Metabolic control during the SARS-CoV-2 lockdown in a large German cohort of pediatric patients with type 1 diabetes: Results from the DPV initiative
Johanna Hammersen and Felix Reschke contributed equally to this study.
Parts of the data have been included in an abstract presented at the JAPED conference in June 2021.
Funding information: Deutsche Diabetes Stiftung, Grant/Award Number: FP-0433-2020; German Center for Diabetes Research, Grant/Award Number: 82DZD14A02; German Diabetes Association; German Robert-Koch-Institute
Abstract
Objective
To assess if metabolic control worsened during the SARS-CoV2 lockdown in spring 2020 in youth with type 1 diabetes (T1D) in Germany.
Methods
Data from 19,729 pediatric T1D patients from the diabetes prospective follow-up (DPV) registry were available. Data sets from four time-periods between January 1 and June 30, 2020, were compared with data from the whole year 2019 in the same patient; differences were adjusted for seasonality, increasing age, and longer diabetes duration. HbA1c values from laboratory measurements and estimates derived from continuous glucose monitoring (CGM) were aggregated into a combined glucose indicator (CGI), expressed in analogy to HbA1c.
Results
Based on regression models adjusted for differences of sex, age, diabetes duration, and migratory background between the four time-periods, CGI values in 2020 were slightly higher than in 2019, for example, by 0.044% (0.042–0.046) (median [95% CI]) in the second lockdown month, time-period 3. Insulin dose and BMI-SDS were also marginally higher. In 2020, there were fewer hospitalizations (e.g., incidence risk ratio in time-period 3 compared with 2019: 0.52 [95% CI: 0.46–0.58]). In a subgroup of patients reporting CGM data in both years, metrics in 2020 improved: time in target increased, and mean sensor glucose fell, for example, by 2.8% (2.7–2.9), and by 4.4 mg/dl (4.3–4.6) in time-period 3.
Conclusion
Before, during, and after the lockdown in spring 2020, metabolic control in youth with T1D in Germany did not differ significantly from the preceding year. Further effects of the ongoing pandemic on pediatric T1D patients need to be evaluated.
1 INTRODUCTION
To contain the spread of the SARS-CoV-2 pandemic, multiple social distancing measures were implemented in Germany on March 16, 2020. Schools and daycare institutions were closed all over the country, employees were largely recommended to work in home-office, children of parents with essential jobs attended emergency care. Team sport activities were prohibited, gyms and public playgrounds were closed. After 2 months, in mid-May 2020, the restrictions were gradually suspended over a period of ~6 weeks. In most federal states, schools and daycare institutions were opened, sports clubs were allowed to offer training to at least some extent, and social distancing regulations were lifted, so that daily life was partially adapted back to normal.
The social distancing measures led to a sudden change of daily life, which may have had a great influence on diabetes management in children and adolescents with type 1 diabetes (T1D), and which may have worsened metabolic control in these patients. On the one hand, the amount of time spent at home—with or without close parental supervision—may have increased, on the other hand, due to the short-term notification, children of parents with essential jobs had to attend daycare institutions without diabetes-trained personnel. The educational staff's training and experience has a significant impact on glycemic control in pediatric T1D patients.1-3 Metabolic control in this group may have deteriorated due to reduced physical activity,4 by changes of nutritional habits,5 by effects of the lockdown on the patients' psychological well-being,6 by diminished utilization of healthcare measures, or by reduced parental guidance. In pediatric patients, a delayed diagnosis of T1D, and an increase of diabetic ketoacidosis (DKA) at manifestation have been observed.7-9 Recent studies revealed short-term improvement in glycemic control in patients with T1D during isolation, both in children and adults, but to a lesser degree in teenagers.10, 11 In children and adolescents with T1D, availability of sensor-assisted insulin therapy seems to have an influence on metabolic control during the time of isolation.12, 13 Since the SARS-CoV-2 pandemic is still ongoing, more evidence is needed to counsel diabetes teams for optimal caregiving.14
This study aims at assessing if the SARS-CoV-2-related lockdown worsened metabolic control in a large cohort of pediatric T1D patients in Germany documented in the diabetes prospective follow-up (DPV) registry, a multicenter quality improvement initiative for patients with diabetes covering more than 90% of youth with T1D in Germany. It is a population-based analysis including data obtained in real life from a very large number of pediatric patients with T1D.
