A poplar B-box protein PtrBBX23 modulates the accumulation of anthocyanins and proanthocyanidins in response to high light
Chaofeng Li and Jinli Pei contributed equally to this work.
Funding information: Forest Science Peak Project of College of Forestry, Fujian Agriculture and Forestry University, Grant/Award Number: 71201800701; JSPS Grant-in-Aid for Young Scientists, Grant/Award Number: 20K15553
Abstract
Flavonoids, which modulate plant resistance to various stresses, can be induced by high light. B-box (BBX) transcription factors (TFs) play crucial roles in the transcriptional regulation of flavonoids biosynthesis, but limited information is available on the association of BBX proteins with high light. We present a detailed overview of 45 Populus trichocarpa BBX TFs. Phylogenetic relationships, gene structure, tissue-specific expression patterns and expression profiles were determined under 10 stress or phytohormone treatments to screen candidate BBX proteins associated with the flavonoid pathway. Sixteen candidate genes were identified, of which five were expressed predominantly in young leaves and roots, and BBX23 showed the most distinct response to high light. Overexpression of BBX23 in poplar activated expression of MYB TFs and structural genes in the flavonoid pathway, thereby promoting the accumulation of proanthocyanidins and anthocyanins. CRISPR/Cas9-generated knockout of BBX23 resulted in the opposite trend. Furthermore, the phenotype induced by BBX23 overexpression was enhanced under exposure to high light. BBX23 was capable of binding directly to the promoters of proanthocyanidin- and anthocyanin-specific genes, and its interaction with HY5 enhanced activation activity. We identified novel regulators of flavonoid biosynthesis in poplar, thereby enhancing our general understanding of the transcriptional regulatory mechanisms involved.
1 INTRODUCTION
In plants, secondary metabolites encompass a diverse variety of phytochemicals and natural products, and play important roles in resistance to stresses, such as pathogen attacks, herbivory, extreme light intensity, low temperature and drought (Moore, Andrew, Külheim, & Foley, 2014). Flavonoids are important secondary metabolites derived from the phenylpropanoid pathway, and comprise anthocyanins, flavones, flavonols, proanthocyanidins (PAs), flavanones, chalcones, dihydrochalcones and dihydroflavonols (Barbehenn & Constabel, 2011; Ma, Reichelt, Yoshida, Gershenzon, & Constabel, 2018).
Flavonoids are biosynthesized from the L-phenylalanine as the major precursor. Numerous structural genes, including phenylalanine ammonia-lyase (PAL), cinnamate 4-hydroxylase (C4H) and 4-coumarate-CoA ligase participate in the general phenylpropanoid pathway and result in the biosynthesis of flavonoids, lignin, phenolic glycosides and phenolic acids (Fraser & Chapple, 2011). The initial enzymatic steps of the flavonoids branch involve the activities of chalcone synthase (CHS), chalcone isomerase (CHI) and flavanone 3-hydroxylase (F3H), which are responsible for the synthesis of key intermediates in the flavonoid biosynthetic pathway (Cain, Saslowsky, Walker, & Shirley, 1997). Desaturation of dihydroflavonols is catabolized by flavonol synthase (FLS) to produce flavonols. Meanwhile, PA and anthocyanin biosynthesis share the downstream enzymatic steps catalysed by dihydro-flavonol 4-reductase (DFR) and anthocyanin synthase (ANS) (Wilmouth et al., 2002). Anthocyanidin reductase (ANR) and leucoanthocyanidin reductase (LAR) are the two enzymes specific to PA biosynthesis, and UDP glucose-dependent flavonoid-3-O-glucosyltransferase (UFGT) is specific to the anthocyanin branch (Xu et al., 2014).
Transcriptional regulation of flavonoid biosynthesis mainly involves the interaction of R2R3-MYB and basic helix–loop–helix (bHLH) transcription factors (TFs), and WD40 repeat proteins (Gonzalez, Zhao, Leavitt, & Lloyd, 2008). These proteins form MYB–bHLH–WD40 (MBW) complexes to activate or suppress the expression of structural genes (Lai, Li, & Yamagishi, 2013; Stracke et al., 2010). In addition, R2R3-MYB TFs determine the specificity of structural genes bound by MBW complexes (Matsui, Tanaka, & Ohme-Takagi, 2004; Xu, Dubos, & Lepiniec, 2015). The R3-MYB TFs may participate in flavonoid biosynthesis by negatively regulating MBW complexes (Hu et al., 2016; Zhuang et al., 2019). A number of additional transcriptional regulators, such as SPL9, NAC78, and TCP3, are also involved in flavonoid biosynthesis (Tohge, de Souza, & Fernie, 2017). These TFs function cooperatively with MBW complexes to promote or suppress flavonoids biosynthesis.
Biotic and abiotic stresses, such as extreme light intensity, salinity, mechanical wounding and low temperature, can induce flavonoid accumulation by regulation of MBW complexes and other TFs (Catalá, Medina, & Salinas, 2011; He, He, & Ding, 2018; Zhao et al., 2013). Nutrient factors, such as phosphate limitation, sucrose and carbon: nitrogen balance, are also important factors that influence flavonoid accumulation (Gaude, Nakamura, Scheible, Ohta, & Dörmann, 2008; Liu, Yang, Gao, Ma, & Bi, 2018; Shi & Xie, 2014). In addition, phytohormones play vital roles in flavonoid biosynthesis. For example, auxin, abscisic acid (ABA), jasmonic acid (JA), gibberellin, cytokinin, ethylene, salicylic acid (SA) or brassinosteroids often act as crucial crosstalk components that either promote or repress PA and anthocyanin accumulation (An et al., 2018; Loreti et al., 2008; Nakata et al., 2013; Ullah et al., 2019; Yuan et al., 2015).
The B-box (BBX) proteins, which contain one or two B-box domains close to the N-terminus, comprise a superfamily of zinc-finger TFs (Gangappa & Botto, 2014; Zhang & Lin, 2017). In addition, a number of plant BBX proteins contain a CCT domain at the C-terminus (Khanna et al., 2009). Thirty-two BBX proteins have been identified and classified into five subgroups in Arabidopsis, of which many interact with and mediate the regulatory activity of ELONGATED HYPOCOTYL 5 (HY5) and CONSTITUTIVE PHOTOMORPHOGENIC 1 (COP1) (Xu, 2019). The BBX4 and BBX18–23 TFs interact with COP1 or HY5 to promote seedling photomorphogenesis (Chang, Maloof, & Wu, 2011; Ding et al., 2018; Wang, Sarmast, Jiang, & Dehesh, 2015; Xu, Jiang, Li, Holm, & Deng, 2018; Yan et al., 2011), whereas BBX24/25, BBX28 and BBX30/31/32 negatively regulates the photomorphogenesis functions of COP1 or HY5 (Heng et al., 2019; Holtan et al., 2011; Jiang et al., 2012; Lin et al., 2018). In addition, BBX may bind directly to several members of the phytochrome-interacting factor (PIF) TF family (PIF1, PIF3 and PIF4) and Early Flowering 3 (ELF3) during photomorphogenesis (Ding et al., 2018; Wang et al., 2015). BBX proteins have been identified and characterized in numerous other plant species, including rice, chrysanthemum, soybean, pear, potato, maize and grapevine (Cao et al., 2017; Fan et al., 2014; Shalmani et al., 2019; Wei et al., 2020; Yang et al., 2014).
