γ-Glutamyl-transpeptidase CsGGT2 functions as light-activated theanine hydrolase in tea plant (Camellia sinensis L.)
Abstract
Theanine is an important secondary metabolite endowing tea with umami taste and health effects. It is essential to explore the metabolic pathway and regulatory mechanism of theanine to improve tea quality. Here, we demonstrated that the expression patterns of CsGGT2 (γ-glutamyl-transpeptidase), participated in theanine synthesis in vitro in our previous research, are significantly different in the aboveground and underground tissues of tea plants and regulated by light. Light up-regulated the expression of CsHY5, directly binding to the promoter of CsGGT2 and acting as an activator of CsGGT2, with a negative correlation with theanine accumulation. The enzyme activity assays and transient expression in Nicotiana benthamiana showed that CsGGT2, acting as bifunctional protein, synthesize and degrade theanine in vitro and in planta. The results of enzyme kinetics, Surface plasmon resonance (SPR) assays and targeted gene-silencing assays showed that CsGGT2 had a higher substrate affinity of theanine than that of ethylamine, and performed a higher theanine degradation catalytic efficiency. Therefore, light mediates the degradation of theanine in different tissues by regulating the expression of the theanine hydrolase CsGGT2 in tea plants, and these results provide new insights into the degradation of theanine mediated by light in tea plants.
1 INTRODUCTION
As one of the most important economic plants, tea (Camellia sinensis) has become the second most-consumed beverage worldwide, after water. Theanine, an important secondary metabolite endowing tea with umami taste and health effects in the tea plant, accounts for 1%–2% of the constituents in dry tea leaves (Camfield et al., 2014; Lyon et al., 2011; Sharma et al., 2018; Yamada et al., 2009; Zhang et al., 2014). Theanine is an amino acid that is not used in protein synthesis but that stores nitrogen in tea plants, and its synthesis and degradation may be the key factor for nitrogen balance in tea plants (Ruan et al., 2012). Studies have shown that theanine serves as both a reservoir of nitrogen and an initiator for skeletal carbon compounds during germination (Deng et al., 2010). Therefore, it is essential to explore theanine metabolic pathway and its regulatory mechanism for elucidating the role of theanine in tea plant nitrogen metabolism. Up to now, theanine synthetase has been reported one after another (Fu et al., 2021; Sun et al., 2019; Wei et al., 2018), but theanine hydrolase and its regulatory mechanism are rarely reported in tea plant.
In tea plants, theanine synthetase (CsTSΙ) transcripts were specifically expressed in roots and were expressed at very low levels in other tissues (Fu et al., 2021). Widely held belief is that theanine is synthesized from glutamic acid (Glu) and ethylamine, mainly through CsTSΙ in roots, then transported to leaves and buds via the vascular tissues, where it would then be converted into new compounds (Deng et al., 2008; Dong et al., 2020; Sasaoka et al., 1965). In addition, four tea plant GSs (Glutamine synthetase) CsGS1.1, CsGS1.2, CsGS1.3 and CsGS2 have TS activity in planta (Fu et al., 2021; Liu et al., 2017). In our previous study, we determined that the γ-glutamyl-transpeptidase CsGGT2 from tea plants could catalyze the synthesis of theanine from glutamine and ethylamine in vitro (Sun et al., 2019). However, the biological functions and regulatory mechanisms of CsGGT2 in tea plants are still largely unknown.
Most studies suggested that the accumulation of theanine in the aboveground parts of tea plants was realized by transport from the roots (Ashihara, 2015; Dong et al., 2020). In the aboveground tissues of tea plants, theanine content and distribution are affected by light (Fu et al., 2021), theanine can be degraded to ethylamine through exposure to sunlight or heat (Kito et al., 1968), and tea growing in areas with less sunshine produces high concentrations of theanine (Vuong et al., 2011). Therefore, light is considered a necessary factor for theanine degradation in tea shoots (Kito et al., 1968). However, the photo-regulated theanine hydrolase and its regulatory mechanism in tea plants remain unknown.
Recently, pyridoxine biosynthesis enzymes (CsPDXs) were reported to hydrolyze theanine to l-glutamic acid and ethylamine in vitro (Fu et al., 2020). The transcription factor CsWRKY40 regulates theanine hydrolysis by activating CsPDX2.1 in tea leaves during withering (Cheng et al., 2022). Nonetheless, CsPDX2.1 only has significant enzyme activity in weakly acidic environments, and the enzyme activity decreases rapidly when the pH exceeds 6.5 (Fu et al., 2020). However, the crude enzyme extracts from tea leaves showed theanine hydrolytic activity at an optimum pH of 8.5 (Tsushida & Takeo, 1984), indicated that tea plants may have other theanine degradation pathways.
In the glutathione cycle, GGT catalyzes the transfer of γ-glutamyl from glutathione and related γ-glutamyl amino groups to amino acids and peptides to synthesize new compounds and can also specifically catalyze the cleavage of γ-glutamate in glutathione and other γ-glutamyl-containing compounds (Han et al., 2007; Kushwaha & Srivastava, 2014; Ou-Yang et al., 2018). Since the reaction does not require any additional ATP and has a wide range of substrate specificity, there is great interest in how GGT may participate in the enzymatic synthesis of theanine (Hu et al., 2012; Mu et al., 2019; Yang et al., 2021). However, the involvement of GGT in theanine degradation has not been reported in detail.