2 METHODS
2.1 Data source
Data originate from the DPV registry, in which participating German, Austrian, Swiss, and Luxembourgian diabetes treatment centers document data from diabetes-related visits for quality improvement and scientific research. Twice a year, pseudonymized data are transferred to the Institute of Epidemiology and Medical Biometry at Ulm University, where data are validated, anonymized, and aggregated into a cumulative registry. Since the governmental regulations imposed to contain the pandemic varied between the countries represented in the DPV registry, only data of German diabetes centers were included in this analysis.
2.2 Ethical approval
The DPV Initiative as well as the analyses of anonymized data have been approved by the ethics committee at the University of Ulm. Participating centers obtained local data protection approval.
2.3 Study population
2.3.1 Data were retrieved from the DPV registry in September 2020
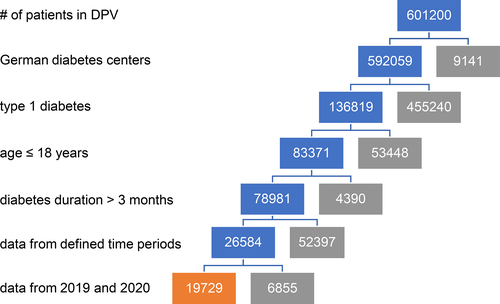
Data were limited to patients with visits or telemedicine contacts in one or more defined time-periods between January 1, and June 30, 2020, and in the whole year 2019, as control. Time-period 1, before the lockdown, ranged from January 1 to March 14, 2020, time-period 2 from March 15 to April 15, 2020, and time-period 3, from April 16 to May 15, 2020, covered the first and second month of the lockdown. Time-period 4 from May 16 to June 30, 2020 reflects the time after the strict lockdown. Data from every time-period in 2020 were compared with those from the whole year 2019 in the same patient. Only patients with paired data (time-period in 2020 and data from 2019) were considered. In total, 19,729 patients with T1D aged less than 18 years and with a diabetes duration longer than 3 months (to exclude data at onset) with visits in 231 German centers were included in the study (Figure 1).
2.3.2 Variables
Demographic characteristics of patients included sex, age at onset of diabetes, and age and duration of diabetes at each contact. In accordance with the definition of the European Union/European Commission, a migratory background was assigned if the patient or at least one parent was born outside of Germany.15 DKA was defined as presence of metabolic acidosis with a pH below 7.3 and/or bicarbonate levels below 15 mmol/L. Clinical variables documented at each visit and considered in this study were daily insulin dose (in units per kg body weight), use of continuous glucose monitoring (CGM) systems (rtCGM or iscCGM), use of an insulin pump, body weight standard deviation score (SDS), height SDS, and body mass index (BMI) SDS based on the German Health Interview and Examination Survey for Children and Adolescents (KiGGs).16 HbA1c values were mathematically standardized to the reference range of 4.05%–6.05% (IFCC 20.8–42.6 mmol/L) of the diabetes control and complications Trial applying the multiple of the mean method in order to correct for different laboratory methods.17, 18 From CGM data, mean sensor glucose values, and time in target range (70–180 mg/dl, 3.9–10.0 mmol/L; TiR) were derived. The percentage of TiR shows a stable correlation with HbA1c values, as reported in a detailed meta-analysis of 18 studies providing paired TiR—HbA1c data.19 Here, estimated HbA1c values were deducted from TiR data according to the above-mentioned meta-analysis: HbA1c (%) = (155.4—TiR [%])/12.762.19 To account for the short time-periods used in our analysis, for each patient and time-period, the median of HbA1c values estimated from CGM data, and the median of available laboratory-measured HbA1c values were integrated into a combined glucose indicator (CGI) expressed in “%,” in analogy to HbA1c values.