Recently, BBX proteins have been shown to participate in the flavonoid pathway, especially in anthocyanin biosynthesis (Allan & Espley, 2018). In Arabidopsis, BBX21/22/23 are positive regulators of anthocyanin accumulation (Datta, Hettiarachchi, Johansson, & Holm, 2007; Xu et al., 2016; Zhang et al., 2017), whereas BBX24/25/31/32 negatively regulate anthocyanin biosynthesis in response to several environmental factors (Job, Yadukrishnan, Bursch, Datta, & Johansson, 2018; Tripathi, Carvallo, Hamilton, Preuss, & Kay, 2017; Yadav et al., 2019). In apple, MdBBX1/20/22 and MdBBX33 promote anthocyanin biosynthesis in response to ultraviolet-B (UV-B) radiation or low temperature (An et al., 2019; Bai et al., 2014), whereas MdBBX37 is a negative regulator of anthocyanin accumulation via light signalling (An et al., 2020). In pear, PpBBX16 and PpBBX18 positively regulate light-induced anthocyanin accumulation (Bai et al., 2019), whereas PpBBX21 interacts competitively with PpHY5 and PpBBX18 to inhibit the formation of the activator complex to repress anthocyanin biosynthesis (Bai et al., 2019). OsBBX14 interacts with OsHY5 to promote anthocyanin biosynthesis in rice (Bai et al., 2016). Although BBX- mediated flavonoid accumulation is predominantly induced by light-signalling pathways, the regulatory mechanisms remain largely unknown.
Poplars (Populus L. spp.) are economically important tree species rich in flavonoid metabolites, of which PAs and anthocyanins generally account for 30% of the leaf dry weight (Tsai et al., 2006; Tuskan et al., 2006). As with many other plant species, the flavonoid biosynthetic pathway of poplar is also conserved, but is rather more complex. For example, the majority of flavonoid biosynthetic enzymes are encoded by multiple genes in poplar, whereas in Arabidopsis these enzymes are encoded by single genes (Morreel et al., 2006; Veljanovski & Constabel, 2013). A number of poplar MBW complexes associated with flavonoid biosynthesis have been characterized and identified. Overexpression of MYB115/134 in poplar specifically promotes PA accumulation (Mellway, Tran, Prouse, Campbell, & Constabel, 2009; Wang et al., 2017), whereas MYB117/118/119 only activate anthocyanin biosynthesis under high-intensity light, wounding and other stresses (Cho et al., 2016; Wang et al., 2020). MYB6 is a multifunctional regulator that positively regulates anthocyanin and PA accumulation, and negatively regulates secondary wall formation (Wang et al., 2019). In addition to activators, some R2R3-MYB repressors have also been characterized. MYB57 and MYB182 reduce PA and anthocyanin accumulation by down-regulating structural and regulatory genes (Wan, Li, Ma, & Luo, 2017; Yoshida, Ma, & Constabel, 2015). MYB165/194 participate in additional processes, including inhibition of the accumulation of PAs, anthocyanins, hydroxycinnamic acid–tartaric acid esters, phenolic glycosides and shikimates (Ma et al., 2018). These R2R3-MYB TFs interact with several bHLH TFs (bHLH1/079/131) and the WD40 protein TTG1 to regulate flavonoid biosynthesis (James et al., 2017; Yoshida et al., 2015).
Although many comprehensive studies have showed the associations of transcription regulators with biotic and abiotic stresses (Dai et al., 2019; Wang et al., 2019), the interactions of poplar B-box proteins with biotic and abiotic stresses in the flavonoid pathway are largely unknown. Furthermore, genome-wide identification and analysis of the BBX TF family in poplar is incomplete, and BBX proteins that regulate flavonoids biosynthesis in response to environmental stress or phytohormone signalling have not been reported previously.
In this study, we identified 45 BBX TFs from the P. trichocarpa Torr. & A.Gray genome. The evolutionary relationships, gene structure, chromosomal distribution and genomic duplication of the BBX TFs were analysed. Expression of 16 candidate BBX genes associated with flavonoid metabolism were characterized in various tissues and under stress or phytohormone treatments by RNA sequencing (RNA-seq), and reverse-transcription quantitative real-time PCR (RT-qPCR) analyses. We demonstrated that BBX23 participated in the PA and anthocyanin biosynthesis pathway by regulating MYB TFs and structural genes, and that irradiation with high-intensity natural light (hereafter termed high light) enhanced their regulation. In addition, BBX23 interacted physically with HY5 to enhance this activation activity. Collectively, the present results provide insight into the roles and mechanisms of BBX TFs in the regulation of flavonoid biosynthesis in poplar.
2 MATERIALS AND METHODS
2.1 Plant materials and growth conditions
Populus trichocarpa (genotype “Nisqually 1”) plants were cultivated in glass bottles containing woody plant medium (WPM) before transfer to soil for stress or phytohormone treatment. Populus tomentosa Carrière clone 741 plants were used for stable genetic transformation experiments. Thirty-day-old seedlings of Nicotiana benthamiana were used as a leaf transient expression system.
Plants of P. trichocarpa and P. tomentosa were cultivated in glass bottles in a temperature–light gradient incubator at 25°C under a long-day (16 hr/8 hr, light/dark) photoperiod, 272 μmol m−2 s−1 supplemental light, and 55–65% relative humidity. All plants were grown in solid WPM. For stress-treatment experiments, P. trichocarpa plants were transferred to soil and cultivated in a controlled environment in a greenhouse (16 hr/8 hr [light/dark] photoperiod, 334 μmol m−2 s−1 supplemental light, 40–50% relative humidity, 25°C ± 2°C under light, and 21°C ± 2°C in the dark) and were treated when 2 months old.
Seeds of N. benthamiana were sterilized with 70% ethanol (30 s) and 1% sodium hypochlorite (10 min), and then germinated in plates on MS medium (Murashige & Skoog, 1962). After10 days, the seedings were transferred into soil and grown in a controlled environment in a greenhouse (16 hr/8 hr [light/dark] photoperiod, 334 μmol m−2 s−1 supplemental light, 40–50% relative humidity, 25°C ± 2°C under light, and 21°C ± 2°C in the dark) and were used for agroinfiltration when 30 days old.
2.2 Stress treatments
For treatment with high light, the poplar plants in the greenhouse were moved and exposed to full natural sunlight for 10 days during July in Fuzhou, Fujian province, China (maximum radiation 2004 m−2 s−1, maximum biologically effective UV-B radiation [UV-Bbe] 6.36 KJ m−2 d−1; average radiation 1,235 μmol m−2 s−1, average UV-Bbe 3.78 KJ m−2 d−1). The photosynthetically active radiation was measured with an illuminance meter (OHSP350P, Hopocolor, Zhejiang, China). The UV-Bbe was measured with a radiometer (IL2000, International Light, Newburyport, MA, USA) equipped with a model 254 nm photodetector (SED 240/NS254/W). Light intensity was measured at 08:00, 10:00, 12:00, 14:00, 16:00 and 18:00 during the days of the experiments. The illuminance meter was positioned above the poplar plants (close to the upper shoot tip) with five measurement points around the seedlings. Three measurements were recorded as technical replicates, and the average of all measurements considered to be the average radiation. Similarly, UV-Bbe was measured at the six time points during the day, and the data were normalized to 300 nm using weighting factors from the Caldwell action spectrum (Flint & Caldwell, 2003). For UV-B treatment, plants were irradiated under PL-S 9 W UV-B narrowband lamps (Philips, Amsterdam, Netherlands) with filters to block UV-C light. The plants were exposed to 0.18 KJ m−2 d−1 UV-Bbe before treatment with the UV-B lamps and to 1.44 KJ m−2 d−1 UV-Bbe during treatment (Mellway et al., 2009). For blue light treatment, plants were irradiated with Optimax OFK-450A LED lamps (Spectronics). For dark treatment, plants were transferred from the greenhouse to a temperature-light gradient incubator with a 24 hr dark photoperiod (Loreto et al., 2007). For cold treatment, plants were transferred to a temperature-light gradient incubator maintained at 4°C. For wounding treatment, young leaves were punctured with sterile pliers for approximately 30% of the total area of each leaf (Wang et al., 2017). For the high light, UV-B, blue light, dark, cold acclimation and wounding treatments, plants of uniform growth that were grown concurrently in the greenhouse were used as controls. For infection with the pathogen Alternaria alternata (Fr.) Keissler, selected leaves (3–5 leaves per line) were excised and inoculated as previously described (Huang, Liu, Jia, Fang, & Luo, 2012). For ABA treatment, a solution of 100 μM ABA dissolved in water was applied as described by Ullah et al. (2019). For JA treatment, a 1 M solution of the precursor methyl jasmonate dissolved in 0.1% (vol/vol) ethanol was applied. A volume of 3 ml was applied to the surface of three leaves at the uppermost nodes of each line (Jiang et al., 2014). The treated leaves were immediately covered with ultra-thin plastic film to retain moisture (Li, Brader, & Palva, 2004). An aqueous solution containing the same volume of 0.1% (vol/vol) ethanol was applied as the control. For salt treatment, plants in the greenhouse were irrigated with 150 mM NaCl every second day for 2 weeks; the control plants were irrigated with water (0 mM NaCl).