In this study, the expression patterns of CsGGT2 and its correlation with theanine accumulation were analyzed in tea plants under light. It was confirmed that CsGGT2 directly regulated by CsHY5 was involved in theanine degradation in tea plants, mediated by light signals. In addition, the gene function of CsGGT2 were characterized in vitro and in planta. Enzyme kinetics analysis, surface plasmon resonance (SPR) with an aptasensor, transient expression in Nicotiana and gene suppression in tea plants all confirmed that CsGGT2 acts as bifunctional protein of theanine synthase and hydrolase, and prefers to hydrolase in different tissues of tea plants due to their affinities for different substrates. This research contributes new, unambiguous information about the theanine hydrolase CsGGT2 involved in theanine degradation regulated by light in tea plants. It not only provides a good reference for research into theanine metabolism in tea plants but also provides a new theoretical basis for light signalling participation in the regulation of tea quality.
2 MATERIALS AND METHODS
2.1 Plant materials and treatments
Tissue-specific samples were harvested from the Camellia sinensis cultivar ‘Shuchazao' that was grown in the tea plantation of Anhui Agricultural University in Hefei, China. All the samples were collected and immediately frozen in liquid nitrogen and stored at −80°C until use. The 2-year-old ‘Shuchazao' tea cuttings, grown hydroponically in a greenhouse, were used for gene suppression. The composition of the nutrient solution was used as described in previous studies (Liu et al., 2019; Yang et al., 2021). New shoots were collected from tea plants (‘Shuchazao') grown in Chaohu Tea Plantation (Chaohu, China). For deionized water and salt stress treatment, new shoots were exposed to deionized water or 150 mM NaCl solution and collected at 6, 12, 24, 48, 72 and 96 h after the onset of treatment.
For light and dark treatments, 2-year-old tea cuttings, grown hydroponically in a greenhouse, were given two treatments: i: White light treatment (Light intensity: 10 μmol photons m-2 s-1 for 14 h per day); ii: Dark treatment (Light intensity: 0 μmol photons m-2 s-1). After these treatments had been applied for 10 days, the first leaf, second leaf, third leaf, fourth and fifth leaves (mixed sample), tender stems and white fibrous tea roots were collected and frozen at −80°C for further analysis.
2.2 RNA isolation, complementary DNA (cDNA) preparation and sequence analysis
Total RNA was extracted using an RNAprep Pure kit (Tiangen), the first-strand cDNA was synthesized using PrimeScript RT Master Mix (Takara). The open reading frame (ORF) sequences were amplified using KOD-Plus-Neo (TOYOBO). The polymerase chain reaction (PCR) products were purified with a Gel Extraction Kit (TransGen), ligated into the pEASY-Blunt Zero Cloning Vector (TransGen) and subsequently transformed into Trans1-T1 Phage Resistant Chemically Competent Cells (TransGen). The amplified full-length sequences were identified by sequencing.
2.3 Quantitative real-time PCR (qRT-PCR) analysis
qRT-PCR was performed using gene-specific primers (Supporting Information: Table S1). qRT-PCR analyses were performed with ChamQ Universal SYBR qPCR Master Mix (Vazyme). Data were analyzed with Opticon monitor software (Bio-Rad). CsGAPDH was used as an internal control and set as 0. Relative expression was determined from cDNA synthesized from at least three independent RNA extractions as technical replicates and analyzed using the 2−ΔCq method (Schmittgen & Zakrajsek, 2000).
2.4 Transient GUS activity assays
The promoter of CsGGT2 was inserted into pCAMBIA1391-β-Galactosidase reporter gene (GUS) to activate the GUS reporter gene, then transformed into Agrobacterium tumefaciens strain GV3101 (pSoup-p19) (Weidi). Five-week-old tobacco leaves were used for Agrobacterium-mediated transformation. The injected tobaccos were grown for approximately 2 days under normal (with light) and dark conditions, respectively. GUS activity was measured as previously described (Liu et al., 2019).
2.5 Yeast one-hybrid (Y1H) and dual-luciferase assay
Y1H and dual-luciferase assays were conducted as previously described (Lin et al., 2021). The cloned promoter fragment of CsGGT2 was inserted into the pAbAi vector, and CsHY5 was recombined into the pGADT7 expression vector to create prey plasmids. pGADT7-53 and p53-pAbAi were used as the positive control, whereas empty pGADT7-T and p53-pAbAi were used as the negative control.
For the dual-luciferase assay, CsHY5 was inserted into the pGreen II 0800 62-SK vector (SK), and the promoter fragment of CsGGT2 was inserted into the pGreen II 0800 62-luciferase (LUC) vector (LUC) (Hellens et al., 2000). The methods of vector transformation and tobacco transfection were described by Wu et al. (2020). At 3 day after infiltration, leaf discs were collected from the infiltrated areas and assayed using dual-luciferase assay reagents from Yeasen, and the LUC signal was visualized with a CCD system (Tanon-5200). Dual-luciferase assays were conducted with three replicates.
2.6 Electrophoretic mobility shift assay (EMSA)
The interaction of the CsHY5 protein and the promoter of CsGGT2 was assessed using an EMSA. The full-length CsHY5 was cloned and inserted into the pMAL-c5x vector, and the recombinant protein rCsHY5 was expressed and purified by the following method. The purified protein rCsHY5 was further incubated with biotin-labelled probes, mutation probes or competitors in the binding buffer. EMSA was performed in a 6.6% nondenatured polyacrylamide gel using a chemiluminescent EMSA kit (Beyotime) according to the method described by Gao et al. (2021).
2.7 ChIP-qPCR
The chromatin immunoprecipitation (ChIP) assay was performed using the EpiQuik™ Plant ChIP Kit (Epigentek) according to the manufacturer's instructions. Briefly, 2 g of the mature tea leaves was cut into small flakes and then immersed in 20 mL of 1% formaldehyde solution for cross-linking. The leaves were powdered in liquid nitrogen, and the soluble chromatin was collected, which was further immunoprecipitated by a specific CsGGT2 polyclonal antibody or nonimmune immunoglobulin G (IgG) in the microwells provided by the kit. After reverse cross-linking, the immunoprecipitated DNA fragments were analyzed by qRT-PCR with the specific primers shown in Supporting Information: Table S1.
