonylurea were treated with insulin for a median (IQR) time of 0.20 (0.09–1.21) years after diagnosis of ABCC8/KCNJ11 variant. For patients currently treated with insulin (n = 6), median treatment duration with insulin was 11.4 (4.5–15.8) years. The median (IQR) dosage of sulfonylurea was 0.10 (0.03–0.19) mg/kg/day in patients with ABCC8 variants and 0.11 (0.07–0.39) mg/kg/day in patients with KCNJ11 variants at the current follow-up visit. The median (IQR) dosage of sulfonylurea was lower, if patients were changed early (<6 months after diagnosis of K+-channel variant) to sulfonylurea with 0.07 (0.07–0.16) mg/kg/day, while it was 0.10 (0.04–0.19) mg/kg/day if patients were switched >6 months after diagnosis of K+-channel variant. A comparison between patients treated with OAD at the most recent appointment and patients receiving insulin therapy showed no statistically significant differences regarding current age, age at diabetes diagnosis, height, weight, BMI, A1c, gender, migration background or associated neurological disease.
Five patients were temporarily without pharmacological therapy, and two other patients were without therapy until the end of follow-up. The median (IQR) time without medication was 4.0 (3.5–4.4) years for the five patients who were temporarily off medication. Taking all seven patients into account, the median time without medication was 4.4 (3.5–7.3) years. We compared patients with neonatal transient diabetes (n = 6, one patient with transdient diabetes was classified as MODY) and neonatal permanent diabetes (n = 13) and observed no significant differences regarding age, duration of diabetes, height, weight, BMI, current A1c value, gender, migration background and the presence of a neurological problem.
We observed a trend towards lower A1c values in patients treated with sulfonylureas compared to patients on insulin therapy. This trend was seen for the whole cohort and also for patients with long-term follow-up data, but was not statistically significant.
4 DISCUSSION
Neonatal diabetes is a rare disease and has been estimated to occur in 1:20,000–1:500,000 live births.8, 16-18 According to our registry, the current incidence of neonatal diabetes in Germany and Austria is 1:64,261 live births and the incidence of neonatal diabetes due to ABCC8/KCNJ11 variants is 1:339,032 live births. Therefore, potassium channel variants account for about 20% of all cases with neonatal diabetes in our cohort. The exact proportion of ABCC8/KCNJ11 variants in relation to all neonatal diabetes cases is unclear. In a previous publication, they accounted for about 40% of all cases and were the most common cause in families without consanguinity, but represented only about 12% of all cases in families with consanguine parents.1 The number of documented cases has increased significantly in recent years and decades, presumably due to better availability of molecular genetic testing and thus more complete recognition of these cases.
With 30 patients included in this study, the number of patients with an ABCC8 or KCNJ11 variant as the cause of diabetes beyond the neonatal period was more frequent than suspected, as only few cases with MODY 12/13 are reported in the literature.19, 20
The time between diagnosis of diabetes and classification as K+-channel variant was only slightly longer in patients with MODY diabetes compared to patients with neonatal onset. Many MODY cases have been diagnosed in the last 5 years, meaning that awareness seems to have increased significantly in the last few years. As there are direct therapeutic consequences (switching to sulfonylureas), these variants should also be considered in patients beyond the neonatal period if they do not have one of the more common forms of MODY diabetes. The oldest patient in our cohort was slightly above 50 years old, showing that diabetes due to an ABCC8 or KCNJ11 variant can occur at any age.
With 22 cases and a median duration of over 11 years follow-up in our study, this is a large group with a long follow-up, given the rarity of ABCC8/KCNJ11 variants. Other publications with a higher number of patients and a similar follow-up time accumulate patients from many countries and ethnicities.3, 21
Our data confirm the results from previous publications regarding the possibility of switching to sulfonlyurea in patients with ABCC8/KCNJ11 variants. 8/11 patients (72%) with KCNJ11 variants and 6/9 (67%) patients with ABCC8 variants who still needed pharmacological therapy were treated exclusively with sulfonylureas at the most recent visit. These numbers are slightly lower compared to the publications of Bowman et al.3, 21 Considering the low number of patients included in the publications of Bowman et al. and in our cohort, the results can be regarded as similar.
