The ERF072 Transcription Factor Directly Regulates MtSOC1-Like Expression and Mediates Drought-Accelerated Flowering in Medicago truncatula
Zhiwei Wang and Jingwei Li contributed equally to this study.
ABSTRACT
Flowering time is a key agricultural trait that indicates the yield of Medicago truncatula. Although drought stress affects flowering time in this species, the molecular mechanism underlying the enhancement of flowering to facilitate drought tolerance remains unclear. Accelerated flowering at the onset of drought enables drought escape in Medicago truncatula, ethylene-responsive factors are an important class of transcription factors whose members are involved in drought stress processes in numerous plants. In this study, MfERF072 overexpression accelerated flowering in Medicago truncatula. In addition, the knockdown of this gene did not affect flowering time, MfERF072 overexpression enhanced drought and decreased the flowering time of Medicago truncatula under drought stress. Moreover, a more pronounced phenotype was observed. In contrast, the knockdown of this transcription factor reduced drought tolerance and delayed flowering time. Furthermore, yeast one-hybrid and two-luciferase assays confirmed that ERF072 binds directly to the promoter of the flowering integration MtSOC1-like gene in Medicago truncatula. This consequently promotes floral transition under drought conditions. Our preliminarily data revealed that ERF072 regulates flowering under drought stress. These results may provide insights into new genetic resources for the molecular breeding of Medicago truncatula, ultimately supporting stress tolerance by balancing drought stress responses and flowering time.
1 Introduction
Drought is a major environmental stress affecting plant growth and development (Zhu 2016). Drought stress limits plant growth and development by altering metabolic activities and biological functions, ultimately leading to reduced crop quality and yield (Mahmood et al. 2019; Ozturk et al. 2021). Alternative life history strategies for adaptation to drought stress and development of drought resistance include drought avoidance (reduced water loss to prevent dehydration) and escape (rapid development to complete the pre-drought life cycle) (Kooyers 2015). During drought escape, early flowering allows the parent plant to produce seeds before it dies because of water deficit, consequently enabling plants to adaptively shorten their life histories (Verslues and Juenger 2011; Fang and Xiong 2015; Hwang et al. 2019). Although drought escape generates progeny, there may be trade-offs, such as reduced fruit and seed numbers (McKay et al. 2003). Drought stress limits crop yield, but the molecular modulators and their mechanisms underlying the trade-off between drought resistance and crop growth and development remain elusive. For annuals subjected to chronic drought during the growing season, drought escape may be preferential for species succession. Avoidance mechanisms may be more appropriate if the growing season is acutely interrupted by drought.
For plants to reproduce successfully, flowering must occur at the right time.
Thus, the molecular pathways underlying flowering responses to acute and chronic drought have been widely studied. Flowering locus T (FT), suppressor of overexpression of constant 1 (SOC1), agamous-like 24, orange, and other important flowering integrators have been identified in Arabidopsis (Yu et al. 2002; Helliwell et al. 2006; Navarro et al. 2011; Wang et al. 2022). Further research indicates that AtABF3 and AtABF4 work with NF-YCs to stimulate AtSOC1 transcription during acute droughts, promoting early blooming in Arabidopsis thaliana (Hwang et al. 2019). In acute drought-induced tomato plants, SlVOZ1 directly binds to the promoter of the major flowering integrator gene SINGLE FLOWER TRUSS (SFT) to promote flowering (Chong et al. 2022). Despite preliminary studies on the molecular pathways involved in acute drought escape-induced early flowering, the complex molecular mechanisms involved remain largely understudied.
The Apetala 2 (AP2)/ethylene response factors (ERF) superfamily of transcription factors is one of the largest plant-specific transcriptional regulators. The conserved AP2/ERF DNA-binding structural domain comprises 57–66 amino acids (Okamuro et al. 1997). Moreover, AP2/ERF superfamily members can be classified into three groups according to their structure: the first group contains two AP2/ERF structural domains, the second group contains the AP2/ERF structural domain and the B3 DNA-binding structural domain, and the third group contains only a single AP2/ERF structural domain (Mizoi et al. 2012; Feng et al. 2020). ERFs belong to the AP2/ERF superfamily, and are involved in plant responses to hormonal and abiotic stressors (Gibbs et al. 2015; Jung et al. 2017; Wu et al. 2022; Fu et al. 2024). In wheat, the overexpression of TaERF87 (a member of the ERF group of the AP2/ERF superfamily) enhances chronic drought tolerance, whereas its silencing leads to drought sensitivity (Du et al. 2023). GmDREB1 (a member of the DREB group of the AP2/ERF superfamily) regulates the expression of downstream stress-related genes via a heterodimer with ERF-like transcription factors, thereby enhancing drought tolerance in soybean (Chen et al. 2022). Furthermore, CqERF24 overexpression in Arabidopsis thaliana enhanced drought tolerance by increasing antioxidant enzyme activities and activating stress-related genes, whereas the silencing of this gene in quinoa reduced drought tolerance (Zhu et al. 2023). In contrast, OsDERF1 overexpression downregulates ethylene synthesis and negatively affects drought tolerance (Zhai et al. 2013). Overexpression of ZmEREBP60 enhances drought tolerance and slows down H2O2 accumulation and malondialdehyde content in maize (Zhu et al. 2022). Several AP2/ERF family genes are involved in the regulation of plant drought tolerance. However, the involvement of ERF genes in drought escape mechanisms is yet to be established.