2.4 Statistical analyses
For each patient, data were aggregated per time interval. In descriptive analyses, the median and the first and third quartiles were used for continuous variables, sums were used to aggregate time of observation or the number of DKA events. For each patient, paired analyses with values from time-periods in 2020 and values from 2019 were performed. For dichotomous outcomes, we applied log-binomial models, for event rates negative-binomial models adjusted for age, and diabetes duration and calculated relative risks with 95% confidence intervals. For the continuous outcomes CGI, BMI SDS, and daily insulin dose/kg, we calculated the differences between the respective time-period of 2020 and 2019. To account for seasonality in 2019, we subtracted the difference between all patients “mean value of the same time-period in 2019 and the overall mean in 2019 from the patient's outcome in 2020. Based on each outcome in 2019, we calculated regression coefficients for the relation between age, and diabetes duration and the respective outcome. With these regression coefficients, we account for the patients” increasing age and diabetes duration in 2020 compared with 2019. To do so, the age difference between the respective time-period in 2020 and 2019 was multiplied with the respective regression coefficient, and added to the product of the diabetes duration difference between the respective time-period in 2020 and 2019, and the respective regression coefficient. This sum was subtracted from the seasonality-adjusted value of the patient. This value was used for calculating the adjusted difference between 2020 and the patient's value in 2019. Finally, we adjusted the outcome for differences between the four patient-groups in 2020 related to age, diabetes duration, migration background, and sex via quantile regression on the dependent outcome difference. The outcomes for the differences were then presented as median with 95% confidence intervals and compared against zero via Wilcoxon's signed rank test. Differences between the time intervals in 2020 were analyzed via Wilcoxon's rank sum test.
Frequencies of DKA, number and duration of hospitalization as well as days with CGM usage per patient-year were estimated using negative-binomial regression with the logarithm of individual time under risk as offset, and compared with the results for the same subgroup of patients in 2019. Models were adjusted for the covariates age and diabetes duration. Results are presented as relative risks with 95% confidence intervals. Two-sided p values <0.05 were considered as significant.
Regression analyses for the whole cohort were repeated stratified by age-group (<6 years, 6 to <12 years, and 12–18 years).
All analyses were performed using SAS version 9.4, build TS1M5, on a windows server 2016 mainframe computer.
3 RESULTS
3.1 Description of the study population
Nineteen thousand seven hundred and twenty-nine patients were included in the study (Figure 1). For each patient, data sets from the defined time-periods in 2020 were compared with data from 2019 of the same patient. In time-period 1, 2, 3, and 4, paired data sets of 17,047, 4819, 7373, and 9888 patients were available, respectively (Table 1). In the year 2019, representing the whole cohort of patients, 52.5% of the individuals were male, 25.7% had a migratory background, 60.6% administered insulin with an insulin pump, 75.0% of the patients used CGM. The patients' median age was 12.6 years (1st and 3rd quartile: 9.4; 15.0) and the median duration of T1D was 4.0 years (1.7; 7.1) (Table 1).
| 2019 | Time-period in 2020 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | n | Whole year | n | 1 (Jan 1–Mar 14) | n | 2 (Mar 15–Apr 14) | n | 3 (Apr 15–May 14) | n | 4 (May 15–Jun 30) |
| Age (years) | 19,729 | 12.6 (9.4; 15.0) |
17,047 | 13.1 (9.9; 15.6) |
4819 | 13.5 (10.3; 15.3) |
7373 | 13.2 (9.9; 15.7) |
9888 | 13.4 (10.4; 15.8) |
| Male sex (%) | 19,729 | 52.5 | 17,047 | 52.7 | 4819 | 52.3 | 7373 | 52.9 | 9888 | 52.1 |
| Migratory background (%) | 19,729 | 25.7 | 17,047 | 25.9 | 4819 | 26.9 | 7373 | 27.4 | 9888 | 25.8 |
| Diabetes duration (years) | 19,729 | 4.0 (1.7; 7.1) |
17,047 | 4.5 (2.3; 7.6) |
4819 | 4.6 (2.4; 7.8) |
7373 | 4.5 (2.4; 7.7) |
9888 | 4.9 (2.6; 8.0) |
| HbA1c (%) | 19,475 | 7.5 (6.8; 8.2) |
16,193 | 7.7 (7.0; 8.5) |
2572 | 7.8 (7.0; 8.7) |
5492 | 7.7 (7.0; 8.5) |
8465 | 7.6 (6.9; 8.4) |
| Number of HbA1c values | 19,729 | 4 (3; 5) | 17,047 | 1 (1; 1) | 4819 | 1 (0; 1) | 7373 | 1 (0; 1) | 9888 | 1(1; 1) |
| Proportion of time spent with CGM (%) | 19,729 | 57.0 | 17,047 | 62.8 | 4819 | 44.8 | 7373 | 55.2 | 9888 | 62.1 |
| CSII usage (%) | 19,488 | 60.6 | 16,513 | 61.1 | 3710 | 58.5 | 6404 | 61.2 | 9056 | 62.7 |
3.2 Parameters of metabolic control in 2020 compared to the preceding year
For parameters of metabolic control, differences between the four time-periods in January to June 2020 and the preceding year were calculated in paired measurements per patient, and corrected for seasonality, age, and diabetes duration. Based on regression models adjusted for sex, age, diabetes duration, and migratory background, CGI values reflecting metabolic control before, during, and after the lockdown were slightly higher compared to the preceding year, for example, 0.044% (0.042–0.046) (median [95% confidence interval (CI)]) in the second lockdown month (time-period 3, p < 0.001; Table 2).
