The transcriptional repressor OsPRR73 links circadian clock and photoperiod pathway to control heading date in rice
Funding information: National Basic Research Program of China, Grant/Award Number: 2016YFD0100802; National Natural Science Foundation of China, Grant/Award Numbers: 31601283, 31701054
Abstract
The phase transition from vegetative to reproductive growth is triggered by internal and external signals that participate in circadian clock in plants. We identified a rice floral inhibitor OsPRR73 encoding a CONSTANS protein. Overexpression of OsPRR73 resulted in late heading under both long-day (LD) and short-day (SD) conditions. Knockout mutants led to early heading under LD conditions but no change under SD. OsPRR73 mRNA accumulated at noon and exhibited a robust oscillation under constant light (LL) and constant darkness (DD) conditions. OsPRR73 overexpression exerted negative feedback on endogenous OsPRR73 expression and altered diurnal expressions of key flowering genes and circadian clock genes. OsPRR73 bound to the promoters of the floral gene Ehd1 and the circadian gene OsLHY, and significantly suppressed their expression at dawn. In LL and DD, the oscillatory patterns of the circadian genes OsLHY, OsTOC1, OsGI and OsELF3 were varied in OsPRR73OX and osprr73 mutants. OsPRR73 expression was decreased in osphyb mutants, and overexpression of OsPRR73 complemented the early heading date phenotype of osphyb, indicating OsPRR73 works downstream of OsPhyB. Therefore, OsPRR73 is involved in a feedback loop of the rice clock and connects the photoperiod flowering pathway by binding to the Ehd1 promoter in rice.
1 INTRODUCTION
Life on Earth must adapt to environmental rhythms, such as light–dark cycles and temperature oscillations, due to the planet's rotation. Circadian clocks are the endogenous timekeeping networks that ensure organisms are able to adapt to periodic changes and have prominent effects on the coordination of multiple physiological processes, termed output pathways (Nohales & Kay, 2016).
The working mechanism of the plant circadian clock has been well studied in Arabidopsis thaliana, revealing a complicated circuit that is composed of multiple transcriptional–translational feedback loops (Greenham & McClung, 2015; McClung, 2019). The core loop consists of two MYB-related transcription factors, CIRCADIAN CLOCK-ASSOCIATED 1 (CCA1) and LATE ELONGATED HYPOCOTYL (LHY), which are expressed near dawn, and TIMING OF CAB2 EXPRESSION 1 (TOC1), a member of the PSEUDO-RESPONSE REGULATOR (PRR) family that is expressed near dusk. CCA1 and LHY directly repress TOC1 expression by binding the evening element (EE) located in the promoter of TOC1 (Alabadí et al., 2001). TOC1 is a DNA-binding transcriptional repressor of CCA1 and LHY (Gendron et al., 2012; Huang et al., 2012). Additional interconnected loops are composed of clock-associated genes regulating the core components. There are five PRR genes in Arabidopsis, designated PRR9, PRR7, PRR5, PRR3 and PRR1 (TOC1); these peak in succession at approximate 2-hr intervals, with PRR9 expression peaking early in the morning and PRR1/TOC1 expression peaking early in the evening (Matsushika, Makino, Kojima, & Mizuno, 2000). PRR9, PRR7 and PRR5 also function as transcriptional repressors like PRR1/TOC1 in the Arabidopsis clock that directly associate with the promoters of CCA1 and LHY (Farré, Harmer, Harmon, Yanovsky, & Kay, 2005; Nakamichi et al., 2010). PRR9 and PRR7 are direct transcriptional targets of CCA1 and LHY (Adams, Manfield, Stockley, & Carré, 2015; Kamioka et al., 2016). The core component TOC1 represses LUX ARRHYTHMO (LUX) in the evening (Gendron et al., 2012; Huang et al., 2012). The EARLY FLOWERING 3 (ELF3), ELF4 and LUX proteins, which form the Evening Complex (EC), are under negative control by CCA1 and LHY (Lu et al., 2012) and in return repress the morning-expressed proteins PRR9 and PRR7 (Dai et al., 2011; Dixon et al., 2011; Helfer et al., 2011; Herrero et al., 2012; Nusinow et al., 2011). CCA1, LHY and TOC1 reduce GIGANTEA (GI) expression, and in turn, GI induces CCA1 and LHY expression through an unknown mechanism (Adams et al., 2015; Huang et al., 2012; Kim et al., 2013; Lu et al., 2012). Linking these loops, Arabidopsis is able to finely adjust its responses with regard to multiple physiological processes during a diurnal cycle. The same way of adaptability is supposed to be in other plants' clocks, while there still remains a largely unknown in the mechanism.