Medicago falcata is widely distributed in northern China and is mostly used as a wild resource with good drought resistance and palatability. In addition, it is used as a nutrient-rich graze feed for cattle, sheep, and other livestock (Yue and Zhou 2004). Medicago truncatula is a perennial herbaceous legume with an annual, diploid, self-pollinated, small genome (approximately 500 Mbp), and a short reproductive period (3–4 months). Moreover, it is capable of symbiotic nitrogen fixation with rhizobacteria. These traits make it a model organism for the study of genetic transformations in legumes. In addition, it is one of the most important and economically valuable fodder crops worldwide. Drought is an important abiotic stress factor that affects the growth and development of pasture grasses, consequently restricting the rapid development of the pasture industry and grass-fed animal husbandry. The growth, development, and stress responses of plants have been the main subjects of research on ERF transcription factors; however, their drought escape mechanisms remain largely understudied.
In this study, we analysed the expression patterns of ERF072 at specific growth stages and investigated its mechanism in M. truncatula drought escape using subcellular localisation, genetic transformation, and yeast one-hybrid experiments. Additionally, we determined the physiological and biochemical indices to provide a theoretical basis for further research on the biological function of drought escape in M. truncatula, and provide theoretical references for the improvement of the germplasm and community succession of this plant.
2 Materials and Methods
2.1 Plant Material
The seeds of Medicago falcata, a wild species, were collected from the Hulunbeier grasslands. Full M. falcata L seeds were selected, sterilised using a 75% alcohol solution and washed eight times with double-distilled water to remove any residual alcohol. A sterile filter paper was used to absorb the water on the surface of the seeds. The seeds were then spread evenly in sterile Murashige and Skoog (MS) solid medium, protected from light, and vernalised at 4°C for 3 days. Seedlings with unfolded cotyledons were then cultured in a plant incubator, transferred to sterile soil (nutrient soil: vermiculite = 1:1), and incubated under long-day conditions (16:8 h light/dark cycle, with a 24°C: 22°C temperature cycle, respectively) and 60% relative humidity. M. truncatula R108 seeds (China Agricultural University) were wall-broken with 98% H2SO4 for 8 min and sterilised with 5% NaClO for 15 min. Next, the seeds were germinated on sterile MS solid medium. The M. truncatula seeds were cultivated in the same light, temperature and humidity environments as M. falcata L seeds.
2.2 Cloning and Vector Construction of ERF072
We screened the resistance gene MfERF072 from wild-type M. falcata transcriptomic data evaluated in the laboratory (Zhang et al. 2020). Next, we analysed its expression pattern under externally applied 2 mg/L ABA stress (Supporting Information S1: Figure S1A), low temperature (4°C) (Supporting Information S1: Figure S1B), 200 mM NaCl (Supporting Information S1: Figure S1C), high temperature (35°C) (Supporting Information S1: Figure S1D), and 15% PEG-simulated drought (Supporting Information S1: Figure S1E) of wild M. falcata (Gopal and Iwama 2007; Feng et al. 2015). The expression of MfERF072 tended to increase and then decrease under simulated drought and ABA stress. Thus, it was hypothesised that this gene is involved in the regulation of drought resistance mechanisms in the ABA pathway. Therefore, we selected it for further research in the present study (Supporting Information S1: Figure S1A-E). The full-length coding sequence of MfERF072 (No. MN970144) was amplified using gene-specific primers. The primer sequence of MfERF072 gene is shown in Supporting Information S2: Table S1, and the full-length sequence is shown in Supporting Information S2: Table S3. The target gene was recombined with the binary expression vector pCAMBIA1307 driven by the 35S promoter to obtain the recombinant overexpression vector OE-pCAMBIA1307-MfERF072. The recombinant interference vector RNAi-pCAMBIA1307-ERF072 was constructed by inserting the MfERF072 fragment with tasiRNA into the 35S promoter-driven binary expression vector pCAMBIA1307 (Fu et al. 2024).
2.3 The ERF Phylogenetic Tree and Conserved ERF072 Motifs
The full-length amino acid sequences of the AP2 and MfERF072 proteins extracted from M. truncatula (https://medicago.legumeinfo.org/download/) and M. falcata respectively, were used for phylogenetic analyses. The obtained genes were subjected to multiple sequence comparisons using the Clustal W algorithm in MEGA 11.0 software. Bootstrap value is set to 1000, and other default parameters in MEGA 11.0 are used (Tamura et al. 2013). Phylogenetic trees were visualised using the iTOL online tool (https://itol.embl.de/).