| 2020 time-period 1 | 2020 time-period 2 | 2020 time-period 3 | 2020 time-period 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Parameter | # of patients | Median | # of patients | Median | # of patients | Median | # of patients | Median |
CGI (%) (first; third q.) |
16,442 | 7.74 (7.05;8.52) |
3669 | 7.84 (7.05;8.79) | 6485 | 7.72 (6.99;8.58) | 9158 | 7.64 (6.90;8.48) |
Diff. 2019 –time-period (%) (95% CI) |
16,341 | 0.044** (0.043;0.046) | 3651 | 0.044** (0.041;0.046) | 6451 | 0.044** (0.042;0.046) | 9115 | 0.039** (0.038;0.042) |
Daily insulin dose (U) (first; third q.) |
16,244 | 0.82 (0.65;1.03) | 3265 | 0.86 (0.68;1.08) | 5759 | 0.84 (0.67;1.06) | 8744 | 0.84 (0.67;1.05) |
Diff. 2019 –time-period (U) (95% CI) |
16,074 | 0.018** (0.017;0.018) | 3231 | 0.016** (0.015;0.017) | 5694 | 0.018** (0.017;0.018) | 8658 | 0.015** (0.015;0.016) |
BMI-SDS (first; third q.) |
16,390 | 0.37 (−0.25;1.00) | 3433 | 0.39 (−0.22;1.02) | 5933 | 0.39 (−0.23;1.02) | 8918 | 0.40 (−0.25;1.03) |
| Diff. 2019 –time-period (95% CI) | 16,235 | 0.028** (0.027;0.028) | 3402 | 0.031** (0.030;0.033) | 5878 | 0.028** (0.027; 0.029) |
8845 | 0.029** (0.028; 0.030) |
- Note: Differences were corrected for age, diabetes duration, and seasonality; p-values for difference ≠ 0 are indicated (*: <0.05; **: <0.001).
Absolute CGI values were 7.74% (7.05; 8.52) (median [first quartile; third quartile]), 7.84% (7.05; 8.79), 7.72% (6.99; 8.58), and 7.64% (6.90; 8.48) in time-period 1, 2, 3, and 4, respectively (Table 2), and 7.52% (6.84; 8.28) in 2019.
In all time-periods of 2020, the estimated insulin dose administered per kg body weight, adjusted for increasing age, longer diabetes duration, and seasonality, was slightly higher than in 2019, for example, the difference was 0.018 (0.017–0.018) (median [95% CI]) units per kg body weight per day in the second lockdown month (Table 2). Similar results were obtained for BMI SDS of T1D patients: in all time-periods of 2020, the BMI SDS was significantly, but only slightly higher than in the preceding year; for example, for the first month of the lockdown, the corrected difference was 0.031 (0.030–0.033) (Table 2).
For each time-period in 2020, the event rates of DKA, and hospitalization were compared with estimated event rates of the respective subgroup of patients in 2019, expressed as incidence risk ratios. Whereas before and after the lockdown, in time-periods 1 and 4 of 2020, the relative risk to experience a DKA was lower compared with 2019, 0.73 (0.56–0.96) in time-period 1 [p = 0.03], and 0.69 (0.49–0.98) in time-period 4 (p = 0.04), patients experienced more episodes of DKA in the first month of the lockdown compared with the preceding year, with an incidence risk ratio of 1.57 (1.02–2.41) (p = 0.04; Figure 2).