Flowering time control is an essential output pathway of circadian clock and is an important agronomic trait in rice that exhibits a significant response to day length (Andrés & Coupland, 2012). The photoperiodic pathway is the major pathway involved in the regulation of heading date in rice because it is not sensitive to the vernalization process (Song, Shim, Kinmonth-Schultz, & Imaizumi, 2015). The photoperiodic pathway is generally conserved between rice and Arabidopsis. CONSTANS (CO) directly activates FLOWERING LOCUS T (FT), functioning as a florigen that is crucial for flowering in Arabidopsis (Amasino, 2010; Kobayashi & Weigel, 2007; Samach et al., 2000). OsGI, an ortholog of GI, is rhythmically expressed and activates expression of Heading Date 1 (Hd1), which is the ortholog of Arabidopsis CO, under both short days (SDs) and long days (LDs) (Hayama, Yokoi, Tamaki, Yano, & Shimamoto, 2003). Grain number, plant height, and heading date 7 (Ghd7) encodes a CCT domain protein and delays flowering by repressing Early heading date 1 (Ehd1) under LDs (Xue et al., 2008). Ehd1 is a grass-specific B-type response regulator that promotes flowering by upregulating Hd3a and RFT1, another ortholog of FT, under both SD and LD conditions (Doi et al., 2004; Komiya, Yokoi, & Shimamoto, 2009). Recent studies have revealed that Hd1 represses Ehd1 expression by interacting with GHD7 and GHD8, a putative HEME ACTIVATOR PROTEIN 3 (HAP3)/NF-YB subunit of a CCAAT-box binding transcription factor (Goretti et al., 2017; Nemoto, Nonoue, Yano, & Izawa, 2016; Zhang et al., 2017).
In addition, previous studies have shown that the circadian clock plays an essential role in regulating the key agricultural trait flowering time (Hsu & Harmer, 2014). For example, LATE BLOOMER1 of pea, is a GI ortholog and promotes flowering initiation (Hecht et al., 2007). The Photoeriod-1 gene, a homolog of PRR7, is involved in flowering regulation in barley (Hordeum vulgare) and wheat (Triticum aestivum) (Shaw, Turner, & Laurie, 2012; Turner, Beales, Faure, Dunford, & Laurie, 2005). SbPRR37, a PRR7 homolog in sorghum (Sorghum bicolor), controls photoperiodic flowering (Murphy et al., 2011). Mutation of EAM8, a homolog of ELF3 in barely, causes a delayed flowering phenotype (Faure et al., 2012). In rice, there are homologs of most Arabidopsis clock genes (Murakami, Tago, Yamashino, & Mizuno, 2007; Song, Ito, & Imaizumi, 2010). Osgi mutants exhibit late flowering under SD conditions, and OsGI confers robust diurnal rhythms in the field (Izawa et al., 2011). OsELF3 promotes flowering by repressing Ghd7 at the transcriptional regulation level and maintains circadian accumulation by interacting with HAF1 under LDs (Yang, Peng, Chen, Li, & Wu, 2013; Zhao et al., 2012; Zhu et al., 2018). Ghd7.1/OsPRR37 represses expression of Ehd1 and Hd3a under LDs (Koo, Yoo, Park, Kwon, & Lee, 2013; Liu et al., 2018; Yan et al., 2013). Thus, photoperiodic flowering control in rice is tied to an endogenous circadian clock. However, it still remains largely unknown between rice circadian clock and photoperiod pathway in flowering control.