2.8 Heterologous protein expression and purification
The full-length ORF of CsGGT2 was subcloned into the expression vector pMAL-c5x, which contains a maltose-binding protein (MBP) tag (42.5 kDa). The recombinant protein rCsGGT2 (with MBP tag) were purified by maltose-binding resin (Sun et al., 2019). Protein concentration was determined by a photometric method, and the correct sizes of the proteins were confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
2.9 Enzymatic activity assay
Enzyme activity analysis of the recombinant protein was performed according to a previous study with slight modifications (Sun et al., 2019). The basic reaction buffer contained 20 mM Tris-HCl (pH 7.4), 20 mM NaCl, 1 mM Ethylene Diamine Tetraacetic Acid, and 1 mM DL-Dithiothreitol. To assay the theanine synthesis activity, reaction mixtures of 1 mL were established containing 100 µL of recombinant protein rCsGGT2 (50 µg) and 900 µL basic reaction buffer with 20 mM glutamine and 20 mM ethylamine hydrochloride as substrates. To assay the theanine hydrolase activity, reaction mixtures of 1 mL were set up with 100 µL of recombinant protein rCsGGT2 (50 µg) and 900 µL basic reaction buffer with 20 mM theanine as substrate. The reaction mixtures were incubated at 37°C for 24 h and terminated by heating at 96°C for 5 min.
To assay the optimal pH for enzyme activity, the reaction mixtures were assayed at different pH values (5.0, 6.0, 7.0, 8.0, 9.0, 10.0, 11.0 and 12.0) in 1 mL reaction buffer (pH adjusted using NaOH and HCl) containing 50 µg purified rCsGGT2, and either 20 mM glutamine and 20 mM ethylamine hydrochloride or 20 mM theanine. The reaction mixture was incubated at 37°C for 24 h. To assay the optimal temperature for enzyme activity, the reaction mixtures were assayed at different temperatures (10, 20, 30, 40, 50, 60, and 70°C) in 1 mL reaction buffer (pH 10.0 for theanine synthesis and pH 8.0 for theanine hydrolase) and otherwise as above. The reaction mixtures were incubated for 24 h. To assay the optimal reaction time, the reaction mixtures were assayed at different times (2, 4, 6, 8, 12, 24 and 36 h) in 1 mL reaction buffer (pH 10.0 for theanine synthesis and pH 8.0 for theanine hydrolase). The reaction temperature was set as 30°C for theanine synthesis and 40°C for theanine hydrolase. The samples were centrifuged at 12,000 rpm for 15 min and then analyzed by high-performance liquid chromatography-mass spectrometry (HPLC-MS) (Sun et al., 2019) and gas chromatography-mass spectrometry (GC-MS) (Cheng et al., 2017).
2.10 Enzyme kinetics analysis
To investigate the substrate affinity of the recombinant protein rCsGGT2, the kinetic parameters for theanine at pH 8.0°C and 40°C for 24 h, and for glutamine and ethylamine hydrochloride were determined in Tris-HCl buffer at pH 10.0°C and 30°C for 24 h. A total of 50 µg purified recombinant protein rCsGGT2 was incubated in reaction mixtures, which contained 900 µL basic reaction buffer with different concentrations of either glutamine and ethylamine hydrochloride or theanine as substrates in a final volume of 1 mL. The reactions were stopped by heating at 96°C for 5 min after a 24 h incubation and analyzed by GC-MS and HPLC-MS.
2.11 SPR analysis
SPR analysis was performed using a Biacore T200 system according to the manufacturer's instructions, with some modifications. Purified proteins (MBP protein or recombinant protein rCsGGT2) were desalted and diluted to 10 μg/mL with acetate buffers of different pH values (pH 4.0, 4.5, 5.0 and 5.5). The optimized coupling pH was determined by a preconcentration experiment. Immobilization of the recombinant protein on the sensor chip CM7 was performed using the Amine Coupling Kit (GE Healthcare Life Sciences) according to laboratory experience in working with small molecules and the manufacturer's instructions (Wang et al., 2018; Zhu et al., 2015). Approximately 6000 resonance units (RU) of coupling proteins were immobilized on each channel of the CM7 chip, a vector protein channel was defined as a reference channel (MBP protein), and the recombinant protein was defined as sensing channel. Three compounds (glutamine, ethylamine hydrochloride and theanine) were prepared in 1×PBS-P + buffer (0.02 M phosphate buffer, 2.7 mM KCl, 0.137 M NaCl and 0.05% v/v surfactant P20, pH adjusted to pH 7.4) at different concentrations, then injected onto the immobilized chip for 210 s at a flow rate of 10 µL/min. The concentrations of the tested substrates ranged from 0 to 1000 mM. Finally, each sensorgram from the sensing channel was normalized by subtracting signals from the reference channel. The experiments were carried out at 25°C.
2.12 Transient expression in Nicotiana benthamiana
The ORF of the CsGGT2 transcript was subcloned into the pK7WGF2 vector with specific primers (Supporting Information: Table S1). The constructed vector pK7WGF2–CsGGT2 and empty vector pK7WGF2 were transformed into A. tumefaciens strain GV3101 (pSoup–p19) (Weidi), respectively. A. tumefaciens strain, carrying pK7WGF2–CsGGT2 plasmid, was injected into tobacco (Nicotiana benthamiana) leaves. The A. tumefaciens strain containing pK7WGF2 was infiltrated as the control. The detailed transient expression process in tobacco leaves referred to a previous study (Fu et al., 2021). After 2 days of injection treatment, 10 mM theanine or 10 mM ethylamine hydrochloride were reinjected into the whole leaf, which had been infected by the A. tumefaciens strain and still maintained a good growth state. The samples were harvested after normal culturing for 24 h and then immediately frozen in liquid N2. The specific detection methods used were described in previous studies with slight modifications (Cheng et al., 2019). Freeze-dried tobacco leaves were weighed equally (100 mg) and used for detection. The theanine content was determined by high performance liquid chromatography tandem secondary mass spectrometry (HPLC-MS/MS), and the ethylamine content was determined by GC-MS. In this study, each test data point was determined from the mixed samples of three independent tobacco leaves, and each treatment had three independent data points. Nine independent tobacco leaves were used.