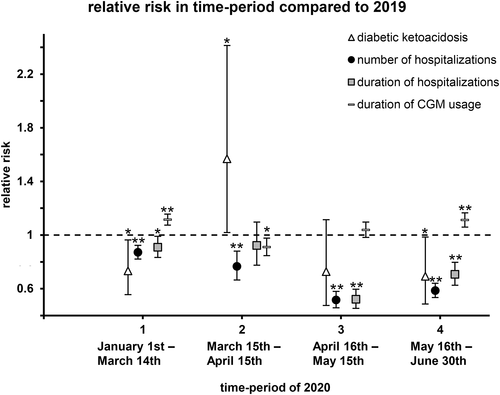
In all time-periods of 2020, before, during, and after the lockdown, the relative risk for hospitalization was lower compared to the preceding year, most notably in the second lockdown month, when the relative risk for hospital admission was 0.52 (0.46–0.58) (p < 0.001) compared with 2019. The differences were least distinct in time-period 1, but became more obvious during and after the lockdown (Figure 2). Similar results were found for the duration of hospitalizations: in all time-periods of 2020, hospital stays were shorter than in 2019; except for time-period 2, these differences were significant. The differences between the duration of hospitalization in 2020, and 2019 were most distinctive in time-period 3, the second lockdown month, when patients spent approximately half as many days in hospital than in the preceding year, with a ratio of 0.52 (0.45–0.60) (p < 0.001; Figure 2).
To analyze differences in CGM usage before, during, and after the lockdown in comparison to the preceding year, days of CGM usage per patient-year for each time-period in 2020 was estimated in regression models and related to data of the same subgroup of patients in 2019. In time-period 2, the first lockdown month, patients used CGM less frequently than in 2019, with a relative risk of 0.91 (0.85–0.98) (p = 0.01); in the second lockdown month, there was no significant difference in CGM usage compared 2019. In the other time-periods of 2020, CGM was more frequently used than in the preceding year, with a relative risk of 1.11 (1.07–1.16) (p < 0.001), and 1.11 (1.06–1.07) (p < 0.001) in time periods 1, and 4, respectively (Figure 2).
3.3 Analysis of metabolic control in different age groups
To determine if age had an impact on metabolic control, regression models were repeated stratified by age groups. The subgroup of patients aged <6 years comprised 1981 individuals, 7417 patients were aged 6 to less than 12 years, 10,331 individuals 12–18 years. Stratification of the regression models by age groups gave similar results as observed in the whole cohort for the differences between a time-period in 2020 and 2019 for the CGI values, the daily insulin dose, and the BMI SDS. Only in children aged <6 years, BMI SDS in all time-periods of 2020 was lower than in the preceding year. For example, in the second lockdown month, time-period 3, the estimated median BMI SDS difference compared to 2019 in children aged <6 years was −0.035 (−0.038 to −0.030).
For all age groups, the DKA rates in time-periods 1 and 4 of 2020 did not differ significantly from the preceding year. In time-period 2, children aged 6 to less than 12 years of age had a higher DKA rate than in 2019, the relative risk for an episode of DKA compared to 2019 was 2.30 (1.07–4.95) (p = 0.03). In time-period 2, the subgroup of patients aged less than 6 years did not experience any DKA. In time-period 3, patients aged 12–18 years had a lower DKA rate than in 2019 with a relative risk of 0.57 (0.32–0.99) (p = 0.047).
To summarize, outcomes of the age-stratified analyses resembled those of the whole cohort.
3.4 Analysis of metabolic control in a subgroup of patients with available paired CGM data
For a subgroup of 2770 patients, CGM data were available for a time-period in 2020, and 2019. Paired analyses were possible for 1900, 1116, 1242, and 1286 individuals in time-period 1, 2, 3, and 4, respectively. Demographic characteristics of the patient subgroups resembled those of the whole cohort: 52.6% of the individuals were male, 24.8% had a migratory background, and 65.6% used CSII. For the CGM-subgroup, the patients' median age was 12.1 years (first and third quartile: 8.8; 14.7), and they had a median duration of T1D of 3.6 years (1.5; 6.7). Laboratory-measured HbA1c values were available for 1699, 191, 471, and 757 patients in time-period 1, 2, 3, and 4, respectively.
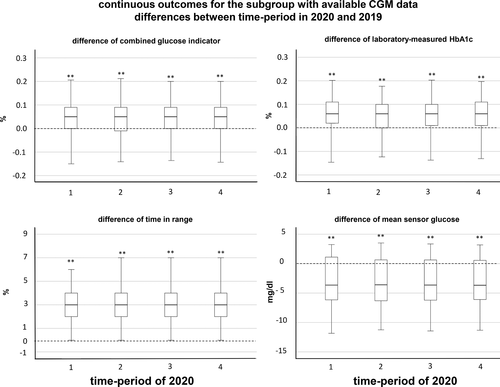
As for the whole cohort of patients, differences of metabolic parameters between the time-periods in 2020 and 2019 were calculated in paired measurements per patient, and corrected for seasonality, age, and diabetes duration. Similar to the results obtained for the whole cohort, before, during, and after the lockdown, CGI values determined in regression models adjusted for sex, age, diabetes duration, and migratory background were slightly higher than in the preceding year, for example, by an estimated median of 0.047% (0.041–0.053) in time-period 2 (p < 0.001). Absolute CGI values were 7.87% (7.15; 8.69), 7.98% (7.04; 8.95), 7.85% (7.04; 8.84), and 7.85% (7.03; 8.64) in time-period 1, 2, 3, and 4, respectively.