2.4 Covariance Analysis of ERF Family Genes
The relevant files were downloaded from the M. truncatula genome database (https://medicago.legumeinfo.org/download/), A. thaliana database (http://plants.ensembl.org/Arabidopsis_thaliana/Info/Index) and Oryza sativa database (http://plants.ensembl.org/Oryza_sativa_chaomeo/Info/Index). The relevant files were downloaded, and we extracted the covariate pairs belonging to the ERF family. Covariate relationships were analysed and plotted using TBtools software (Wang et al. 2012).
2.5 Generations of Transgenic Plants
The recombinant plasmids OE-pCAMBIA1307-MfERF072 and RNAi-pCAMBIA1307-ERF072 were transformed into Agrobacterium tumefaciens EHA105. Leaves of 4-week-old M. truncatula were detached and disinfected with 5% NaClO for 8 min. The sterilised leaves were immersed in A. tumefaciens EHA105 carrying the target vector and stored in a 0.08 kPa vacuum for 20 min. They were subsequently drained of surface liquid with sterile filter paper and placed on SH3α medium. Next, they were cultured at 24°C in the dark and passaged in SH3α at 2-week intervals. Once the leaves had differentiated into healing tissue, the healing tissue was transferred to SH9 medium. The plants were cultured under light at 24°C and passaged in SH9 at 4-week intervals until they differentiated into seedlings.
The seedlings were then transferred to 1/2 MS medium for rooting, and the rooted seedlings were transferred to sterile soil for culture. Plant viability was verified through PCR and RT-qPCR to identify transgenic seedlings (Li et al. 2018). The phenotypic identification material under normal conditions and drought stress was the T3 generation.
We obtained regenerated transgenic T0 plants through genetic transformation and tissue culture, identified positive plants using PCR and RT-qPCR, harvested T0 seeds, seeded T0 seeds in 1/2 MS medium containing hygromycin resistance for screening, and transplanted the surviving T1 M. truncatula. Surviving plants were detected via PCR, and T1 seeds were cultured and collected. T3 M. truncatula with a survival rate of 100% was screened using this method, and the gene copy number of this generation was detected using Southern blot (Supporting Information S1: Figure S2).
We counted the number of planting days to observe the phenotype; we considered the first flower blooming as the flowering time, whereas the average flowering time of 10 plants in the same strain was considered the average flowering time. Statistics for all flowering times are shown in Supporting Information S2: Table S4.
2.6 Subcellular Localisation
The MfERF072 gene with a removed stop codon was recombined into the pBE vector, and the recombinant vector pBE-MfERF072 before transformation into A. tumefaciens GV3101. The primer sequences are listed in Supporting Information S2: Table S1. The lower epidermis of 4-week-old Nicotiana benthamiana leaves was infiltrated, and the expression of fluorescent proteins was visualised using a NIKON AX orthogonal laser confocal microscope.
2.7 RNA Extraction and RT-qPCR Analysis
The total RNA of M. truncatula was extracted using RNAiso Plus (TaKaRa).
Furthermore, the cDNA with removed genomic DNA was obtained as a template for quantitative PCR using the PrimeScript RT reagent kit with the gDNA Eraser Reverse Transcription Kit (TaKaRa). The mean and standard deviation values were calculated from the results of three biological replicates, and the results were plotted using GraphPad Prism9.5.1. The primers used in this study are listed in Supporting Information S2: Table S1, and the calculation data of RT-qPCR in this study are shown in Supporting Information S2: Table S5.
2.8 Southern Blot Hybridisation
The genomic DNA of M. truncatula was extracted using the sodium dodecyl sulphate (SDS) method (Chang 2022). The DNA was completely digested using Hind III and Xba I, and the products were purified and separated using 0.8% agarose gel electrophoresis (1 V/cm, 12 h). The recovered DNA was transferred to a nylon membrane using the capillary transfer method and dry baked for 2 h at 80°C for immobilisation, MfERF072-OE and ERF072-RNAi transgenic plants were hybridised with the MfERF072 fragment and 3 × flag, respectively, as hybridisation probes. The fixed membranes were placed in a salmon sperm DNA hybridisation bag containing 5 mL of high-efficiency hybridisation solution and 5 μL of denaturing salmon sperm DNA. Next, there were pre-hybridised for 2 h at 65°C by shaking in a water bath. The hybridisation solution was subsequently replaced with a fresh one, the labelled probes were added, and the hybridisation was carried out by shaking in a water bath at 65°C for 10 h. The hybridisation solution was then replaced with a fresh solution. After hybridisation, the membrane was removed from the hybridisation bag, immersed in 2× SSC and 0.1% SDS solution, and washed by shaking twice for 5 min each time.