Two patients with ABCC8 variants and one patient with KCNJ11 variant were continuously treated with insulin and never transferred to sulfonylurea. It is conceivable that still not all diabetologists know about the option of treating patients with K+-channel variants with sulfonylureas. Alternatively, some patients or parents may not want to change therapy if insulin therapy is working well. With a median sulfonylurea dose of about 0.10 mg/kg/day at the most recent visit, the dose in our cohort was lower than in the mentioned publications with 0.37 mg/kg/day in ABCC8 and 0.23 mg/kg/day in KCNJ11 patients.3, 21 Dosage of sulfonylurea was slightly lower if patients were switched shortly after classification as K+-channel variant. However, the differences were not very large and these data were only available from a small number of patients. Our observation is consistent with a study in 58 patients with KCNJ11 associated NDM, also showing a correlation between the age at which sulfonylurea therapy was started and the dose required, and might be due to a declining sensitivity to sulfonylurea with age, or to a lower ß cell mass in patients treated with insulin.22 Therefore, diagnosis should be made as early as possible and, if functionally relevantABCC8/KCNJ11 variants are detected, a change to sulfonylurea should be considered early.
The median time between diabetes onset and the classification as ABCC8/KCNJ11 variant was just over 1 year in our whole cohort, and the median time until insulin was stopped was 0.20 years after classification as potassium channel variant, that is, about 1–2 months after classification. However, data on the duration of insulin therapy after classification were only available for eight patients. Compared to other reports,3, 5, 22 the time between diabetes onset and the start of sulfonylurea was relatively short. However, the IQR (0.14–6.54 years) is quite wide. Presumably, this depends on how much experience the respective center has with neonatal diabetes and how rapidly genetic results are available. There were no significant differences between patients treated with OAD and those receiving insulin therapy at the most recent visit. However, the numbers of cases were very small. We can only speculate about possible reasons why insulin was reintroduced in some patients, as our registry does not collect data on treatment decisions (physician-induced vs. patient preference). However, our data indicate that poor metabolic response to OAD does not seem to be a major driver.
Transient forms of diabetes mellitus are known in patients with ABCC8- and KCNJ11-variants.23 In our group, 2/11 patients with ABCC8 variants were without medication at the most recent visit, while 3/11 ABCC8 patients and 2/11 KCNJ11 patients did not receive pharmacological treatment temporarily. Our observation of a higher proportion of transient courses in ABCC8 compared to KCNJ11 variants is consistent with the current literature.24, 25
We observed a decrease of height SDS in patients with ABCC8 and KCNJ11 variants during follow-up. Low birth weight and height are already known in patients with neonatal diabetes,23 reflecting the necessity of insulin for prenatal growth. The decrease of height SDS might be caused by a lack of catch-up growth after intrauterine growth retardation, or by the genetic variant. Metabolic control was quite good in all patients, so that a poor metabolic situation is unlikely to be the cause of the poor growth.
We observed neurological complications in almost 20% of cases, more frequently in patients with KCNJ11 variants. The mechanism underlying neurological involvement in patients with potassium channel variants is not yet fully understood,26, 27 and the reported frequency varies,3, 21, 22 probably because there is a large spectrum of symptoms, ranging from mild attention deficits to severe neurological impairment. Microvascular complications were described in about 10% of patients by other groups in collectives with a similar follow-up time3, 21 and were observed in 35.3% of patients in our collective with long-term follow-up. As the observation periods were similar, one might only speculate about possible explanations for this finding. However, it should be mentioned that the number of cases in all reports is low.
4.1 Limitations and strengths
Our study has many strengths. The DPV registry covers about 80%–90% of pediatric patients with diabetes in Germany and Austria, with the coverage for infants with diabetes assumed to be even higher.28 This allowed us to access a relatively high number of cases and to identify a large number of patients with ABCC8/KCNJ11 variants. The DPV registry has a standardized data collection for clinical parameters related to diabetes diagnosis, management and outcomes. Our study is limited by the fact that we had to rely on the genotype information from the treatment center and that we did not have direct access to the original laboratory reports. However, all clinicians who contribute data are qualified according to national/international standards. As we did not collect information on the specific pathogenic variants of each case, correlation between genotype and phenotype was not possible. Furthermore, the rarity of the disease leading to small numbers of cases allowed us only to do a description of cases, and limited the possibility for statistical evaluations and conclusions need to be drawn with caution.