Paired laboratory-measured HbA1c values in a time-period of 2020 and 2019 were available for 1635, 181, 448, and 725 individuals in time-period 1, 2, 3, and 4, respectively. Before, during, and after the lockdown, HbA1c values were higher than in 2019, for example, by an estimated median of 0.061% (0.047–0.072) in time-period 2 (Figure 3; p < 0.001). The differences were slightly more pronounced than those observed for the CGI values.
In time-periods 1, 2, and 4, the DKA rates were not significantly different from those in 2019; in the second month (time-period 3) of the lockdown, the number of documented DKA episodes was too low for further analyses. Except in time-period 2, for which there was no significant difference, hospitalization rates were lower in 2020 than in the preceding year, e.g. with a relative risk for hospital admission of 0.53 (0.35–0.79) in time-period 3 compared to 2019 (p = 0.002).
In adjusted and corrected regression models, in all time-periods of 2020, time in the target range (70–180 mg/dl) was higher than in the preceding year; in time-period 3, the estimated median difference was 2.8% (2.7–2.9) (Figure 3). In accordance with these results, estimated mean sensor glucose values were significantly lower than in the preceding year in all time-periods of 2020, for example, by 4.4 mg/dl (4.3–4.6) in time-period 3 (Figure 3).
Thus, in the subgroup of patients for whom CGM profiles were available, CGI values were marginally higher in all time-periods of 2020 than in 2019, but CGM metrics before, during, and after the lockdown improved compared with the preceding year: time in target increased, and mean sensor glucose fell.
4 DISCUSSION
The aim of this study was to assess if metabolic control of pediatric T1D patients in Germany worsened during the first lockdown in spring 2020. In summary, data from a population-based cohort of 19,729 pediatric patients with T1D treated in Germany show no clinically relevant difference in metabolic control before, during, and after the first lockdown in spring 2020 compared to the preceding year 2019.
The effect of SARS-CoV-2-associated lockdowns on glycemic control in adults,20-22 children,13, 23 and adolescents.11, 24 with T1D has been investigated in various studies and countries. They mostly revealed stable metabolic control,13, 24 or even an improvement during or after social isolation.20-23, 25
Changes of daily life brought about by the lockdown may have had effects on diabetes management. Negative effects on metabolic control in pediatric T1D patients may result from a lack of physical activity. Previous studies showed significant changes in lifestyle for adolescents during the SARS-CoV-2 pandemic, such as a modified sleep–wake rhythm, different eating habits, and increased screen time.26 Moreover, there are indications of increased social withdrawal and an increased rate of depression and anxiety disorders among adolescents as a result of the lockdown.27, 28 In some parts of the world, for example, in India, glycemic control deteriorated due to non-availability of insulin and glucose test-strips.29
On the other hand, the lockdown situation may have had positive effects on metabolic control of pediatric T1D patients, for example, by an increased amount of time spent at home under parental guidance,30 or by a slowdown of daily activities. Improved glycemic regulation has been described for 20 adult patients with T1D from Italy, who stayed at home during the lockdown, but not for the control group of T1D patients with essential jobs, who continued working and did not change their daily routine.20 Spending more time in their private environment may have helped adolescents to perform diabetes management, since they may feel ashamed of performing diabetes management in public.31
In general, parents, children, and adolescents showed an enormously rapid adaptation to the changes in daily life due to the contact restrictions, and positive and negative effects of the lockdown on metabolic control may have been in balance.
In our patients, an increase of BMI SDS and insulin dose during the lockdown was observed, which is consistent with other studies32-35 and may deteriorate long-term outcome of T1D. In order to mitigate effects of the SARS-CoV2 pandemic on metabolic control in pediatric T1D patients, diabetes care teams should encourage patients to stay physically active.
Surprisingly, for the number and duration of hospitalizations, a difference between 2020 and the preceding year was already noted before the lockdown. A perceived danger in health care settings even before the political decisions to implement social distancing on March 14th, 2020, may have altered patients' and healthcare providers' behavior and may thus have led to postponement or cancelation of visits.