It was then incubated in blocking solution under gentle shaking for 30 min, then in antisomal solution with gentle shaking for an additional 30 min. The membrane was washed twice with washing solution by shaking for 15 min each time and equilibrated in the assay buffer for 5 min. Next, it was placed in 10 mL of chromogenic substrate solution and protected from light for 16 h to develop colour. The reaction was terminated by rinsing with 50 mL TE buffer for 5 min.
2.9 Drought Treatment and Measurement of Physiological and Biochemical Indicators
For drought stress treatments, transgenic M. truncatula were grown in a growth chamber. For pot soil-drying experiment, wild-type (WT), MfERF072-OE and ERF072-RNAi plants were grown in the same pots containing a mixture of soil and vermiculite (2:1, 500 g/pot). Ten pots were grown independently for phenotypic observation (n = 10 biological replicates; one plant/pot). The saturated soil water content (SWC) was approximately 40%. Water was withheld from day 45 after sowing (45 DAS), and SWC was measured on every other day. The SWC reached < 10% approximately 15 days after water withdrawal, the SWC reached below 10%, ERF072-RNAi and CK showed wilted and nonflowering symptoms, whereas
MfERF072-OE came into bloom. The plants were photographed, re-watered, and their survival rates were estimated after 5 days of recovery (Chen et al. 2020).
MfERF072-OE2 and ERF072-RNAi1, and WT plants were subjected to a 15-day drought treatment by withholding water, whereas a separate control group was maintained under normal cultivation conditions. Leaves of MfERF072-OE, ERF072-RNAi, and WT M. truncatula leaves cultured under long-day conditions and drought-treated leaves were collected separately. Superoxide dismutase, peroxidase, catalase, superoxide anion, and malondialdehyde contents were measured using plant-soluble sugars according to the manufacturers' instructions. These experiments were repeated three times with six biological replicates for each group.
2.10 Yeast One-Hybrid Assay
Four cis-acting progenitors—GGCGGCT, TCATGTCGA, GTCGGT, and TCAACA—were screened from the promoter of the MtSOC1-like gene. Three tandem copies of each progenitor, including the first and second 5 bp fragments, were cloned into the pAbAi vector to construct the bait vectors. Sequences are listed in Supporting Information S2: Table S1. The full-length CDS of MfERF072 was ligated into the pGADT7 vector to generate a prey vector (pGADT7-MfERF072). The bait vector was then transformed into the Y1HGold yeast (Saccharomyces cerevisiae). Transformants were then selected on SD/-Ura plates, and the lowest inhibitory concentration of AbA in the bait strains was determined. Next, pGADT7-MfERF072 or pGADT7-empty vectors were introduced into Y1HGold yeast strains in a vacuum. Positive co-transformed cells were screened on SD/-Leu medium supplemented with the lowest inhibitory concentration of AbA and incubated at 30°C for 3 days. Positive controls (pAbAi-p53 and pGADT7-53) and negative controls (pAbAi-p53 and pGADT7) were treated similarly.
2.11 Dual-Luciferase Assay
A 2000 bp sequence upstream of the MtSOC1-like gene was ligated to pGreenII 0800-LUC as a reporter gene. Similarly, MfERF072 CDS was ligated to pGreen 62SK as an effector. The bacterial solution was injected into young leaves of tobacco leaves. Luciferase (LUC) was measured using luciferin (Promega) and used as an internal control. The primers used for oligonucleotide synthesis are listed in Supporting Information S2: Table S1.
2.12 Western Blot Analysis
The leaves of MfERF072-OE and WT plants were pulverised into a fine powder using liquid nitrogen. Total protein was extracted by adding plant tissue lysate and subsequently transferred to a centrifuge tube containing an equal volume of 2× SDS loading buffer. After spotting, electrophoresis was performed at 120 V for 1 h. The gel was subsequently inverted and placed beneath a PVDF membrane. It was then covered with filter paper and a sponge before being transferred to the membrane using an electric field strength of 100 V for 60 min at 4°C. The PVDF membrane was incubated with the primary antibody Flag and treated with the secondary antibody (HRP-labelled goat anti-mouse IgG [H + L]). Next, a mixture containing an equal amount of BeyoECL Moon A and B solutions was added dropwise, followed by imaging using a western blot exposure instrument. These reagents were supplied by SIAT (Su et al. 2022).
2.13 Statistical Analysis
All experiments included at least three biological replicates, and all data were analysed using GraphPad Prism (GraphPad Software). All data are expressed as mean ± SD of all results were calculated, and Student's t-test was used to analyse statistically significant differences.