2.13 Gene suppression of CsGGT2 in tea plants
Candidate antisense oligonucleotides (AsODNs) were selected using Soligo software (https://sfold.wadsworth.org/cgi–bin/soligo.pl) with CsGGT2 as input sequence (Supporting Information: Table S1). To silence CsGGT2 in the tea leaves that were still attached to the plant, 50 μM AsODN–CsGGT2 solution was injected into the third and fourth leaves of ‘Shuchazao' tea cuttings until the solution filled the whole leaf. Tea leaves injected with the sense oligonucleotides (sODNs–CsGGT2) was injected as control (Supporting Information: Table S1). After 24 h of incubation, the leaves were harvested and kept at -80°C before analysis (Jing et al., 2018; Zhao et al., 2020). Fresh shoots taken from tea plants were treated with deionized water or 150 mM NaCl in a beaker. CsGGT2 was silenced during the treatment by injecting 50 μM AsODN–CsGGT2 solution into the third leaves, sODNs–CsGGT2 was still used as control. After different durations (6, 12, 24, 48, 72 and 96 h) of treatment, the samples were separately collected and immediately stored at –80°C until use.
2.14 Western blot analysis
Total protein was isolated from the leaves of tea cuttings subjected to AsODN–CsGGT2 or sODN–CsGGT2 treatments. The amount of total protein was measured with a Bradford protein assay kit (Beyotime). A total of 20 µg total protein was separated by 12.5% SDS-PAGE and then transferred onto polyvinylidene difluoride (PVDF) membranes (Beyotime). The blot was blocked in tris buffered saline with tween-20 (TBSTw) (20 mM Tris-HCl, 137 mM NaCl, 0.1% Tween-20, pH 7.6) containing 5% milk overnight at room temperature (RT), incubated with primary antibody at RT for 3 h, and washed five times with TBSTw. The blot was then incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (at a dilution of 1:10,000) at RT for 1.5 h and then washed five times with TBSTw. Chemiluminescence was performed with BeyoECL Plus (Beyotime) and detected with X-ray film. Plant β-actin protein (Sangon) was used as an internal control in this study.
2.15 Analysis of theanine and ethylamine
For the analysis of theanine contents in different tissues of tea plants, theanine was extracted with hot water, as previously described (Tsushida & Takeo, 1984). Briefly, freeze-dried tea tissue samples (100 mg) were ground into powder, dissolved in 1 mL of distilled water, and then heated for 30 min at 100°C. After filtering through a 0.22-µm filter, the liquid extracts were analyzed by HPLC. The parameters and methods were according to Dong et al. (2020). For the bifunctional identification of CsGGT2 for the synthesis and degradation of theanine in vitro and in tobacco cells, samples were collected and subjected to HPLC-MS/MS and GC/MS analysis, and the determination methods were performed as previously described (Cheng et al., 2017; Sun et al., 2019).
2.16 Statistical analysis
All experiments were carried out in triplicate, and the data collected in this study were subjected to analysis of variance and expressed as means ± standard deviations. SPSS version 16.0 software (Statistical Product and Service Solutions) was used for the analysis of variance and multiple comparisons. Significant differences at the p < 0.05 confidence level were analyzed by Duncan's multiple range test and Student's t test.
3 RESULTS
3.1 Photo-regulated CsGGT2 is negatively correlated with theanine accumulation in tea plants
It has been reported that CsGGT2 have the ability to synthesize theanine in vitro (Sun et al., 2019). To explore the expression patterns of CsGGT2 in different tissues and verify the correlation with theanine accumulation in tea plants, the contents of theanine in various tissues were measured by HPLC. Theanine accumulated in roots, to 23.96 mg g-1, and accumulated in bud (13.00 mg g-1), stem (10.41 mg g-1) and first leaf (7.69 mg g-1). The contents of theanine continued to decline with the maturity of tea leaves and were the lowest in the fifth leaf among these tissues, at only approximately 0.86 mg g-1. The qRT-PCR results indicated that CsGGT2 was specifically expressed in mature leaves with a low theanine content and was present at very low levels in the stems and roots, with a high theanine content. Therefore, the expression levels of CsGGT2 and the content of theanine in different tissues showed a high negative correlation (R = −0.921). Moreover, the expression of CsGGT2 increased with the increase in leaf maturity from the bud to the fourth leaf, and the upward trend of the expression was significant (Figure 1a). Interestingly, CsGGT2 showed higher expression in strong light organs, than that of the stem and root, which were exposed to weak light and not exposed to light (Figure 1a; Supporting Information: Figure S1). Taken together, these data clearly show an obvious difference in the expression of CsGGT2 between the organs exposed the strong and weak light of tea plants, and the theanine accumulation in different tissues may be regulated by light.