In the first lockdown month, the DKA rate was increased. This may be a result of the rapid adaptation that was needed to cope with the new situation, and from preselection of patients who contacted their diabetes teams during social isolation. Moreover, an increase of DKA rates during the lockdown in patients from centers with high COVID-19 mortality rates has been observed in the cohort of T1D patients from the SWEET registry,36 possibly reflecting reluctance of families to seek emergency care.
Facing the increased DKA rate during the first lockdown month, diabetes care teams should focus on some important aspects in diabetes management training: self-measurement of ketones should be propagated among patients and their families, and sick-day management should be reiterated. A language barrier is generally associated with higher HbA1c levels in pediatric T1D patients.37 During the lockdown in spring 2020, migratory background has been described as an independent risk factor for DKA at T1D manifestation in pediatric patients.38 Especially during the pandemic, rapid adaptation to new regulations is necessary, so that difficulties in diabetes management related to language barriers or cultural differences may be aggravated. Therefore, special attention should be paid to patients with a migratory background.
During the lockdown, face-to-face visits with healthcare providers were suddenly reduced, and may have been replaced by telehealth visits.39 The composition of the groups of patients seen by telemedicine and those with laboratory-measured HbA1c values may have differed significantly, since they may have been influenced by patients, their families, and by healthcare providers: some individuals may not have appeared at their healthcare provider because they may have been afraid of a SARS-CoV2 infection, especially during the first months of the pandemic, when diabetes was propagated as a risk factor for severe illness from COVID-19. Moreover, patients and families whose glycemic control was assessed as rather stable, and those who were capable of providing CGM data to their diabetes team may be overrepresented in the group providing CGM data only. To account for this special and new situation, we used both HbA1c values estimated from TiR metrics, and laboratory HbA1c values, resulting in an integrative parameter of metabolic control, the CGI. In the last years, CGM-based metrics have increasingly been used to evaluate metabolic control. HbA1c values deducted from TiR data19 as well as those calculated from mean sensor glucose values40 show a strong correlation with laboratory-measured HbA1c values, so that they can be considered as reliable indicators of glycemic control. As TiR is much more commonly used in patient care compared to mean glucose, we selected this equation, which provides the validation of our calculations.
The CGI can be helpful in assessing T1D patients' metabolic control during the pandemic. A recent analysis of web-based surveys shows a rapid and distinct increase in telemedicine usage for routine diabetes care in different countries.41 We expect a wider usage of CGM-derived metrics to assess metabolic control in the future, now accelerated by telemedicine care during the COVID pandemic. National and international benchmarking efforts to improve metabolic outcome in pediatric diabetes care like the SWEET initiative, and the DPV study group, have already included the CGI as a parameter in their reports in 2020 and 2021, which underlines its role for future assessment of metabolic control in pediatric diabetology. We foresee a period of several years, during which laboratory-measured HbA1c and CGM-derived metrics are used simultaneously to assess metabolic control, while in the more distant future, laboratory HbA1c measurements might be replaced entirely.
Since the CGI also depends on CGM usage, CGM usage was analyzed in the whole cohort of patients. In 2020, before and after the lockdown, CGM was more frequently used than in the preceding year, but in the first lockdown month, CGM was used less frequently than in 2019. The short time-periods in 2020 may explain this phenomenon: some patients may not have continuously used CGM, especially during a phase of social isolation, home-schooling, and closer parental guidance.
In the subcohort of patients providing CGM data in both years, an improvement of TiR and mean sensor glucose in the time-periods of 2020 compared with 2019 was observed, underlining the potential of technical means in patient support and self-management. In a recent study on 80 pediatric patients with T1D in Great Britain, improved glycemic control during the lockdown was also observed.42 However, it has to be kept in mind that patients providing CGM results during the lockdown are likely different from the entire patient population, with higher motivation and the ability to use tele-healthcare.
Both for the whole cohort of patients and for the subgroup providing CGM profiles, CGI values in all time-periods of the first half of 2020 were slightly higher than in the preceding year. Whereas this difference is not clinically relevant, it is still astonishing, especially in the patients with CGM profiles, for whom a reduction of mean sensor glucose, and an increase of TiR was observed. The difference of laboratory-measured HbA1c values was even slightly more pronounced. In part, the disparity of higher HbA1c values on the one hand, and improved glycemic control in CGM profiles may be due to the fact that HbA1c values reflect glycemic control during the preceding 2–3 months, while TiR calculations usually reflect the previous 10–14 days. Some patients may pay special attention to their diabetes management for a concise period of time before providing CGM data to their diabetes team, resulting in documented TiR values that are better than average.