2.3 Phylogenetic, gene structure and conserved motifs analysis
A multiple sequence alignment comprising all identified BBX proteins in poplar and Arabidopsis was generated using ClustalW software. The alignment was used to construct a phylogenetic tree using the neighbour-joining (NJ) method with MEGA 7.0 software. TreeView 2 was used to visualize the phylogenetic tree.
The exon intron structure of P. trichocarpa BBX proteins was predicted using the Gene Structure Display Server (http://gsds.cbi.pku.edu.cn/) based on the coding sequence (CDS) and corresponding genomic sequence for each protein. The conserved B-box, CCT and other motifs were identified using the Multiple Em for Motif Elicitation (MEME) program.
The website PopGenIE (http://www.popgenie.org/) was used for chromosomal localization and mapping of all BBX TFs identified in the P. trichocarpa genome.
2.4 RNA isolation and reverse-transcription quantitative real-time PCR
Total RNA was extracted from various tissues of P. trichocarpa and P. tomentosa using the RNeasy Plant Mini Kit (QIAGEN, Beijing, China) following the manufacturer's instructions. Reverse-transcription PCR was performed using the NovoScript® Plus All-in-one first Strand cDNA Synthesis SuperMix (gDNA Purge, Novoprotein, Shanghai, China) to synthesize the first-strand cDNA. The cDNA was stored at −20°C and used subsequently as the template for gene cloning and RT-qPCR analysis. The RT-qPCR assay was performed in accordance with a previously published protocol (Li et al., 2013).
2.5 RNA-seq analysis
To perform RNA-seq analysis, young leaves or mixed samples of poplar plants were collected and frozen immediately in liquid nitrogen. Total RNA was isolated as described in the preceding section. Construction of the cDNA library, verification of the library quality and sequencing were performed by Biomarker (Beijing, China) using an Illumina HiSeq 4000 platform in accordance with standard protocols. The clean reads were mapped to the P. trichocarpa reference genome (Pertea, Kim, Pertea, Leek, & Salzberg, 2016; Yang et al., 2017). Differentially expressed genes (DEGs) were identified and annotated using the DESeq2 tool (Love, Huber, & Anders, 2014). The Kyoto Encyclopedia of Genes and Genomes (KEGG) database and PlantGSEA software were respectively used to annotate and analyse the biological functions of the DEGs (Yuan et al., 2015).
2.6 Cloning of BBX23 and its promoter
The full-length CDS (without the termination codon) of BBX23 was amplified and cloned into the plant binary vector pCAMBIA1302 containing the Cauliflower mosaic virus (CaMV) 35S promoter and the green fluorescent protein (GFP) reporter gene.
The upstream flanking sequence (~2000 bp) of the BBX23 protein was amplified by PCR with gene-specific primers (Table S1). The PCR products were cloned into the plant binary vector pCAMBIA1305 containing the histochemical β-glucuronidase (GUS) reporter gene.
The 35S:BBX23:GFP and proBBX23:GUS constructs were introduced into Agrobacterium tumefaciens strain GV3101.
2.7 Poplar transformation
Leaf discs from P. tomentosa sterile tissue-cultured seedlings were used for A. tumefaciens transformation as described previously (Li et al., 2015). Selected young leaf discs were infected with A. tumefaciens strain GV3101 harboring the appropriate construct (35S:BBX23:GFP, proBBX23:GUS, or BBX23-pYLCRIPSR/Cas9). Putative transgenic seedlings were selected on WPM supplemented with hygromycin (9 mg/L) or kanamycin (25 mg/L).
2.8 GUS activity assay
Various tissues from 2-month-old proBBX23:GUS-overexpressing transgenic poplar plants were collected for GUS staining following a previously described method (Li et al., 2015). The samples were soaked in GUS-staining solution for 20 min at 37°C, then the dye and chloroform were removed with 65% ethanol for 15 min at 65°C in the dark. The stained samples were stored in 75% ethanol before observation.
2.9 Measurement of flavonoids content
Fresh leaves (from the third to the sixth leaves, 0.5 g) of 3-month-old wild-type (WT) and transgenic poplar lines were harvested to measure the contents of flavonoid (anthocyanins, soluble PAs, insoluble PAs and flavonols). The butanol/hydrochloric acid assay (Porter, Hrstich, & Chan, 1985) was used for PA analysis. A methanol/ hydrochloric acid assay (Martin, Oswald, & Graham, 2002) was used to measure the anthocyanins content. Catechin, procyanidin B1 and cyanidin-3-O-glucoside were used to generate standard curves for soluble PAs, insoluble PAs and anthocyanins. Flavonols content was determined by reverse-phase high-performance liquid chromatography using an Essentia LC-16 HPLC (SHIMADZU, Kyoto, Japan) equipped with Shim-pack VP-ODS (4.6 × 150 mm, 5 μm) columns. Quercitrin, kaempferol and isorhamnetin were used to generate standard absorption curves. Section pretreatment, loading and quantities were performed as described previously (Luo et al., 2011; Yoshida et al., 2015).
2.10 Histological staining
Dimethylaminocinnamaldehyde (DMACA) reacts specifically with PAs and flavan 3-ol monomers to produce blue chromophores (Li, Tanner, & Larkin, 1996). Therefore, the intensity of DMACA staining reflects the inherent PA content of plant tissue. The fourth fresh leaf of 3-month-old poplar lines was stained with DMACA as described by Wan et al. (2017).
2.11 Yeast one-hybrid assay
Yeast one-hybrid (Y1H) assays were performed using the Matchmaker Gold Yeast One-Hybrid System Kit (TaKaRa) following the manufacturer's instructions. In brief, the promoter fragments (~2000 bp) were cloned and ligated into the pAbAi vector, and the CDS of BBX23 was ligated into the pGADT7 vector after PCR amplification. The pAbAi vector was linearized and transformed into yeast (Saccharomyces cerevisiae) strain Y1H Gold. Transformants were grown on SD/−LU medium to screen positive clones. The BBX23-pGADT7 prey vector was transformed into Y1H Gold cells harbouring the Bait-pAbAi and screened on SD/+A/−LU medium.