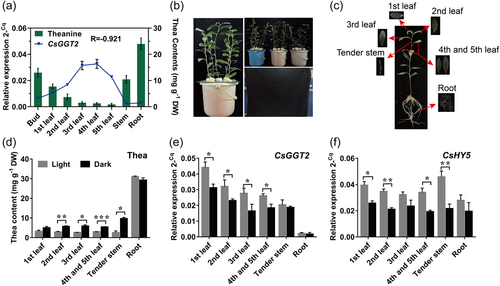
To further investigate whether the metabolism of theanine by CsGGT2 was regulated by light, we conducted hydroponic experiments on 2-year-old tea cuttings with white light treatment (light intensity: 10 μmol photons m-2 s-1) and dark treatment (light intensity: 0 μmol photons m-2 s-1) (Figure 1b). After a 10-day treatment, we harvested the first leaf, second leaf, third leaf, fourth and fifth leaves (mixed samples), tender stems and roots (Figure 1c). As shown in Figure 1d, dark treatment significantly increased the accumulation of theanine in the mature leaves and tender stem, while it did not change the contents significantly in root and the first leaf. In addition, dark treatment significantly decreased the expression of CsGGT2 in aboveground tissues except stem and root of tea plants (Figure 1e). These results indicated that light was negatively correlated with theanine accumulation in the aboveground tissues of tea plants. Compared with the dark treatment, the expression of CsGGT2 was up-regulated under the light and was also negatively correlated with theanine accumulation in tea plants.
3.2 Light-mediated CsHY5 bind to the promoter of CsGGT2 and activated CsGGT2
Based on the above results, we speculated that the increased theanine concentration in the dark treatment might be caused by the suppression of CsGGT2 expression levels and the absence of light. To further validate whether the gene function of CsGGT2 is regulated by light, we carried out molecular cloning and bioinformatics analysis of the promoter of CsGGT2. As shown in Figure 2a, the sequence analysis of promoter revealed that the region contained putative regulatory elements involved in light responsiveness, the G-box. There were two G-box elements at 58 bp (CACGTC) and 171 bp (CACGTG) upstream of the CsGGT2, respectively. Transient expression assays with the β-glucuronidase reporter gene (GUS) in the leaves of tobacco (Nicotiana benthamiana) showed that light could activate the activity of the promoter of CsGGT2 (pro-CsGGT2) in vivo (Figure 2a). G-box is the target sequence of the key regulator ELONGATED HYPOCOTYL5 (HY5) in light signalling pathways (Jing & Lin, 2020; Zhang et al., 2011). Therefore, the expression patterns of CsHY5 were studied in the above six tissues subjected to light and dark treatments. Our results indicated that CsHY5 transcript levels were repressed by dark treatment, which was consistent with CsGGT2 expression but opposite to the accumulation of theanine (Figure 1f).
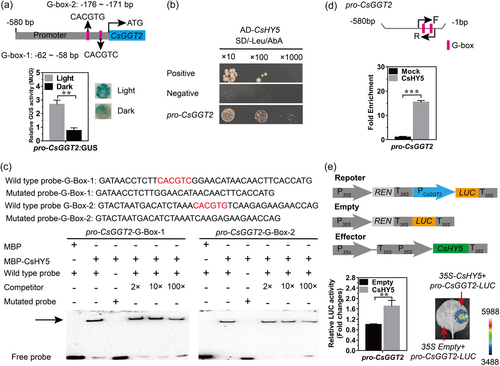
To explore whether CsHY5 regulates CsGGT2, yeast one-hybrid (Y1H) and EMSAs were performed. The results revealed that CsHY5 could directly bind to the pro-CsGGT2 in yeast cells (Figure 2b). In addition, the CsHY5 protein has been shown to bind to the first G-box-1 (CACGTC) and the second G-box-2 (CACGTG) of pro-CsGGT2, the purified rCsHY5 protein delayed the biotinylated probe, and the binding affinity was gradually reduced by the concentration gradient of cold probes (without biotin labelling) (Figure 2c). Additionally, the presence of CsHY5 protein had no significant effect on the mobility of the mutant biotinylated probe (Figure 2c). To further verify whether CsHY5 could target the pro-CsGGT2 in tea plants, a pair of gene-specific primers were designed containing the two G-box elements for the pro-CsGGT2, followed by ChlP-PCR analysis with a specific CsGGT2 antibody on tea plant leaves. The results in Figure 2d show a 15-fold enrichment of the G-Box elements from pro-CsGGT2, indicating the in vivo binding of CsHY5 to the promoter of CsGGT2 in tea plants. A dual luciferase (LUC) transactivation assay revealed that CsHY5 acted as an activator to promote the expression of CsGGT2 (Figure 2e), which was negatively correlated with theanine accumulation.
All the above results confirmed that theanine accumulation through CsGGT2 was negatively regulated by CsHY5, which bound directly to the CsGGT2 promoter that contained the G-box elements. CsHY5 acted as an activator of CsGGT2 and participated in theanine metabolism regulated by light signals. In our previous study, it was reported that CsGGT2 catalyze the synthesis of theanine from glutamine and ethylamine in vitro (Sun et al., 2019). Combined with the results of CsGGT2 gene expression patterns and correlation with theanine in different tissues of tea plants, we speculate that CsGGT2 may have different biological functions in tea plants, including theanine synthesis and degradation.
3.3 Bifunctional identification of CsGGT2 that synthesize and degrade theanine in vitro and in planta
To confirm our conjecture that CsGGT2 has the dual activities of theanine synthetase and theanine hydrolase, the enzyme activities of the recombinant protein rCsGGT2 were determined in vitro. The recombinant protein rCsGGT2 was produced using an Escherichia coli expression system and purified through affinity chromatography with amylose resin. The molecular weight of the purified rCsGGT2 protein with the MBP tag were approximately 104.3 kDa, as determined by SDS-PAGE (Supporting Information: Figure S2). The enzyme activity assays were performed under the preliminary conditions of 37°C for 24 h, and the reaction mixtures for theanine synthesis and theanine degradation were analyzed by HPLC-MS/MS and GC-MS, respectively. The results revealed that the recombinant protein rCsGGT2 is bifunctional protein and could not only synthesize theanine from glutamine and ethylamine hydrochloride but also degrade theanine into glutamic acid and ethylamine in vitro (Figure 3a,b).