Here, we characterized the function of OsPRR73, the other PRR7 homologous protein in rice. We investigated the heading dates of OsPRR73 overexpression and knockout lines and examined the free-running rhythms of clock-associated genes under constant light (LL) and constant dark (DD) conditions. We found that Ehd1 and OsLHY are the direct downstream targets of OsPRR73 and that OsPRR73 works downstream of the light receptor OsPhyB. These findings demonstrate that OsPRR73 is a component of the morning loop in the rice circadian clock that contributes to photoperiodic flowering regulation under LDs.
2 MATERIALS AND METHODS
2.1 Plant growth and harvest conditions
Zhonghua 11 (ZH11; O. sativa ssp. japonica) was used as the wild-type (WT) genotype in this study. OsPRR73-overexpressing plants were grown under natural long days (NLDs) in Wuhan (30.3°N, 114.2°E) in 2015 and 2016 summer and under natural short days (NSDs) in Hainan (18.3°N, 110.0°E) in 2015 winter. Positive and negative T2 families of one single copy OsPRR73-overexpressing plants (OsPRR73OXS1, OsPRR73OX for short) were cultivated under artificial SDs (10 hr light/14 hr dark) with a shelter in the field in Wuhan in 2015 summer. The osprr73 mutants were grown in Wuhan in the summer of 2016 and 2017 and in Hainan in 2016 winter. OsPRR73-FLAG plants were cultivated in the field in Wuhan in the summer of 2019. The osprr73ehd1 and ehd1 mutants were grown in Wuhan in the summer of 2019 and 2020. Field management, including irrigation, fertilizer application and pest control, essentially followed standard agricultural practices. In 2020, seven plants of WT, OsPRR73OX and osprr73 mutants were grown in the growth chambers under both SDs (10 hr light at 32°C, humidity 70%/14 hr dark at 25°C, humidity 50%) and LDs (14 hr light at 32°C, humidity 70%/10 hr dark at 25°C, humidity 50%).
For diurnal expression analysis, the penultimate leaves were obtained every 4 hr beginning 35 days after germination from the plants, which were grown in growth chambers under either SDs (10 hr light at 32°C, humidity 70%/14 hr dark at 25°C, humidity 50%) or LDs (14 hr light at 32°C, humidity 70%/10 hr dark at 25°C, humidity 50%). Samples were harvested beginning at Zeitgeber time 0 (ZT0; 0 hr after the lights were turned on). Within the continuous light and continuous darkness experiments, 3-week-old plants were grown under SDs for 5 days and then transferred to darkness conditions or under LDs for 5 days and then transferred to light. The samples were harvested for 2 days under continuous darkness or light conditions.
2.2 Phenotypic data collection
Heading date was scored when the first panicle became visible. Plant height was measured from the ground to the tip of the tallest tiller of the plant. The other yield traits, including the length of the main panicle (LP), the number of primary branches, the number of secondary branches, the number of spikelets per panicle and the 1,000-grain weight (GW), were measured after the crop harvest.
2.3 Vector construction and rice transformation.
To construct the OsPRR73 overexpression vector, the full-length cDNA of OsPRR73 was amplified and cloned into the PCAMBIA1301S vector, which was driven by the Cauliflower Mosaic Virus 35s promoter. To generate osprr73 mutants, an 18-bp OsPRR73-specific sgRNA was designed (Figure S1a) and assembled into the binary expression vector pCXUN-CAS9, in which the sgRNA was driven by the U3 promoter. A single ehd1 mutant and osprr73ehd1 double mutants were obtained by CRISPR/Cas9-based genome editing technology (Ma, Chen, Zhu, Chen, & Liu, 2015). To produce OsPRR73-FLAG plants, full-length cDNA was inserted into the overexpression vector Pu1301-3XFLAG under the control of the maize (Zea mays) ubiquitin promoter. The primers for the constructs and the targets for CRISPR/Cas9-based genome editing technology are listed in Table S1. The constructs were introduced into ZH11 by Agrobacterium tumefaciens (EH105)-mediated transformation.