3 Results and Analyses
3.1 Cloning, Multiple Sequence Alignment, and Evolutionary Tree Analysis
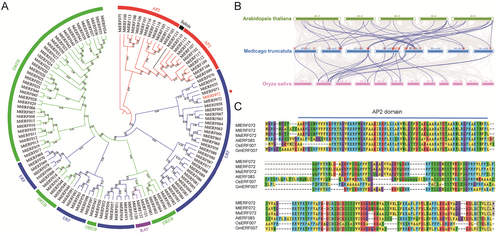
A phylogenetic tree was made using the full amino acid sequence of M. truncatula to learn more about the connection between MfERF072 and other ERF proteins. The study of tree structure revealed that MfERF072 is a direct homologue of MtERF072 (Medtr3g053690), a member of the M. truncatular ERF family (Figure 1A) (Shu et al. 2016). MfERF072 has a 95.11% match with MtERF072 at the amino acid level, and no studies of MtERF072 have been reported. In addition, we performed a joint analysis of the M. truncatula, A. thaliana, and Oryza sativa ERF genes. The results showed that 34 MtERFs exhibited covariance with A. thaliana and 21 MtERFs exhibited covariance with O. sativa; however, MtERF072 did not appear in any covariance pairs, suggesting that this gene may function differently on different species (Figure 1B). Multiple sequence alignments of the AP2 conserved structural domains of MfERF072 with homologues of ERF072 from other plant species (the sequence is detailed in Supporting Information S2: Table S2) showed that MfERF072 shares very high similarity at the amino acid level with the homologues of M. truncatula, M. sativa, A. thaliana, O. sativa, and Soybean (Figure 1C). Analyses indicate that the structural domain of ERF family member ERF072 is most conserved in leguminous crops and may perform the same function, and is relatively unconserved in the evolution of graminaceous, cruciferous and leguminous crops, which may be functionally distinct. We supplemented the expression pattern of MfERF072 gene in 20-day-old wild M. falcata 15% PEG simulated drought, ABA, 200 mM NaCl, low temperature at 4°C, high temperature at 35°C, and externally applied 2 mg/L ABA stress (Figure S1), and the results showed that the MfERF072 gene could be induced to be expressed by drought, high salt, ABA, and high temperature, with a significant response in drought and the response was most significant under the induced conditions of ABA, and the inflection point of the response level appeared on the time gradient, so it is suggested that MfERF072 may respond to drought and be regulated by ABA. We cloned MfERF072 homologous gene MtERF072 from M. truncatula. The AP2 domain of MfERF072 and MtERF072 was identical, and the amino acid similarity of the full-length protein sequence was 95.11%; subcellular localization showed that both MfERF072 and MtERF072 were located in the nucleus; the expression pattern analysis of different tissues showed that the expression trend of MtERF072 in different tissues of M. truncatula was consistent with that of MfERF072 in M. falcata, so we believe that there is a functional similarity between MfERF072 and MtERF072.
3.2 Expression Pattern and Subcellular Localisation of ERF072
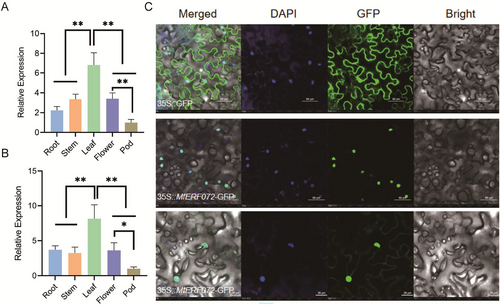
The expression pattern of the MtERF072 gene in roots, stems, leaves, flowers, and pods of M. truncatula under normal growth conditions using RT-qPCR. The results showed that the relative expression of MtERF072 was highest in leaves and lowest in pods (Figure 2A). The expression pattern of the MfERF072 gene in roots, stems, leaves, flowers, and pods of M. falcata under normal growth conditions using RT-qPCR. The results showed that the relative expression of MfERF072 was highest in leaves and lowest in pods, It was consistent with the expression trend of MtERF072 gene (Figure 2B). MfERF072 and MtERF072 driven by the cauliflower mosaic virus 35S promoter fused to GFP in tobacco leaves. MfERF072 and MtERF072 was found to be expressed in the nucleus (Figure 2B), consistent with findings that it functions as transcription factor.