2.12 Yeast two-hybrid assay
Yeast two-hybrid (Y2H) assays were performed with the Matchmaker Gold Yeast Two-Hybrid System Kit (TaKaRa). The BBX23 fragments were cloned independently and ligated into the pGADT7 vector, whereas the HY5 fragments were independently ligated into the pGBKT7 vector. These recombinant plasmids were co-transformed into yeast strain Y2H Gold cells. The co-transformed yeast cells were cultured on SD/−LT medium. Positive clones were transferred to SD/−AHLT medium and 5-bromo-4-chloro-3-indolyl α-D-galactopyranoside (X-α-gal) was used as the substrate for transcriptional activity analysis.
2.13 Transient expression and dual-luciferase assay
The full-length CDS of BBX23 and HY5 was amplified and ligated into the pCAMBIA2300 vector driven by the CaMV 35S promoter and a mini CaMV 35S promoter. The two constructs were used as effectors for a dual-luciferase assay. The promoter fragments (~2000 bp) were independently cloned and ligated into the pGreen-0800-35mini vector (Hellens et al., 2005) to produce various luciferase (LUC) reporters. These constructs including firefly LUC and Renilla (REN) LUC were used as reporters for the assay. All vectors were individually transformed into A. tumefaciens strain GV3101.
The reporters and effectors were infiltrated simultaneously into N. benthamiana leaves as described in Methods S1 (supporting information S1). After incubation for 48 hr in the dark, the Dual-Luciferase Report Assay Kit (Promega) was used to analyse LUC activity with a GloMax® 20/20 luminometer (Zhang, Li, & Zhu, 2018). Relative expression levels were calculated based on the ratio of LUC/REN activity.
2.14 Bimolecular fluorescence complementation assay
For the bimolecular fluorescence complementation (BiFC) assay, the full-length CDS of BBX23 was cloned into the p2YN vector containing the N-terminal sequence of the enhanced yellow fluorescent protein (YFP), whereas the CDS of HY5 was cloned into the p2YC vector containing the C-terminal sequence of the enhanced YFP (Walter et al., 2004). The vectors were individually transformed into the A. tumefaciens strain GV3101.
The two vectors were infiltrated simultaneously into N. benthamiana leaves as described in Methods S1 (supporting information S1). Observation of the YFP signal was undertaken via the same method as that used for subcellular localization analysis. The excitation wavelength was 515 nm.
2.15 Chromatin immunoprecipitation–quantitative real-time PCR analysis
Three-month-old P. tomentosa plants expressing GFP tag were used for chromatin immunoprecipitation–quantitative real-time PCR (ChIP-qPCR) analysis. Tissue (about 0.1 g) of the third leaf from the shoot tip was cross-linked by vacuum inoculation, then ground in liquid nitrogen. The treated samples were added to sodium dodecyl sulfonate (SDS) lysis buffer for ultrasonic disruption (sonication for 10 s followed by an interval of 10 s, for a total of 15 min; repeated three times). Immunoprecipitation was performed with GFP and nonspecific preimmune mouse IgG antibodies (Saleh, Alvarez-Venegas, & Avramova, 2008). The promoter fragments containing the G-box element that were coimmunoprecipitated by the corresponding antibody were determined by RT-qPCR.
2.16 Primers
The gene-specific primers used in this study are listed in Table S1.
2.17 Accession numbers
The GenBank accession numbers for sequences generated in this study are listed in Table S2.
2.18 Statistical analysis
All data comprised at least three biological replicates. Statistical analysis between the control and test groups was performed using Student's t test program. Significant differences among data were evaluated using one-way ANOVA (*p < 0.05; **p < 0.01; ***p < 0.001). The statistical analysis for pairwise comparisons was conducted using Dunnett's test.
3 RESULTS
3.1 Identification and phylogenetic relationships of poplar BBX TFs
Thirty-two Arabidopsis BBX amino acid sequences were used as queries for a BLAST search of the P. trichocarpa genome database (version 3.0). A total of 45 BBX genes were identified. These genes were named PtrBBX1 to PtrBBX45 in the order of their accession number (Table S3). The deduced protein of the P. trichocarpa BBX TFs ranged in length from 95 to 514 amino acids, the predicted protein isoelectric point ranged from 3.95 to 9.3, and their molecular weight vary from 10,414.75 to 56,453.83 (Table S3). All 45 BBX proteins were predicted to be localized in the nucleus (Table S3). We also identified BBX TFs in other poplar species (38 in P. alba, 49 in P. deltoides, 38 in P. euphratica, 42 in P. tremula, 40 in P. tremuloides and 36 in P. tremula × P. tremuloides), and named these proteins based on the relevant genome databases (Table S3).
To evaluate the evolutionary relationships among the poplar and Arabidopsis BBX TFs, the amino acid sequences were used to construct a neighbor-joining (NJ) phylogenetic tree. The BBXs were resolved into five subfamilies, which were denoted I, II, III, IV and V (Figure 1a). In comparison to Arabidopsis, all poplar species possessed an expanded BBX family, which was approximately 1.5-fold larger in P. trichocarpa (Figure 1a and Table S3). Interestingly, expansion in the poplar species was biased and was concentrated mainly in subfamilies I, II, IV and V, whereas subfamily III was similar in size to that of Arabidopsis (Figure 1a and Table S3).
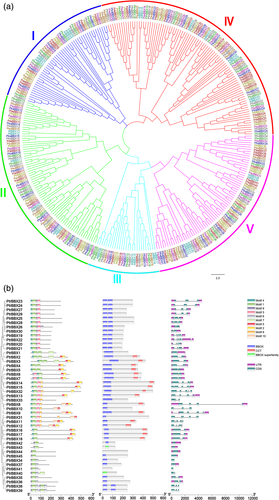
3.2 Gene structure, conserved motifs and synteny analysis
Analysis of the gene structure and conserved motifs of P. trichocarpa BBXs revealed that subfamilies I and II contained two B-box and one CCT domain, subfamily III contained one B-box and one CCT domain, subfamily IV contained two B-box domains and subfamily V contained one B-box domain (Figure 1b). Therefore, the number of conserved motifs in each BBX protein ranged from two to seven, while the full-length genomic sequences of the PtrBBX family ranged from 288 bp (PtrBBX41) to 11,258 bp (PtrBBX8), and the PtrBBX gene sequences contained 1–6 exons (Figure 1b).
Chromosomal location analysis revealed that 42 BBX genes were distributed on 16 of the 19 chromosomes, and three BBX genes (PtrBBX2/7/39) were mapped conclusively to unanchored scaffolds (Figure S1). Tandem and segmental duplication occurred frequently during evolution and expansion of the subfamilies. One tandem duplication cluster (PtrBBX34/40/41) was located on chromosome 4, whereas nine pairs of duplicated segments were identified (Figure S1). A comparative collinearity map was generated with Circos to further investigate the evolutionary relationships between PtrBBX and AtBBX, PtraBBX and PdeBBX family members (Figures S1 and S2). A total of 11 PtrBBX–AtBBX, 18 PtrBBX–PdeBBX and 24 PtrBBX–PtraBBX orthologous gene pairs were identified (Figures S1 and S2). This result suggests that the BBX proteins of P. trichocarpa, Arabidopsis, P. deltoides and P. tremula might share a common ancestor. In addition, positive selection analysis of 71 BBX gene pairs showed that the Ka/Ks ratios were all less than 1, and were less than 0.5 in 54 gene pairs (Table S3). Therefore, we presume that the P. trichocarpa BBX family has undergone strong purifying selection with limited functional divergence, which occurred after segmental duplication and whole-genome duplication.
3.3 Tissue-specific expression patterns of PtrBBX genes and in response to stress or Phytohormone treatment
We performed a RNA-seq experiment to analyse the expression profiles of the 45 BBX TFs under 10 stress or phytohormone treatments. The results reveal that 33 genes were significantly induced by various stimuli (Figure 2a and Data S1).