2.4 DNA extraction and southern blot hybridization
Fresh leaves were harvested from field-grown plants, and the total genomic DNA was isolated by the CTAB method. The hpt probe for Southern blot hybridization was amplified as described previously in Ling, Zhou, Chen, and Lin (2016). The experimental procedures for Southern analysis followed the methods described.
2.5 RNA extraction and quantitative real-time RT-PCR
Total RNA was extracted from the leaves or seedlings using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. RNA (3 μg) was treated with DNase I (Invitrogen) and then reverse transcribed using a SuperScript III First Strand cDNA Synthesis Kit (Invitrogen). FastStart Universal SYBR Green Master (Roche) was used in real-time PCR analysis with a StepOnePlus Real-Time PCR System (Life Tech). The measurements were obtained using the relative quantification method. The primer pairs for qRT-PCR analysis are listed in Table S1.
2.6 Subcellular localization of OsPRR73
The full-length coding sequence of OsPRR73 was amplified by PCR with the primers listed in Table S1, and it was fused with XbaI into PM999-YFP. After the sequence of the fragment was verified to be in the right direction, it was co-transfected into rice protoplasts with CFP-NLS. CFP-NLS is a nuclear marker, the NLS sequence was cloned from NLS-RFP (He et al., 2019) and was fused after CFP with the primers listed in Table S1. The rice protoplasts were isolated as described previously in Zong et al. (2016). Fluorescence images was obtained using a confocal microscope (Leica, Germany) after incubating the transformed cells in the dark at 28°C for 16 hr.
2.7 Yeast one-hybrid (Y1H) assay
Following the method described by Wang et al., PJG4-5 was used as the AD vector (Sun et al., 2015). The OsPRR73-AD fusion effector was transformed with lacZ reporters that carried different regions of the promoter of Ehd1 into yeast strain EGY48, and the transformants were further grown on SD/−Trp-Ura dropout medium for 2 days. The transformants were further grown on SD/−Trp-Ura dropout medium with 20 mg/ml X-gal (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside) for colour development. The full procedure for the yeast hybrid assays was performed according to the Yeast Protocols Handbook (Clontech).
2.8 Luciferase transcriptional activity assay with protoplasts
To determine the transcriptional activation and repression activity of OsPRR73, we constructed plasmids in which the full-length OsPRR73 was fused with the GAL4 DNA-binding domain (BD-OsPRR73), as described in our previous work (Weng et al., 2014). Both plasmids were co-transformed with a LUC reporter containing the 4× upstream activation sequence region and a mini 35S promoter sequence fused to LUC cDNA into rice protoplasts. For verification of the transcriptional repression activity of OsPRR73 on the promoter of Ehd1, we followed the previously reported method (Zong et al., 2016) and constructed an OsPRR73-None vector containing a GAL4-binding domain as an effector and a pEhd1-190fLUC vector as a reporter, in which almost 2 kb of the Ehd1 promoter was fused into the firefly luciferase (fLUC) sequence. AtUbi::rLUC (Renilla luciferase) was used as an internal control, and the fLUC/rLUC ratio was calculated as the relative luciferase activity. The activity of the LUC reporters was measured with a Dual-Luciferase Reporter Assay System (Promega). All the primers for vector construction are listed in Table S1, and the plasmids used in this assay were purified with a Plasmid Midi Kit (Qiagen).
2.9 ChIP assay
The ChIP experiment was performed as described (Cheng et al., 2018). Approximately 2 g of leaf tissue from 40-day-old seedlings were collected at AM 10:30 under NLD condition and was cross-linked in 1% formaldehyde under a vacuum. Chromatin was extracted and fragmented to 200–500 bp by sonication. The antibody used was anti-FLAG (Sigma M8823). The precipitated and input DNA samples were analysed by quantitative RT-PCR with the primers listed in Table S1. Three replicates of each sample were analysed, and the values were normalized to those of the input samples.