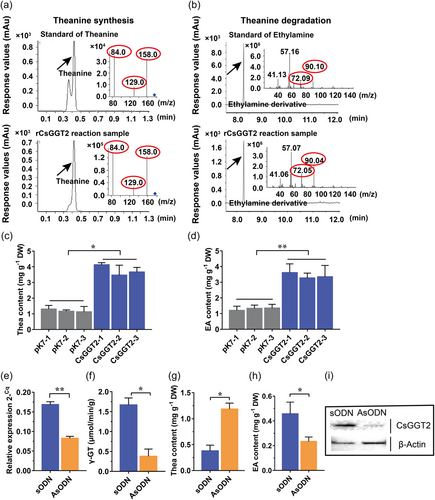
Limited by the lack of an efficient tea transgenic system, we used the Nicotiana benthamiana transient expression system to further characterize the functions of CsGGT2 in tobacco cells. Transient CsGGT2 overexpression in N. benthamiana plants was realized, and then 10 mM ethylamine hydrochloride solution or 10 mM theanine solution was injected into the leaves of transgenic tobacco plants overexpressing CsGGT2. In the leaves overexpressing CsGGT2, injection of ethylamine hydrochloride resulted in significantly higher theanine contents compared with the control (empty vector expressed in N. benthamiana and injected with 10 mM ethylamine hydrochloride), and injection of theanine resulted in significantly higher ethylamine contents than in the control (empty vector expressed in N. benthamiana and injected with 10 mM theanine) (Figure 3c,d). These results showed that CsGGT2 from tea plants have the abilities to synthesize and degrade theanine in tobacco cells. Interestingly, the overexpression of CsGGT2 in tobacco leaves had a more significant effect on theanine degradation (Figure 3d).
To further analyze the gene function of CsGGT2 in theanine metabolism in tea plants, the expression levels of CsGGT2 were suppressed in the leaves of tea cutting by the gene-specific antisense oligodeoxynucleotide (AsODN)-interfering gene-specific suppression strategy. The expression levels of CsGGT2 in tea leaves treated with AsODN–CsGGT2 were significantly reduced compared with the control (sense oligodeoxynucleotide, sODN–CsGGT2) (Figure 3e). Western blot analysis showed that AsODN treatments resulted in a decrease in the protein levels of CsGGT2 (Figure 3i). These results indicated that the AsODN method is effective for CsGGT2 gene silencing in tea leaves. In CsGGT2-silenced tea leaves, the γ-GT total enzyme activity decreased significantly (Figure 3f). Compared with the control (sODN), the content of theanine in CsGGT2-silenced tea leaves were significantly increased, accompanied by a significant decrease in ethylamine content (Figure 3g,h), which suggested that the silencing of CsGGT2 was likely to inhibit its hydrolase activities. All the above results reinforced that CsGGT2, as bifunctional protein, involved in the synthesis and degradation of theanine in vitro and in planta. In addition, transient expression and CsGGT2-silenced assays also suggested that CsGGT2 may have different catalytic efficiencies for different substrates, but is more inclined to hydrolyze theanine in tea plants.
3.4 Recombinant protein rCsGGT2 had higher substrate affinity for theanine than that for ethylamine hydrochloride
To explore the thermodynamic properties of the recombinant protein rCsGGT2 for theanine synthesis and theanine degradation, the optimum pH, temperature and reaction time were obtained by measuring enzyme activities with either 20 mM glutamine and 20 mM ethylamine hydrochloride or 20 mM theanine as substrates. The recombinant protein rCsGGT2 showed the highest theanine synthesis activity at pH 10.0 and the highest theanine degradation activity at pH 8.0 (Supporting Information: Figure S3a). It had the highest activity to catalyze glutamine and ethylamine hydrochloride into theanine at 30°C, while the highest activity to catalyze theanine into glutamic acid and ethylamine was seen at 40°C (Supporting Information: Figure S3b). Besides, rCsGGT2 achieved the best reaction efficiency after 24 for theanine synthesis and theanine degradation (Supporting Information: Figure S3c). The long reaction time observed for the in vitro reactions might be related to the conformation of the purified recombinant proteins, inhibition by their products, or the lack of coenzyme factors normally found in vivo.
To investigate the substrate affinity and catalytic efficiency of the recombinant protein rCsGGT2, the kinetic parameter for theanine were determined at pH 8.0°C and 40°C for 24 h, while the kinetic parameters for glutamine (in the presence of 20 mM ethylamine hydrochloride) and ethylamine hydrochloride (in the presence of 0.1 mM glutamine) were determined in Tris-HCl buffer at pH 10.0°C and 30°C for 24 h. The kinetic analysis results fit hyperbolic Michaelis−Menten saturation curves, indicating that rCsGGT2 had estimated km value of 62.90 mM for theanine (Figure 4a), of 0.04 mM for glutamine (in the presence of 20 mM ethylamine hydrochloride) (Figure 4b) and of 185.25 mM for ethylamine hydrochloride (in the presence of 0.1 mM glutamine) (Figure 4c).