This may represent a possible limitation of our study: in 2020, the number of patients with laboratory-measured HbA1c values was lower than in 2019, so that with the CGI as a parameter of metabolic control, the influence of missing data or interpellation of results by the CGI may have altered precision. However, in the subgroup providing CGM profiles, the difference of laboratory-measured HbA1c values was only slightly more pronounced than the CGI difference, and in general, the advantages of the usage of the CGI outweigh the disadvantages in this study.
Further limitations of our study may result from the preselection of patients with contact with diabetes teams in the time of very strict contact restrictions. Some patients may have performed diabetes management on their own, perhaps with provision of insulin, and glucose measurement strips by their pediatrician or family doctor or prescriptions sent by mail. Especially children from families of lower financial means may have been missed, as they may not have had the option to have telehealth visits due to limited availability of electronic devices, and they also may not have been seen in clinic for similar reasons. Patients expected to be at risk of such a situation should be convinced to visit their diabetes care team in future lockdown situations.
Another limitation may result from differences in implementation and removal of contact restriction regulations in different federal states of Germany. To account for these differences, we chose four short time-periods in the first 6 months of 2020, and we limited data from the DPV registry to those documented for patients in Germany.
This analysis is limited to the first lockdown in spring 2020 in Germany, after which many restrictions were temporarily lifted. A recent study shows a linear relationship between the duration of a lockdown and worsening of HbA1c values and diabetes-associated complications in adults with T1D.43 Long-term effects certainly need to be evaluated in future studies.
Nevertheless, this study is characterized by many strengths. By analyzing data from the DPV registry, we were able to perform a population-based study with a very high number of pediatric patients with T1D from Germany. Whereas some previous analyses of metabolic control during the lockdown were designed as clinical trials with a defined number of patients, we had the opportunity to analyze real-world data in routine care, because the DPV registry has implemented a method to upload glucose profiles from CGM devices. Moreover, our study design allows intra-individual comparison of metabolic control before, during, and after the lockdown, always in relation to the whole year 2019. To account for seasonal variation of HbA1c values with higher values during cold weather periods, which has been shown before,44 we corrected the CGI values and other parameters of metabolic control for seasonality, which represents another strength of our study. Introduction of a CGI as a potential future parameter of metabolic control integrating laboratory-measured values and CGM data are another strength of our analysis, which sets it apart from other studies, for example, from the SWEET registry.37
In summary, although an improvement of glycemic control—represented by TiR, and mean sensor glucose—was observed for a subgroup of patients, there was no clinically relevant change of metabolic control in pediatric patients with T1D in Germany in spring of 2020, the lockdown did not worsen metabolic control. Further studies are needed to evaluate long-term effects of the ongoing SARS-CoV-2 pandemic and associated social distancing means on metabolic control of pediatric patients with T1D.
ACKNOWLEDGMENTS
The authors thank all centers participating in the DPV project. For a full list of participating DPV centers, see the supplement. Special thanks to A. Hungele and R. Ranz for support and the development of the DPV documentation software, K. Fink and E. Bollow for the DPV data management (all clinical data managers, Ulm University). This work was partly funded by the Deutsche Diabetes Stiftung (FP-0433-2020). The DPV initiative was funded by the German Center for Diabetes Research (DZD, grant number 82DZD14A02), the German Robert-Koch-Institute, and the German Diabetes Association.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Johanna Hammersen and Felix Reschke designed the study created figures and tables, wrote the initial manuscript, and edited the manuscript. Sascha R. Tittel designed the study, performed data analyses, and edited the manuscript. Angeliki Pappa, Axel Dost, Katharina Köstner, Joachim Rosenbauer, Thomas M. Kapellen, and Tilman R. Rohrer and edited the manuscript. Reinhard W. Holl conceived and designed the study, performed and supervised data analyses, and edited the manuscript. Reinhard W. Holl is the guarantor of the study and accepts full responsibility for the work and the conduct of the study, had access to the data, and controlled the decision to publish. All authors have read and approved the final manuscript. Johanna Hammersen and Felix Reschke contributed equally to the study.
Open Research
DATA AVAILABILITY STATEMENT
To protect patient privacy, patient level data cannot be shared with outside investigators. However, upon request and after agreement from the DPV scientific board, joint research projects are possible.