2.10 Electrophoretic mobility shift assay.
The MBP-fused CCT of OsPRR73 was expressed in the Escherichia coli BL21 strain and purified according to the manufacturer's instructions. HAP proteins, including the His-fused HAP domain of Ghd8 and the HAP domain of OsHAP5C (Li et al., 2016), were together purified with a His tag, which was the same as the one used in our previous study (Shen et al., 2020). 5′ FAM oligonucleotides were synthesized and labelled by the Shanghai Sangon Company (Table S1). The protein and DNA binding reactions were performed according to a LightShift Chemiluminescent EMSA Kit (Thermo Fisher Scientific, 20148). The fluorescence signals were captured with an FLA-5100 (Fuji).
3 RESULTS
3.1 OsPRR73 is a negative regulator of heading date
OsPRR73 contains a PRR domain at the N-terminal region and a CCT domain at the C-terminal region (Figure S1b); the PRR domain and CCT domain share 78.4% and 83.7% amino acid sequence identity, respectively, with the corresponding regions of PRR7 in Arabidopsis (Figure S1c). We generated three OsPRR73 overexpression (OsPRR73OX) lines with single copy target gene that were identified by Southern blot hybridization (Figure S2a). The days to heading of OsPRR73OX were 86.9, which was 16.9 days more than 70.0 days of ZH11 under NLD condition. The other two lines (OsPRR73OXS2 and OsPRR73OXS3) also delayed heading under NLD condition (Figures S2b and S3a,c). Moreover, the OsPRR73OX plants exhibited taller plant heights and larger panicles than the wild type (Figure S3b,d; Table 1). Under ASD condition with 10 hr of light a day, OsPRR73OX flowered 8.1 days later than ZH11 that headed 64.6 days after sowing (Figure S3c), and also exhibited taller plant heights and larger panicles than ZH11 (Figure S3d; Table 1). ZH11 showed a significant difference in heading date between NLD (70.0 days) and ASD (64.6 days), indicating its photoperiod sensitivity. The OsPRR73OX plants still showed later flowering and taller plant heights than the wild type under NSD conditions (Figure S4a,b).
| Conditions | Genotype | Number of plants | SPP | TGW (g) | PL (cm) | NPB | NSB | Yield (g) |
|---|---|---|---|---|---|---|---|---|
| Natural long-day | OsPRR73OX | 17 | 215.9 ± 35.3 | 24.6 ± 2.0 | 29.5 ± 2.9 | 19.9 ± 2.2 | 58.8 ± 11.0 | 35.4 ± 8.4 |
| OsPRR73OX- | 10 | 130.5 ± 14.2 | 27.4 ± 1.7 | 23.7 ± 0.9 | 16.1 ± 1.6 | 32.6 ± 1.8 | 23.9 ± 6.7 | |
| P | 1.3E-07 | 1.2E-03 | 2.1E-06 | 5.9E-05 | 9.5E-08 | 1.1E-03 | ||
| Artificial short-day | OsPRR73OX | 17 | 166.6 ± 20.3 | 26.6 ± 1.1 | 22.6 ± 1.2 | 14.8 ± 2.0 | 31.2 ± 6.9 | |
| OsPRR73OX- | 6 | 106.2 ± 27.5 | 27.6 ± 1.5 | 20.7 ± 1.0 | 10.8 ± 1.3 | 18.3 ± 6.2 | ||
| P | 7.8E-06 | 4.4E-02 | 7.9E-04 | 1.0E-04 | 3.1E-04 |
- Abbreviations: SPP, spikelets per panicle; TGW, 1000-grain weight; PL, panicle length; NPB, number of primary branch; NSB, number of secondary branch.
- Notes: The five yield-related traits are as follows. p Values were obtained between positive and negative plants by Student's t test. All phenotypic values are reported with mean ± SE.