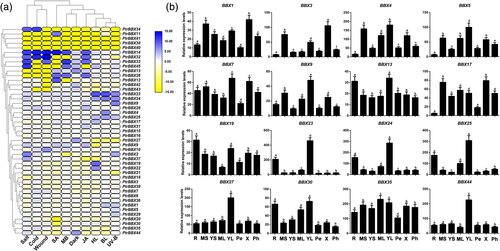
To further determine and confirm involvement of the BBX genes in the flavonoid pathway, the expression patterns of 16 BBX genes in different tissues were quantified by RT-qPCR analysis (Figure 2b). The 16 genes showed high relative expression levels in young leaves and roots, which was consistent with tissues associated with flavonoid biosynthesis and accumulation. PtrBBX23/24/25 showed tissue specificity in the root and young leaf, and PtrBBX27/44 showed specificity in the young leaf, whereas PtrBBX3/4/5/35 showed high expression levels in multiple tissues (Figure 2b). Expression of PtrBBX23/24/25 was induced under the 10 stress and phytohormone treatments, especially by high light, blue light and UV-B radiation (Figure 2a and Data S1), whereas PtrBBX27/44 was only induced following exposure to dark (Figure 2a and Data S1). Expression profiles of the BBX TFs suggest that PtrBBX23/24/25/27/44 may be involved in the flavonoid pathway, while the other BBX genes participate in multiple biological processes.
3.4 Transcriptional activity and subcellular localization of PtrBBX23/24/25/27/44
We examined the transcriptional activity of the BBX TFs using a yeast trans-acting activity system (Li, Ma, Yu, Fu, & Luo, 2018). The yeast harbouring the fusion proteins GAL4BD:PtrBBX23/24/25 survived on SD/−AHT medium and exhibit X-α-gal activity, whereas yeast carrying the fusion proteins GAL4BD:PtrBBX27/44 did not. These results suggest that PtrBBX23/24/25 were transcriptional activators, whereas PtrBBX27/44 were transcriptional repressors (Figure S3a).
We next used a tobacco leaf transient expression system (Sparkes, Runions, Kearns, & Hawes, 2006) to determine the subcellular localization of the BBX proteins. The expression of GFP tagged fusion proteins, (PtrBBX23/24/25/27/44:GFP) in this system showed their localization in the nucleus (Figure S3b), which was consistent with the bioinformatics prediction (Table S3).
3.5 Response of BBX23 to high light and effects on PAs and anthocyanins
To verify that PtrBBX23/24/25 responds to stress treatments, 2-month-old P. trichocarpa WT plants were subjected to stress treatments, comprising high light, salt, cold, wounding, blue light and UV-B radiation for different time periods. A RT-qPCR analysis revealed that PtrBBX23/24/25 expression was induced by all six treatments but with variation in the stress responses (Figures 3b and S4–S6). PtrBBX23 and PtrBBX24 respectively exhibited approximately 13- and 6-fold up-regulation under high light (Figures 3b and S5), whereas PtrBBX25 was significantly induced (more than six-fold) by cold and UV-B radiation (Figure S6). In comparison to the potential multifunctional factor PtrBBX25, PtrBBX23/24 appeared to mainly participate in light signalling mechanism. We, therefore, selected PtrBBX23 for further investigation based on its high expression level.
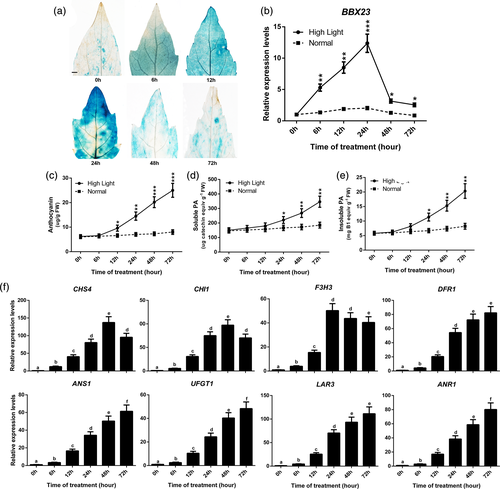
The WT P. tomentosa was transformed with the ProBBX23:GUS construct to determine BBX23-GUS fusion activity. A strong GUS signal was predominantly observed in young leaves and roots (Figure S7a,c), and an extremely weak signal was detected in mature leaves, stems and petioles (Figure S7b,d,e). Subsequent GUS staining was observed in the vessels of leaves (Figure S7a,b), the vascular bundles of roots (Figure S7c) and the cambium of stems and petioles (Figure S7d,e). Hence, we further analysed the expression patterns of PtrBBX23 and flavonoids structural genes in young leaves of transgenic poplar under exposure to high light. GUS activity was detected after 6 hr of treatment and increased continuously until 24 hr of treatment, thereafter it decreased until the end of the experiment at 72 hr (Figure 3a).
Similar results were observed by RT-qPCR analysis of the third leaf of 2-month-old WT P. trichocarpa seedlings under exposure to high light treatment (Figure 3b). Anthocyanins began to accumulate upon with exposure to high light for 12 hr and thereafter increased continuously, whereas anthocyanins barely accumulated throughout the experimental time period in the control (Figure 3c). Accumulation of soluble and insoluble PAs showed a similar trend to anthocyanins and was detected 24 hr of high light (Figure 3d,e). A RT-qPCR analysis showed that the majority of PA and anthocyanin biosynthetic structural genes (CHS4, CHI1, F3H3, DFR1, ANS1, UFGT1, LAR3 and ANR1) were strongly up-regulated after 24 hr of treatment, which was consistent with accumulation of PA and anthocyanin (Figure 3f). Therefore, we speculate that PtrBBX23 plays an essential role in high-light-mediated PA and anthocyanin synthesis.
3.6 BBX23 positively regulates accumulation of PAs and anthocyanins
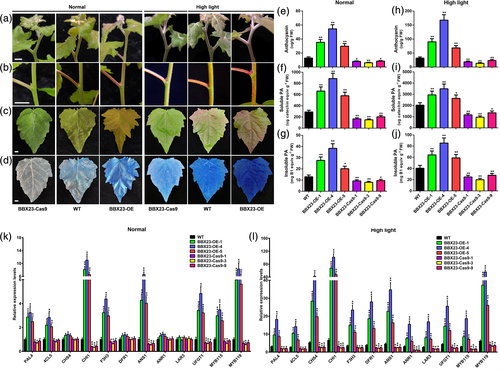
To clarify the biological function of BBX23, transgenic P. tomentosa BBX23-overexpression (BBX23-OE) and BBX23-knockout lines were generated via Agrobacterium-mediated transformation. Transformants were identified by PCR amplification of the hygromycin resistance gene (Figure S8a). Three independent BBX23-OE lines (L1, L4 and L5) that showed elevated BBX23 expression levels were selected for further experiments (Figure S8c). PCR amplification and sequence analysis of 15 BBX23-pYLCRIPSR/Cas9 (BBX23-Cas9) transgenic lines demonstrated that Cas9-sgRNA successfully generated heritable mutations of BBX23. Lines L1, L3 and L9, which showed high mutagenetic efficiencies (96%, 100% and 96%, respectively), were used for further analysis (Figure S8b). The BBX23-Cas9 poplar lines showed a sporadic single-base substitution in the T1 region of BBX24, but no frameshift mutation resulted in comparison with the homologous BBX24 (Figure S9). The lines chosen for further analysis harboured these single-base substitutions. Although these changes were unlikely to disrupt the function of BBX24 fully, it cannot be ruled out that BBX24 function was compromised to a certain degree in the lines selected.