3.3 ERF072 Alters Flowering Time in M. truncatula
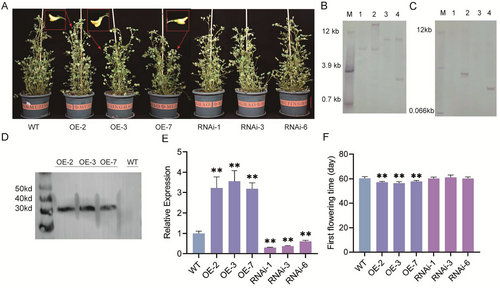
To further confirm the biological function of ERF072, we constructed the MfERF072 overexpression vector and the MtERF072 interference vector and transformed the recombinant vectors into M. truncatula by the Agrobacterium-mediated method. Which showed that three MfERF072-OE M. truncatula plants (MfERF072-OE2, MfERF072-OE3, and MfERF072-OE7) and three ERF072-RNAi M. truncatula plants (ERF072-RNAi1, ERF072-RNAi3, and ERF072-RNAi6) were obtained (Figure 3A), the regenerated plants obtained were subjected to Southern Blot validation (Figure 3B, Supporting Information S1: Figure S2D), Western Blot (Figure 3D) and RT-qPCR (Figure 3E), the results showed that both copy number and expression of ERF072 were increased in MfERF072-OEs plants compared with wild-type (WT), MfERF072-OEs M. truncatula detects MfERF072 protein expression. The regenerated plants obtained were subjected to Southern Blot validation (Figure 3C, Supporting Information S1: Figure S2G) and RT-qPCR (Figure 3E), the results showed the presence of a 3 × flag tag and a downregulation of ERF072 expression in ERF072-RNAis plants compared to wild-type (WT). We cultured MfERF072-OE, ERF072-RNAi, and wild-type (WT) M. truncatula material under long-day light conditions and from the time of germination and transplanting to nutrient soil as the first day, the time of the first flowering of the plant was calculated. The average flowering time of the wild-type (WT) plants was 60.4 ± 1.7 days, the average flowering time of the three strains of MfERF072-OE plants was 57.2 ± 1.1 days (Figure 3F), and the average flowering time of ERF072-RNAi plants of the three strains was 60.6 ± 1.5 days (Figure 3F), and the MfERF072-OE plants flowered 3 ± 1.7 days earlier compared to the control plants, while the ERF072-RNAi plants hardly changed the flowering time. The results showed that overexpression of the MfERF072 gene accelerated flowering in M. truncatula, and disruption of the MtERF072 gene did not alter flowering time in M. truncatula.
3.4 ERF072 Accelerates Flowering in M. truncatula Under Drought Conditions
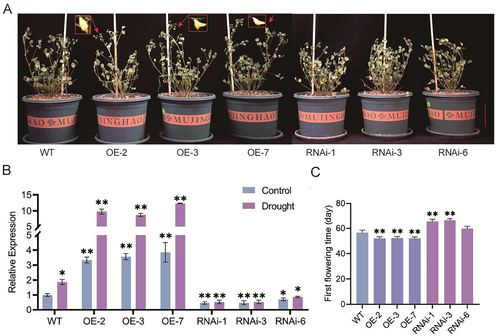
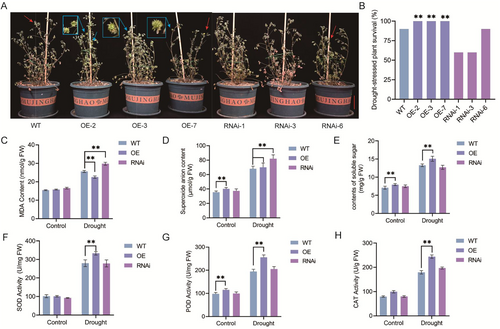
During plant cultivation, we found that MfERF072-OE could still flower normally under water-deficient conditions compared with ERF072-RNAi plants, and we conjectured that ERF072 might be associated with drought resistance (Figure 4A). We subjected 45-day-old MfERF072-OE, ERF072-RNAi, and wild-type (WT) M. truncatula to drought stress by stopping watering. The ERF072 expression levels of WT, MfERF072-OE and ERF072-RNAi were analyzed in control and drought-treated plants, respectively. The RT-qPCR results showed that the expression level of MtERF072 gene in MfERF072-OE plants was higher than that of WT, while the expression level of MtERF072 gene in ERF072-RNAi plants was lower than that of WT. The expression level of MtERF072 gene was higher in drought-treated plants than in control plants. (Figure 4B). The Flowering statistics results showed that the average time to flower after drought stress was 57.8 ± 0.8 days for wild-type (WT) plants, 52.2 ± 1.2 days for MfERF072-OE strain plants (Figure 4C). In contrast, ERF072-RNAi plants did not flower under drought stress. However, when water was restored after a 15-day suspension, about two-thirds of the ERF072-RNAi plants flowered, with an average flowering time of 66.4 ± 1.5 days (Figure 4F). Compared with the long-day culture conditions, the average flowering time under drought stress was advanced by 2.6 ± 1.7 days for wild-type (WT) plants and by 5.0 ± 1.2 days for the MfERF072-OE strain plants. In contrast, the average flowering time of ERF072-RNAi plants was delayed by 5.8 ± 1.5 days, with some plants wilting and failing to flower. Under drought conditions, MfERF072-OE flowered 5.6 ± 1.2 days earlier and ERF072-RNAi flowered 8.6 ± 1.5 days later than wild-type (WT). These results suggest that under drought conditions, ERF072 accelerates flowering in M. truncatula and escapes drought-induced damage.