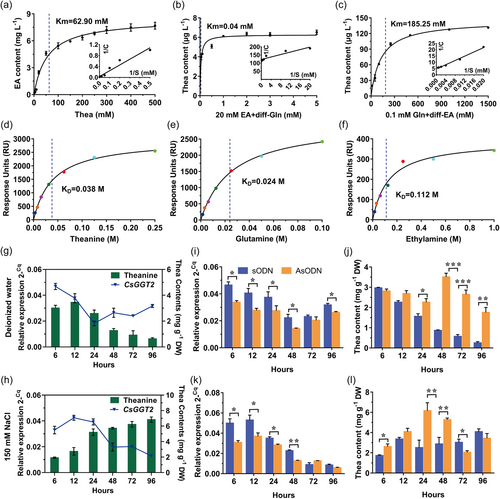
To further validate the affinity of rCsGGT2 to a single substrate, SPR affinity assays were performed. Theanine, glutamine or ethylamine hydrochloride at different concentrations were injected into the SPR flow channels. The equilibrium dissociation constant (KD) value of rCsGGT2 for theanine, glutamine and ethylamine hydrochloride were estimated to be 0.038, 0.024 and 0.112 M, respectively (Figure 4d–f). These results showed that the recombinant protein rCsGGT2 have different substrate affinities for glutamine, ethylamine hydrochloride and theanine in vitro. The affinity of rCsGGT2 to glutamine and theanine was significantly higher than that to ethylamine hydrochloride. Our current findings confirmed that the enzymatic reaction of CsGGT2 is reversible, and preferentially responsible for hydrolase. Since the content of theanine in tea plants is much higher than that of glutamine, CsGGT2 may mainly contribute to theanine hydrolysis in tea plants.
3.5 CsGGT2 is preferentially responsible for theanine degradation in tea plants
To better investigate the functions of CsGGT2 in tea plants, new shoots of tea plants were exposed to deionized water or 150 mM NaCl solution for different hours. As shown in Figure 4g, deionized water treatment resulted in a downward trend in the expression of CsGGT2 before 24 h and then increased slowly within 96 h, the content of theanine in the new shoots were significantly decreased after deionized water treatment for 12 h. The content of theanine remained only 18% after treatment for 96 h compared with the treatment for 12 h (Figure 4g). In contrast, 150 mM NaCl treatments resulted in an upward trend in the expression of CsGGT2 before 12 h and then decreased rapidly within 96 h. Interestingly, the content of theanine increased significantly under the 150 mM NaCl treatments. The content of theanine was increased by 3.55-fold after 96 h of exposure to 150 mM NaCl compared with the treatment for 6 h (Figure 4h).
To ascertain the effect of CsGGT2 on theanine accumulation during the deionized water and salt stress treatments, we used the AsODN method for CsGGT2 gene silencing in tea cuttings. As shown in Figure 4i, the expression of CsGGT2 was significantly suppressed in tea plant leaves compared with the control (sODN) during the process of deionized water treatment. At this time, the downward trend of theanine was also significantly suppressed, and the content even increased compared with the control after treatment for 48 h (Figure 4j). As shown in Figure 4k,l, when the expression of CsGGT2 was significantly suppressed in tea plant leaves during the process of salt stress treatment for 48 h, the upward trend of theanine was promoted after treatment for 48 h. Combined with the above results of substrate affinity for recombinant protein rCsGGT2, we concluded that CsGGT2 is preferentially responsible for theanine degradation compared with theanine synthesis, based on the higher affinity of CsGGT2 protein to theanine in vitro, and the higher concentration of theanine in tea plants.
4 DISCUSSION
4.1 Light-activated CsGGT2 is negatively correlated with the theanine accumulation in tea plants
Theanine is distributed in almost all organs of tea plants, but there are obvious differences in the concentration of theanine in different tissues, and different enzymes regulated by developmental and environmental factors might be responsible for theanine metabolism in these different organs (Deng et al., 2013; Fu et al., 2020; Ku et al., 2010; Lin et al., 2022; Yang et al., 2021). In tea cultivation, shading methods are commonly used to increase the content of l-theanine and enhance the strong and mellow taste (Deng et al., 2013; Ku et al., 2010; Yang et al., 2021). It has been reported that shading treatment results in a gradual increase in theanine in tea plant shoots (Deng et al., 2012, 2013; Gong et al., 2020; Li et al., 2019), and shading is considered to change the theanine allocation from the roots to shoots and significantly increases the content of theanine in tender stems (Yang, Liu, et al., 2021). Therefore, dark treatment may contribute to the increase of theanine accumulation. However, the regulatory mechanism by which light regulates theanine metabolism in tea plants remains unknown.
To exclude the interference of other environmental factors, this study did not apply a shading treatment in tea gardens but utilized an indoor treatment, with or without light (Figure 1b). In this study, the presence of light clearly up-regulated CsGGT2, and the accumulation of theanine among different organs was negatively correlated with the expression of CsGGT2, which is consistent with the above results of higher expression of CsGGT2 in the mature leaves exposed to the strong light with low theanine contents (Figure 1; Supporting Information: Figure S1). Likewise, the absence of light down-regulated CsGGT2 and significantly promoted the accumulation of theanine in the mature leaves and stems of tea plants (Figure 1d), which is consistent with the results of lower expression of CsGGT2 in the stems exposed to the weak light and roots in the dark with high theanine contents (Figure 1a). The results suggested that light promoted the expression of CsGGT2 and was negatively correlated with theanine accumulation.
In this study, CsHY5, a key component in the light signalling pathway (Chen et al., 2013), was verified to directly bind to G-Box, one of the light-responsive elements, upstream of the CsGGT2 (Lee et al., 2007). Photo-activated CsHY5 up-regulated the expression of CsGGT2, which preferentially catalyzed theanine degradation, based on the affinity for different substrates (Figure 4a–f). In summary, light can up-regulate the expression of CsHY5, promote its binding to the G-box element on the promoter of CsGGT2, and act as an activator of CsGGT2 to promote theanine degradation and provide nitrogen and carbon skeleton for other pathway in tea plants (Deng et al., 2010). Moreover, dark treatment suppressed the expression of CsHY5 and indirectly down-regulated the expression of CsGGT2 and suppressed theanine degradation, resulting in an increase of theanine accumulation in tea plants. Theanine was identified in tea by Yajiro Sakato in 1949 (Sakato, 1949) and reported to be degraded through exposure to sunlight in 1968 (Kito et al., 1968). In this study, we report for the first time the mechanism by which photo-regulated CsHY5–CsGGT2 pathway promotes theanine degradation in tea plant (Figure 5).