The shoots, stems and young leaves of BBX23-OE lines were pigmented a more intense red than those of WT P. tomentosa in the greenhouse, whereas BBX23-Cas9 lines were greener than the WT (Figure 4a–c). After transfer outdoors and upon exposure to natural high light for 10 days, this phenotypic difference was enhanced. The intensity of red pigmentation of all plants increased, especially in stems and young leaves (Figure 4a–c). The red pigmentation of BBX23-OE lines covered almost the entire leaf and stem surfaces, whereas the BBX23-Cas9 lines showed only faint colouration (Figure 4a–c). Staining with DMACA revealed that the strongest PA accumulation was detected in BBX23-OE lines, whereas BBX23-Cas9 lines showed the weakest pigmentation (Figure 4d). However, exposure to high light, greatly enhanced the intensity of blue pigmentation in all materials and BBX23-OE lines showed blue-black colouration, in contrast to the other materials analysed (Figure 4d). The BBX23-OE lines showed a significant increase, whereas BBX23-Cas9 lines showed a significant decrease, in accumulation of anthocyanins and PAs compared with those of the WT (Figure 4e–g). High light enhanced accumulation of these secondary metabolites in all lines, among which BBX23-OE lines accumulated the highest quantities, followed by WT and BBX23-Cas9 lines (Figure 4h–j). The flavonols biosynthetic pathway shares many common intermediates and enzymes with anthocyanins and PAs. Hence, we analysed flavonols (quercetin, kaempferol and isorhamnetin) contents in the transgenic lines, but no significant differences under normal and high light treatment were observed (Figure S10a–f). These results indicate that BBX23 promotes accumulation of PAs and anthocyanins, and high light enhances this stimulation, but no significant effect on flavonols content was detected.
3.7 BBX23 promotes expression of structural genes and MYB activators
To identify the genes regulated by BBX23 TF, we performed RNA-seq and compared gene expression profiles of the WT and BBX23-OE transgenic lines. In total, 2,357 DEGs (1,361 up-regulated, 996 down-regulated) were detected in BBX23-OE lines under normal light (Figures 5a, S11a, and Data S2). After exposure to high light, 2,510 DEGs (1829 up-regulated, 681 down-regulated) were detected in BBX23-OE lines (Figures 5a, S11b, Data S2). Of these genes, 795 DEGs were detected under both normal and high light treatment, and 1715 high-light-specific DEGs were detected in the BBX23-OE lines (Figure 5a and Data S2). Among the 795 DEGs, 56.4% were up-regulated in BBX23-OE lines under normal light, and 74.2% were up-regulated under high light treatment (Figure S12).
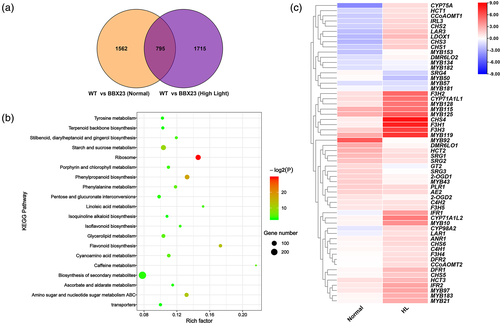
We functionally annotated and classified these 4,072 DEGs. A KEGG enrichment analysis revealed that the DEGs were mainly enriched in the pathways for flavonoids biosynthesis, phenylpropanoid biosynthesis, phenylalanine metabolism, ABC transporters and caffeine metabolism (Figures 5b, S13, and Data S2). Compared with those under normal light, the gene clusters in the flavonoid and phenylpropanoid biosynthesis pathways showed significant changes under exposure to high light (Figures 5b, S13, and Data S2). These results indicate that BBX23 may regulate flavonoid biosynthesis through these genes.
A heat map was generated for the enriched DEGs involved in flavonoid and phenylpropanoid biosynthesis. Nineteen of the 32 DEGs enriched under normal light were up-regulated, whereas 39 of the 43 DEGs enriched under high light treatment were up-regulated (Figure 5c and Data S2). Among structural genes and MYB TFs, MYB115, MYB119, F3H2/3, PLR1 and CYP71A1L1 were significantly up-regulated in the BBX23-OE lines under normal and high light, whereas only MYB57 was down-regulated under both treatments (Figure 5c and Data S2). CHS1/2/3/4/5, LAR2/3, LDOX1, DFR1, IRL3, IFR1/2, F3H1/4/5, ANR1, DFR2, CYP71A1L2, MYB97 and MYB183 were up-regulated in BBX23-OE lines in response to high light (Figure 5c and Data S2). MYB182 was down-regulated under normal light, whereas its homolog was down-regulated under high light (Figure 5c and Data S2).
A RT-qPCR analysis was performed to verify expression of these DEGs and other flavonoid biosynthetic genes. Most structural genes were distinctly up-regulated in the BBX23-OE lines, especially CHI1, F3H3, ANS1 and UFGT1, and these genes were significantly down-regulated in the BBX23-Cas9 lines (Figure 4k). Expression of MYB115 and MYB119 was significantly increased in the BBX23-OE lines and decreased in the BBX23-Cas9 lines (Figure 4k), whereas MYB57, MYB165 and MYB182 showed the opposite trend (Figure S14a). Expression of all structural genes and MYB TFs in all plant materials changed strongly with high significance under high light (Figures 4l and S14b). Among these genes, CHS4, DFR1, ANR1 and LAR3 were significantly induced by high light (Figure 4l). Of the plant materials, the BBX23-OE lines responded most rapidly and strongly to high light, followed by the WT, and the BBX23-Cas9 lines showed the weakest response (Figures 4l and S14b). Expression of FLS, which is a flavonol-specific biosynthetic gene, showed no change in transgenic poplar (Figure S10g). In general, the RT-qPCR analysis results were consistent and further validated the RNA-seq data.
Another important branch of general phenylpropanoid biosynthesis is the lignin biosynthesis pathway. Interestingly, many DEGs were annotated and classified as lignin biosynthesis, including C4H1/2, HCT1/2/3, CCoAOMT1/2, CYP75A/98A2, MYB10, MYB92/125 and MYB10/128 (Figure 5c and Data S2). These data further showed that BBX23 was involved in other biological processes in addition to the regulation of PAs and anthocyanin biosynthesis.
3.8 BBX23 interacts with HY5 in vitro and in vivo
The bZIP TF HY5 often functions as a partner of BBXs to regulate photomorphogenesis, seedling development and light signal transduction (Holtan et al., 2011; Xu, 2019; Zhang et al., 2017). After 6 hr of high light exposure, the transcripts of HY5 were significantly increased in the third leaves and continuously increased until 12 hr of treatment, thereafter decreasing until the end of treatment (Figure S15). The results suggest that HY5 participate in the regulation of high light responses. We performed Y2H and BiFC assays to verify BBX23–HY5 interaction in poplar. In the Y2H assay, the yeast cells harbouring full-length BBX23-AD and HY5-BD grew on SD/−AHLT medium and showed X-α-gal activity (Figure 6a). In the BiFC assay, YFP fluorescence was detected in the nuclei of tobacco cells co-transformed with BBX23-nYFP and HY5-cYFP (Figure 6b).