3.5 Overexpression of MfERF072 Enhances Drought Tolerance in M. truncatula
After 15 days of stopping the water treatment and then rewatering for 5 days, it was found that the control M. truncatula was in the flowering stage, and MfERF072-OE2, 3, 7 produced pods, and the leaf growth condition was better than the control (Figure 5A), and the survival rate of the MfERF072-OE2, 3, 7 strain was 10% higher than that of the wild-type (WT) (Figure 5B); It was also found that ERF072-RNAi1 and ERF072-RNAi3 did not flower, ERF072-RNAi3 strain had more severe leaf wilt than the control, and ERF072-RNAi6 was phenotypically similar to the control (Figure 5A). ERF072-RNAi1 and MffERF072-RNAi3 were 30% less viable than wild-type (WT), and MtERF072 with low interference was not significantly different from wild-type (WT). It indicated that MfERF072-OE was more drought-resistant than ERF072-RNAi. We found the leaves of MfERF072-OE2, ERF072-RNAi3 and WT plants were taken from long-day cultivation and drought treatment, respectively, to test the physiological and biochemical indexes. After drought treatment, the MfERF072-OE2 strain had the lowest levels of malondialdehyde (Figure 5C) and superoxide anion (Figure 5D), and the highest levels of plant soluble sugars (Figure 5E), superoxide dismutase (Figure 5F), peroxidase (Figure 5G), and catalase (Figure 5H). Conversely, the ERF072-RNAi3 strain displayed the opposite trend. These results confirm the role of ERF072 in enhancing drought tolerance in M. truncatula.
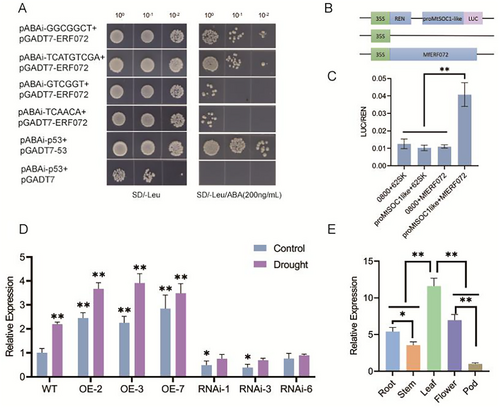
3.6 ERF072 Positively Regulates MtSOC1-Like
To find possible downstream targets of ERF072 transcription factors in controlling flowering time, we selected key flowering genes such as MtSOC1-like, MtSPL1, MtFTa, MtLFY, MtFUL, MtTFL, etc., and utilised plantpan 4.0 for the prediction of ERF072 binding to the promoter elements of the flowering genes, and screened for MtSOC1-like promoter, MtSPL1 promoter, MtFTa promoter and other motifs that might bind the AP2 structural domain. MtSOC1-like is a homologue of SOC, a key flowering gene in tomato that responds to drought escape, and phenotypic analyses indicate that ERF072 is also involved in regulating the drought escape mechanism in thistles and clovers, so it is conjectured that MtSOC1-like (Medtr8g033250) may be a downstream target of the ERF072 transcription factor. We looked at four cis-acting elements in the promoter of the MtSOC1-like gene. These are the GGCGGCT motif, the TCATGTCGA motif, the GTCGGT motif, and the TCAACA motif. We then determined whether MfERF072 could bind to the selected four cis-acting elements by yeast one-hybridisation (Y1H). For Y1H, each of the four ciselements from the MtSOC1-like promoter was cloned into the pAbAi vector to generate decoys, and MfERF072 was cloned into the pGADT7 vector to obtain prey. The prey vector was cotransfected into yeast strain Y1HGold with each of the four bait vectors, respectively. It was easy for the yeast to grow on selective medium (SD/-Leu) with or without AbA when prey vectors and bait vectors for the GGCGGCT motif and TCATGTCGA motif elements were changed together. The yeast that had the prey vector changed along with a bait vector that had GTCGGT motif and TCAACA motif elements did not grow on the selective medium (SD/-Leu) with AbA. This shows that MfERF072 connects to the GGCGGCT motif and TCATGTCGA motif parts of the MtSOC1-like promoter (Figure 6A). We cloned the promoter region of the MtSOC1-like gene and performed a LUC activity assay, which showed a significant enhancement of LUC activity after co-transformation of 35S: MfERF072 with ProMtSOC1-like: LUC in tobacco leaves (Figure 6B,C), validating the direct interaction between MfERF072 and MtSOC1-like. We examined the MtSOC1-like gene in control and drought conditions in WT, overexpressing MfERF072 M. truncatula MfERF072-OE2, MfERF072-OE3 and MfERF072-OE7 plants, and in three interfering MtERF072 M. truncatula ERF072-RNAi1, ERF072-RNAi3, and ERF072-RNAi6 plants for relation expression. The results revealed that the expression level of the MtSOC1-like gene was upregulated in overexpressing MfERF072 plants and downregulated in interfering MtERF072 plants, MtSOC1-like gene expression levels were higher in the drought-treated group than in the control group. The further demonstrated that the ERF072 gene positively regulates MtSOC1-like gene expression and that MtSOC1-like genes respond drought (Figure 6D). We looked at how the MtSOC1-like genes were expressed in the roots, stems, leaves, flowers, and pods of M. truncatula. The results showed that MtSOC1-like was most highly expressed in the leaves, second most highly expressed in the flowers, and least highly expressed in the pods, which is similar to what we saw with MtERFO72 (Figure 6E). These results confirm that ERF072 can directly bind to the MtSOC1-like gene promoter and regulate MtSOC1-like gene expression.