5 CONCLUSIONS
ABCC8/KCNJ11 variants are a rare cause of diabetes and should be considered early in patients with neonatal diabetes, as many of them can be successfully switched to sulfonylurea. However, we also saw a considerable proportion of potassium channel pathogenic variants in patients with MODY diabetes (MODY 12/13). Since there is a clear therapeutic consequence, ABCC8 and KCNJ11 variants should be considered as rare causes of diabetes also in adult patients. The response to sulfonylureas appears to be better if treatment is started early. Our patients had a good metabolic control even after a median follow-up time of 11 years. Neurological development needs to be examined continuously, as neurological comorbidity is frequent and affected one third of our patients, mainly with KCNJ11 variants.
AUTHOR CONTRIBUTIONS
Reinhard W. Holl conceived the study, Katharina Warncke drafted the article, and Alexander Eckert carried out data analysis. Alexander Eckert, Thomas Kapellen, Sebastian Kummer, Klemens Raile, Desiree Dunstheimer, Jürgen Grulich-Henn, Joachim Woelfle, Sandra Wenzel, Sabine E. Hofer, Axel Dost, and Reinhard W. Holl revised the article critically for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
ACKNOWLEDGMENTS
Special thanks to A. Hungele and R. Ranz for support and the development of the DPV documentation software.
List of DPV Centers contributing data to this analysis: Arnsberg-Hüsten Karolinenhospital; UK Augsburg; Kinderklinik Aurich; Bad Kreuznach Diakonie Kinderklinik; Bad Kreuznach Viktoriastift; Berlin DRK Kliniken Pädiatrie; Berlin Lichtenberg Kinderklinik; Berlin Virchow Kinderklinik; Bielefeld Kinderarztpraxis; Bielefeld Kinderklinik Gilead; Bochum Universitätskinderklinik St. Josef; Bonn Universitäts-Kinderklinik; Bremen Kinderklinik Nord; Bremen Zentralkrankenhaus Kinderklinik; Chemnitz Kinderklinik; Coesfeld Kinderklinik; Dresden Universität Kinderklinik; Duisburg St. Johannes Helios; Düsseldorf Universität Kinderklinik; Erlangen Universität Kinderklinik; Essen Universität Kinderklinik; Freiburg Kinder MVZ; Freiburg Universität Innere Medizin; Hagen Kinderklinik; Hamburg Altonaer Kinderkrankenhaus; Hamburg Kath. Kinderkrankenhaus Wilhelmstift; Hameln Kinderklinik; Zentrum f. Kinder- und Jugendmedizin, Universitätsklinikum Heidelberg; Heringsdorf Inselklinik; Innsbruck Universität Kinderklinik; Jena Universität Kinderklinik; Karlsburg Klinik für Diabetes und Stoffwechsel; Kassel Klinikum Kinder- und Jugendmedizin; Kiel Universität Kinderklinik; Leipzig Universität Kinderklinik; Ludwigshafen Kinderklinik St. Anna Stift; Lübeck Universität Kinderklinik; Minden Kinderklinik; Mönchengladbach Kinderklinik Rheydt Elisabeth Krankenhaus; München Kinderklinik Schwabing; Münster St. Franziskus Kinderklinik; Neuburg Kinderklinik; Neunkirchen Marienhausklinik Kohlhof Kinderklinik; Nürnberg Cnopfsche Kinderklinik; Oldenburg Schwerpunktpraxis Pädiatrie; Ravensburg Kinderklinik St. Nikolaus; Salzburg Universitäts-Kinderklinik; Schwerin Kinderklinik; Stade Kinderklinik; Stolberg Kinderklinik; Stuttgart Olga Hospital Kinderklinik; Ulm Universitäts Kinderklinik; Villach Kinderklinik; Wien SMZ Ost Donauspital; Wien Universitäts-Kinderklinik; Winnenden Rems-Murr Kinderklinik. Open Access funding enabled and organized by Projekt DEAL.
FUNDING INFORMATION
The DPV initiative is supported by the German Ministry of Education and Research (BMBF) within the German Center for Diabetes Research (DZD), the diabetes surveillance of the Robert Koch Institute, the German Diabetes Association (DDG) and the EU-IMI2-projects SOPHIA and INNODIA.
CONFLICT OF INTEREST
The authors have no conflicts of interest relevant to this article to disclose.
ETHICS STATEMENT
Analysis of anonymized data within the DPV initiative was approved by the Ethics Committee of the Medical Faculty of the University of Ulm, Germany, and the institutional review boards at the participating centers.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