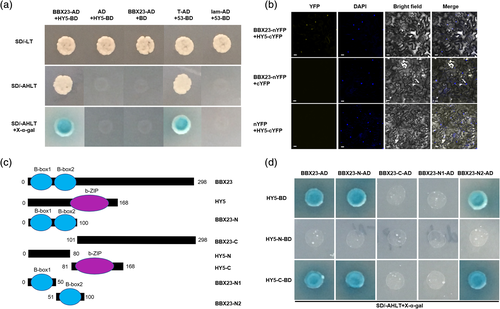
To confirm the interaction response domains of BBX23 and HY5, we truncated the BBX23 protein into four fragments and split the HY5 protein into two fragments in accordance with their conserved motifs (Figure 6c). The fragment BBX23-N2 containing the B-box2 domain was able to interact with HY5-C harbouring a bZIP domain. Interestingly, BBX23-N1, which also contained the B-box1 domain, could not interact with HY5 (Figure 6d). These results suggest that the B-box2 domain of BBX23 and the bZIP domain of HY5 are responsible for interaction of the proteins.
3.9 BBX23 directly binds to the promoters of MYB TFs and structural genes
To explore the specific binding of BBX23 to potential downstream genes, Y1H and dual-LUC assays were performed. Based on the results of RNA-seq and RT-qPCR analysis (Figures 4, 5 and S14), R2R3-MYB TFs and structural genes that were significantly up-regulated in BBX23-OE transgenic plants were selected for further assays. The Y1H yeast strains containing recombinants of BBX20-AD with proCHS4/CHI1/F3H3/DFR1/ANS1/UFGT1/MYB115/MYB119-AbAi successfully grew on SD/+A/−LU medium (Figure 7a).
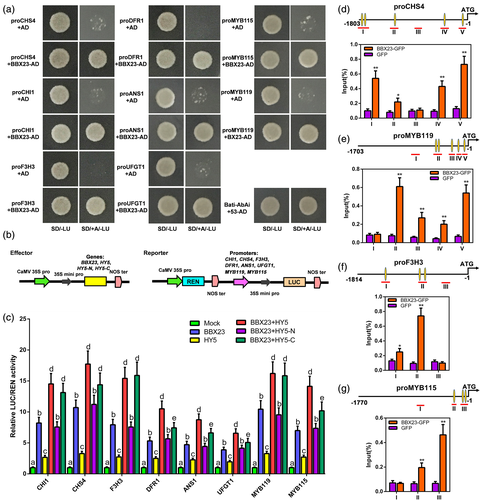
Next, we transiently co-expressed 35S:BBX23 and the specific promoters of these genes in tobacco leaves (Figure 7b). BBX23 indeed activated these reporters in living plant cells (Figure 7c). Given the interaction of BBX23 and HY5, we co-expressed 35S:BBX23, 35S:HY5 and their specific promoters. Their physical interaction enhanced the expression of downstream genes, especially the transcript levels of CHS4/F3H3 and MYB115/MYB119 (Figure 7c). In addition, the bZIP domain at the C-terminus of HY5 played a crucial role in their physical interaction and jointly activated expression of the reporter genes (Figures 6d and 7c).
G-box domains are the binding sites of BBX TFs to their downstream promoters (Chang et al., 2011; Zhang et al., 2017). A search of the PlantCARE database revealed that the promoters of CHS4/F3H3 and MYB115/MYB119 contained several putative G-box elements (Figure S16). A ChIP-qPCR assay showed that BBX23 directly bound to these G-box elements in the promoters of CHS4, F3H3, MYB115 and MYB119 (Figure 7d–g).
Taken together, these results indicate that BBX23 was able to bind directly to the promoters of MYB TFs and structural genes to activate their transcription, thereby promoting the accumulation of PAs and anthocyanins.
4 DISCUSSION
4.1 Characterization of the BBX gene family in poplar
The BBX TF family plays essential roles in a variety of cellular and developmental processes, including photomorphogenesis, skotomorphogenesis, signal transduction and responses to various phytohormones (Gangappa et al., 2013; Xu, 2019; Zhang et al., 2017). However, BBX TFs have not been studied previously in poplar. In this study, 45 BBX genes, classified into five subfamilies, were identified in the P. trichocarpa genome (Figure 1a, Table S3). Each P. trichocarpa BBX protein contained at least one conserved BBX motif (Figure 1b). Notably, phylogenetically related BBX TFs in poplar showed greater structural similarities in the arrangements of exons and introns, and conserved motifs (Figure 1b). These results are consistent with the BBX structural architecture of other plant species (Huang, Zhao, Weng, Wang, & Xie, 2012; Wei et al., 2020).
Poplar has undergone at least three genome duplication events, followed by transposition events and tandem and segmental duplication (Tuskan et al., 2006). Gene duplication and co-synteny analysis showed that segmental duplication may have been more important than tandem duplication in poplar BBX expansion (Figures S1 and S2). In addition, Ka/Ks ratios revealed that the poplar BBX family showed limited functional divergence owing to strong selection pressure for purification (Table S3). These results allowed us to make preliminary predictions of the functions of poplar BBX members, based on their Arabidopsis homologs and the results of previous studies.
4.2 BBX proteins respond to biotic and abiotic stresses and influence flavonoid accumulation
Flavonoids perform multiple functions during plant growth and development, and can be induced by diverse environmental and nutritional cues, pathogen attack and phytohormones (Cominelli et al., 2008; Yuan, Leng, & Wang, 2016). To elucidate the molecular mechanism of flavonoid accumulation in poplar, RNA-seq analysis was conducted on P. trichocarpa WT seedlings subjected to 10 stress or phytohormone treatments. Interestingly, 73% (33) of the BBX family members were induced by single or multiple factors (Figure 2a and Data S1), which suggests that the BBX family may participate in the regulation of flavonoid biosynthesis.
Flavonoids are abundant in poplar and commonly accumulate in the leaves, roots and bark (Gourlay, Ma, Schmidt, & Constabel, 2020; Yoshida et al., 2015). In addition, anthocyanins accumulate mainly in young leaves (Cho et al., 2016), whereas PAs and other phytochemicals are enriched in mature leaves (Ma et al., 2018). We confirmed these findings by tissue-specific expression analysis (Figure 2b). Furthermore, RNA-seq analysis of 16 BBX proteins (Figure 2b) revealed that some responded to stresses that perturbed flavonoids accumulation, and showed temporal and spatial specificity in young leaves and roots.
4.3 BBX23 responds to high light and promotes PA and anthocyanin biosynthesis
Light is required for photomorphogenic processes in poplar, including flavonoids production, hypocotyl elongation and lateral root development (Dash et al., 2017; Mellway et al., 2009). We confirmed that high light is an important environmental factor in MYB-mediated PA and anthocyanin biosynthesis (Wan et al., 2017; Wang et al., 2017). In the present study, we aimed to characterize and isolate the BBX genes that promote flavonoid accumulation, especially PAs and anthocyanins. Based on the combination of results from bioinformatics, RNA-seq and RT-qPCR data, we observed that BBX23/24/25 were transcriptional activators, whereas BBX27/44 were transcriptional repressors (Figure S3). BBX23/24 are the homologs of AtBBX22/23 and BBX25 is the homolog of AtBBX20/21 (Figure 1).
AtBBX20/21/22/23 are positive regulators of photomorphogenesis, and influence light-signal-mediated anthocyanin accumulation by promoting expression of MYB TFs and structural genes (Chang et al., 2008, 2011; Wei et al., 2016; Xu et al., 2018). In pear, their homologs PpBBX16/PpBBX18 positively regulate light-induced anthocyanin accumulation by activating PpMYB1/10 and downstream structural genes (Bai, Tao, Tang, et al., 2019; Bai, Tao, Yin, et al., 2019). In apple, the homologs MdBBX20/MdBBX22 also promote anthocyanin accumulation under UV-B radiation and low-temperature stress (An et al., 2019; Fang et al., 2019). Given the functional conservation of BBXs, we speculate that BBX23/24/25 also promote anthocyanin biosynthesis via light signalling. In addition, MYB25 showed greater sensitivity to UV-B radiation and cold treatment (Figure S6), whereas BBX23/24 predominantly respond to high light exposure (Figures 3a,b, S4 and S5). BBX23 showed a similar expression pattern to that of BBX24 (Figures 2, 3, S4 and S5) but with significantly higher expression levels, which indicates that BBX23 is more suitable for studying the high light-mediated flavonoid pathway.