4 Discussion
Plant flowering is a critical developmental stage that is susceptible to environmental stress. Prolonged exposure to stressors such as drought can lead to yield loss. Under drought stress, most annual plants escape it by flowering and fruiting early to survive and reproduce (Kooyers 2015). M. truncatula also employs a drought escape strategy; however, the molecular mechanism of drought escape for early flowering in M. truncatula has not been elucidated. M. falcata, also known as wild Medicago, is an extremely drought-resistant plant of the genus Medicago in the family Leguminosae (Pennycooke et al. 2008). M. truncatula is a leguminous model plant, which is easy to transform, while M. falcata is difficult to genetically transform (Du et al. 2022). To further demonstrate the function of ERF072 gene, we interfered with the expression of MtERF072 gene in M. truncatula to provide help for the study of ERF072 function. The results showed that under drought stress, the OE-MfRF072 strain exhibited drought tolerance and accelerated flowering, while the RNAi-ERF072 strain exhibited drought intolerance and delayed flowering. A reference for clarifying the potential function of ERF072 in the entire alfalfa family.
The role of the ERF subfamily in the regulation of flowering time is largely understudied. Heterologous expression of tomato SlERF36 in tobacco leads to early flowering and affected stomatal density, electrical conductivity, and photosynthesis (Upadhyay et al. 2013). ERF1, a key member of the ethylene signalling pathway, acts as a negatively regulated of flowering time by repressing FT transcription in A. thaliana (Chen et al. 2021). Similarly, chrysanthemums overexpressing CmERF110 bloomed earlier than wild-type plants, whereas those with suppressed CmERF110 expression bloomed later (Huang et al. 2022). These studies suggest that ERF genes may be involved in the regulation of plant flowering; however, the underlying molecular mechanisms remain unclear.
We demonstrated that MfERF072 directly regulates MtSOC1-like through Y1H and double luciferase experiments, and Y1H showed that MtERF072 directly binds to the promoters of MtSOC1-like gene GGCGGCT motif and TCATGTCGA motif (Supporting Information S1: Figure S1F). In this study, nucleus-localised ERF072 positively induced MtSOC1-like expression, consequently providing a possible mechanism for the balance of drought response and flowering in M. truncatula. Triple mutants of MtSOC1 in thistledown alfalfa do not flower (Poulet et al. 2024), which is consistent with our findings. The ABF3 and ABF4 transcription factors accelerated flowering by regulating the expression of SOC1, a flowering integrator, and by controlling drought in A. thaliana (Hwang et al. 2019). Their ChIP-seq and microarray data analyses that SOC1 directly binds to and represses genes encoding various transcription factors, including the AP2 family of transcription factors (Hwang et al. 2019). This is consistent with our findings.
Drought stress and accelerated flowering in Arabidopsis species involve ABA-dependent and ABA-independent pathways (Riboni et al. 2013; Du et al. 2018). Studies have also linked the SOC1 to the ABA pathway, suggesting that ERF072 may regulates SOC1 through this pathway. This provides directions for subsequent in-depth studies.
In the present study, increased expression of ERF072 expression accelerated the flowering of M. truncatula, whereas decreased ERF072 expression did not cause delayed flowering. ERF072 overexpression not only improved the drought tolerance of this plant, but also promoted early flowering under drought conditions. In contrast, knockdown ERF072 led to delayed flowering or non-flowering traits, suggesting that this gene plays a key role in the regulation of drought escape mechanisms in M. truncatula (Figure 7). In conclusion, ERF072 accelerated flowering by inducing MtSOC1-like expression in response to drought stress in M. truncatula.

Acknowledgements
This study was supported by the Science and Technology Innovation 2030-Major Project (2022ZD04012), the Major Demonstration Project of “The Open Competition” for Seed Industry Science and Technology Innovation in Inner Mongolia (2022JBGS0016), the National Natural Science Foundation of China (31860671, 31101762), the Natural Science Foundation Key Project of Inner Mongolia Autonomous Region (2021ZD03).
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflicts of interest.
Appendix A. Supplementary Data
Supporting data to this article can be found online at. Genome Database of Medicago truncatula (https://medicago.legumeinfo.org/download/); Genome Database of Arabidopsis thaliana (http://plants.ensembl.org/Arabidopsis_thaliana/Info/Index); Genome Database of Oryza sativa (http://plants.ensembl.org/Oryza_sativa_chaomeo/Info/Index).
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Data will be made available on request.