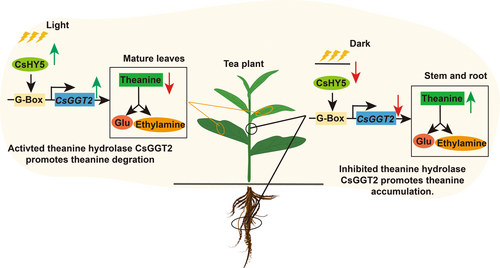
4.2 CsGGT2 is preferentially as theanine hydrolase in tea plants
Recent studies have reported that the content of theanine decreases during withering, and salt stress could induce theanine accumulation (Chen et al., 2020, 2021; Deng et al., 2012). In this study, it was verified that the content of theanine decreases by activating CsGGT2 during deionized water treatment, and salt stress could inhibit theanine hydrolysis by inhibiting CsGGT2 and enhance the resistance to salt stress in new shoots. CsGGT2 was preferentially responsible for theanine hydrolysis in tea plants, although CsGGT2 were confirmed to act as bifunctional protein, involved in both the synthesis and degradation of theanine in vitro and in planta (Figures 3 and 4). Interestingly, CsGGT2 has higher substrate affinities for glutamine and theanine than that for ethylamine hydrochloride by enzyme kinetics and SPR analysis. Besides, CsGGT2 has the highest substrate affinity for glutamine in the presence of ethylamine hydrochloride, and the lowest substrate affinity for ethylamine hydrochloride in the presence of glutamine. This phenomenon may be due to the fact that glutamine can be used as the substrate of CsGGT2 alone and hydrolyzed to produce glutamic acid and water and inhibited the synthesis reaction (Suzuki et al., 2007). Here, we conject that the enzymatic reaction of CsGGT2 may be reversible, and the substrate concentration could be the key factor affecting the reaction direction. It is reported that the content of theanine in tea leaves and roots is significantly higher than that of glutamine and ethylamine in tea plants. The content of theanine in tea leaves collected in October is about 1.96 mg g-1, six times more than that of glutamine (0.29 mg g-1) (Cheng et al., 2017). In the leaves of spring tea and winter tea, theanine content is still significantly higher than glutamine (Cheng et al., 2019). Therefore, we believe that CsGGT2 in tea plants is more likely to take theanine as the substrate, and catalyze the hydrolysis of theanine to produce glutamic acid and ethylamine.
The encoding genes involved in theanine synthesis have been reported successively in tea plants (Fu et al., 2021; Sun et al., 2019; Wei et al., 2018). However, few genes related to theanine hydrolysis have been reported in tea plant. CsPDX2.1 was reported to hydrolyze theanine only in weakly acidic environments with pH less than 6.5 in vitro (Fu et al., 2020), which is contrary to the previous report that the optimum pH of theanine hydrolase extracted from tree leaves by acetone powder was pH 8.5 (Tsushida & Takeo, 1984). In this study, the recombinant protein rCsGGT2 showed the highest catalytic activities under pH 8 for theanine degradation (Supporting Information: Figure S3a), which is consistent with the previous reports that crude enzyme extracts from tea leaves with theanine hydrolytic activity at an optimum pH of 8.5, and GGT functions under alkaline conditions (Lin et al., 2006; Mu et al., 2019; Shuai et al., 2011; Tsushida & Takeo, 1984). These results reinforced that CsGGT2, as one of the key theanine hydrolases, contributed to the degradation of theanine in tea plants. rCsGGT2 showed different optimal temperatures for the synthesis (30°C) and degradation (40°C) of theanine (Supporting Information: Figure S3b), which is consistent with the phenomenon that theanine can be degraded to ethylamine through exposure to heat in tea leaves (Kito et al., 1968; Suzuki et al., 2007). This indicates that CsGGT2 may modify the metabolism pathway of theanine at different environmental temperatures (Calvio et al., 2018). In spring, when temperatures are relatively mild, CsGGT2 may mainly synthesize theanine. In summer, the content of theanine in tea leaves is lower, which is closely associated with the strong-light-induced activation of CsGGT2 and the high-temperature-induced promotion of theanine hydrolase function of CsGGT2 and suppression of theanine synthase of CsGGT2 (Li et al., 2018).
In conclusion, in the mature leaves of tea plants that can effectively receive light signals, the expression of CsGGT2 is higher than that in stems and roots. The higher expression of CsGGT2 promotes the degradation of theanine and reduces the accumulation of theanine in mature leaves, thus realizing the reuse of theanine to provide nitrogen and carbon skeleton for other pathways in tea plants. Similarly, the lower expression of CsGGT2 significantly repressed the degradation of theanine, resulting in the high accumulation of theanine in the stems and roots of tea plants, where the reception of light was limited (Figure 5). This study provides new insights into the mechanism of how light regulates the dynamic accumulation of theanine in tea plants, which may provide molecular tools for the genetic improvement of tea quality and flavour.
ACKNOWLEDGEMENTS
This work was supported by the Ministry of Agriculture of China through the Earmarked Fund for China Agricultural Research System (No. CARS 19); the National Natural Science Foundation of China (No. 31170283); the National key research and development project (2021YFD1601105) and the Science Foundation of Anhui Agricultural University (2021yjs–22).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