Strong GUS activity was predominantly observed in young leaves and roots of P. tomentosa transformed with the ProBBX23:GUS construct (Figure S7), which was consistent with RT-qPCR analyses (Figure 2b). In P. trichocarpa WT seedlings, BBX23 was significantly induced under high light treatment (Figure 3b), and the anthocyanin content rose accordingly (Figure 3c). Overexpression of BBX23 induced anthocyanin accumulation, whereas BBX23 knockout decreased anthocyanin content (Figure 4a–c,e). We also demonstrate that high light is an important environmental factor in BBX23-mediated anthocyanin biosynthesis, and enhances the phenotypic changes and accumulation of secondary metabolites (Figure 4a–c,h).
Notably, unlike its homologs in Arabidopsis, pear and apple, BBX23 was involved in the regulation of PA biosynthesis. In response to high light, the PA content in P. trichocarpa WT seedlings increased significantly with upregulation of BBX23 (Figure 3d,e). Staining with DMACA and measurement of PA content showed that overexpression of BBX23 resulted in a significant increase in total PA content (Figure 4d,f,g). By contrast, BBX23-knockout lines exhibited significantly reduced PA content (Figure 4d,f,g). Similarly, high light promoted PA accumulation in all poplar plant materials generated, especially in BBX23-OE lines (Figure 4d,i,j). Meanwhile, ANR1 and LAR3, two enzymes specific to PA biosynthesis, were induced significantly by high light (Figure 4k,l). Taken together, these results suggest that BBX23 is conserved together with its homologous genes in the regulation anthocyanin synthesis, but shows functional differentiation in PA biosynthesis.
4.4 BBX23 interacts with HY5 and jointly promotes expression of PA and anthocyanin structural genes and MYB TFs
BBX TFs usually exert flavonoid regulation via downstream genes (Bursch et al., 2020; Chang et al., 2008). Indeed, both RNA-seq and RT-qPCR analyses in the present study showed that the majority of structural genes in the flavonoid pathway were significantly activated in BBX23-OE transgenic lines, and certain MYB transcription activators (MYB115/119/MYB134) were also up-regulated (Figures 4k,l, 5c and S14 [supporting information S1]). Moreover, Y1H, dual-luciferase and ChIP-qPCR assays confirmed that BBX23 was able to bind directly to the promoters of these structural genes and MYB TFs (Figure 7). We conclude that BBX23-mediated anthocyanin biosynthesis follows the same pattern as in other plant species, and that PAs synthesis is completed by activation of downstream genes and TFs. Interestingly, BBX23 also inhibited the expression of several MYB repressors (Figures 5c, S14, and Data S2), which indicates that intermediate factors may be involved in the BBX23-mediated PA and anthocyanin pathway.
Plants often use HY5 as a modulator of hypocotyl elongation, anthocyanin biosynthesis and chlorophyll accumulation (Burko, Gaillochet, Seluzicki, Chory, & Busch, 2020; Gangappa & Botto, 2016). For example, in Arabidopsis, HY5 interacts directly with AtBBX20/21/22 and appear to be required for their functions (Bursch et al., 2020; Datta et al., 2007, 2008; Wei et al., 2016). Similarly in pear and apple, HY5 also directly interacts with BBX TFs to promote anthocyanin accumulation (Bai, Tao, Yin, et al., 2019; Fang et al., 2019). We observed that BBX23 was capable of direct interaction with HY5 (Figure 6a,b), and their interaction promoted the expression of downstream structural genes and TFs (Figure 7b,c). Furthermore, the second B-box domain of BBX23 and the bZIP domain of HY5 were essential for their interaction (Figures 6c,d, 7b,c). Recently, HY5 was found to be strongly induced in the fourth leaves of tomato when the fifth to eighth leaves were exposed to a high light level of 1,500 μmol m−2 s−1 (Jiang et al., 2020). We also demonstrate that PtrHY5 can respond to high light (Figure S15). To the best of our knowledge, this is the first report to link high light, BBX genes and HY5 in relation to the regulation of anthocyanin biosynthesis. Interestingly, the transcript of PtrHY5 peaked earlier than PtrBBX23 and structural genes of PA and anthocyanin biosynthesis when exposure to high light (Figures 3b,f and S15), which suggesting that HY5 and BBX23 synergistically regulate the PA and anthocyanin synthesis with a complex mechanism. Therefore, we propose that the full function of BBX23 might require the assistance of additional regulators, such as HY5. The RNA-seq analysis data for BBX23-OE lines revealed that many additional KEGG pathways were enriched (Figures 5b, S13, and Data S2), which suggest that BBX23 may also function in other biological processes.
4.5 Potential functions of BBX23 and other BBX family members in flavonoid pathway
In addition to high light, BBX23 responded to other stress treatments, including UV-B radiation, cold, blue light and wounding (Figures 2a, S4, and Data S1). Apple MdBBX22, which is the homolog of BBX23, regulates UV-B-induced anthocyanin biosynthesis (An et al., 2019). These results suggest that BBX23 may also influence PA and anthocyanin biosynthesis in a high light-independent manner. Likewise, BBX24 is a homolog of BBX23 (Figure 1), and showed similar tissue-specific expression patterns (Figure 2b) as well as responses to stress (Figures 2a, S4, S5, and Data S1) compared with those of BBX23. In addition, BBX25 may be mainly involved in the regulation of anthocyanin accumulation in a UV-B- and cold- dependent manner compared with BBX23/24, (Figures 2a, S6, and Data S1), consistent with its homolog in apple (Fang et al., 2019). On the other hand, BBX27 and BBX44 may negatively regulate anthocyanin biosynthesis by suppressing HY5 transcriptional activity due to their self-activation, evolutionary relationship and expression patterns (Figures 2 and S3; Holtan et al., 2011; Jiang et al., 2012). Similarly, BBX1/3/4/5/7/9/13/17/19/30/35 may also be involved in flavonoid biosynthesis through different regulatory pathways and environmental factors (Figure 2).
ACKNOWLEDGMENTS
We thank Dr. Yingming Fan (Beijing Forestry University, China) for the provision of fungal strain Alternaria alternata (Fr.) Keissler and sample preparation. We thank Dr. Lijun Wang (Southwest University of Science and Technology, China) for extraction of anthocyanins, PAs and flavonols. We are grateful to Mrs. Xia Wang (Southwest University, China) for support and helpful comments. This work was supported by the JSPS Grant-in-Aid for Young Scientists 20K15553 (Chaofeng Li) and Forest Science Peak Project of College of Forestry, Fujian Agriculture and Forestry University (No. 71201800701).
CONFLICT OF INTERESTS
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Chaofeng Li and Chunlan Lian designed the work; Chaofeng Li, Jinli Pei, Xin Yan, Xin Cui, Momi Tsuruta, and Ying Liu performed experiments and analysed data; Chaofeng Li and Jinli Pei drafted the manuscript; Chaofeng Li and Chunlan Lian revised the manuscript. Chaofeng Li and Jinli Pei should be considered joint first author. Chaofeng Li and Chunlan Lian should be considered joint senior author.
Open Research
DATA AVAILABILITY STATEMENT
All data that support the findings of this study are available from the corresponding author upon reasonable request.