Two OsPRR73 knockout mutants, one with a 1-bp deletion (W2 line) and one with a 19-bp deletion (W5 line) in the N-terminal region, were generated (Figure S2c) and hereafter designated osprr73 mutants. The knockout mutants flowered about 3 days earlier and exhibited shorter plant heights and smaller panicles than the wild type under NLD conditions (Figure S3e–h; Table 2; Table S2). No significant difference in heading date was observed between the mutants and WT under NSD conditions, while the osprr73 mutants showed shorter plant heights than WT (Figure S4c,d).
| Genotype | Number plants | SPP | TGW(g) | PL (cm) | NPB | NSB |
|---|---|---|---|---|---|---|
| osprr73 | 17 | 97.2 ± 10.8 | 19.1 ± 1.5 | 18.4 ± 1.0 | 10.9 ± 0.9 | 17.0 ± 4.4 |
| osprr73− | 14 | 126.0 ± 11.6 | 22.1 ± 1.1 | 23.0 ± 1.1 | 12.8 ± 1.2 | 30.1 ± 5.5 |
| p Value | 3.7E-08 | 1.1E-06 | 2.4E-13 | 3.3E-06 | 7.7E-09 |
- Abbreviations: SPP, spikelets per panicle; TGW, 1000-grain weight; PL, panicle length; NPB, number of primary branch; NSB, number of secondary branch.
- p Values were obtained between positive and negative plants by Student's t test. All phenotypic values were presented with mean ± SE.
To precisely evaluate the genetic effects of OsPRR73 on heading date, we investigated the heading dates of WT, OsPRR73OX and mutant plants under strict LD and SD conditions. Similar to NLD and ASD conditions, OsPRR73OX delayed heading by 14.9 days under LD condition and 4.6 days under SD conditions (Figure 1a,c). While osprr73 headed 3.1 days earlier under LD condition but no difference under SD (Figure 1b,c). Taken together, these findings show that OsPRR73 suppresses flowering and increases plant height in rice under LD conditions.
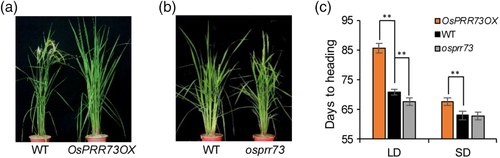
3.2 Spatiotemporal and diurnal expression of OsPRR73.
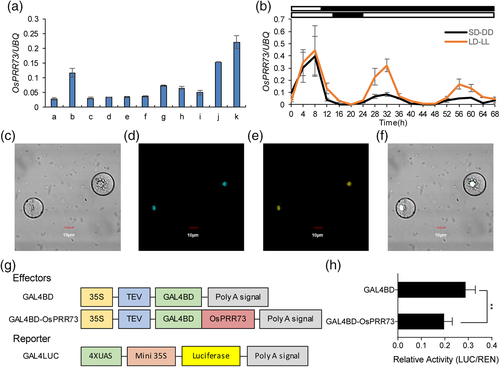
OsPRR73 was constitutively expressed in all investigated tissues and was preferentially expressed in mesophyll under NLD conditions (Figure 2a). To characterize the rhythmic expression of OsPRR73, ZH11 plants were grown under LD and SD conditions for a week and then separated for growth under constant light (LL) or constant darkness (DD) conditions for 48 hr. Under both LD and SD conditions, the transcript levels of OsPRR73 gradually increased in the morning and peaked at noon before gradually decreasing in the afternoon and finally becoming hardly detectable after dusk (Figure 2b). When the plants were transferred into DD conditions from SD conditions, the expression of OsPRR73 remained rhythmic, but the amplitude appeared to be rapidly damped. On the other hand, a later damping of rhythmic amplitude appeared when the plants were transferred into LL conditions from LD conditions. These results suggest that OsPRR73 expression exhibits a diurnal oscillation rhythm with accumulation at noon and is positively regulated by light.
3.3 OsPRR73 acts as a transcriptional repressor.
Fused OsPRR73-YFP and CFP-NLS (nuclear marker) plasmids were co-transformed into protoplasts. A transient expression assay showed that the OsPRR73-YFP fusion proteins colocalized with NLS (Figure 2c–f), indicating that OsPRR73 is a nuclear protein. To identify the transcriptional activity of OsPRR73, we used the Galactose 4 (GAL4) DNA-binding domain to establish a transient system with luciferase (LUC) as a reporter (Figure 2g). BD-OsPRR73 repressed the transcription of the LUC reporter gene compared with the control (Figure 2h). Thus, OsPRR73 had transcriptional repression activity in vivo.
3.4 OsPRR73 regulates heading date by repressing Ehd1 expression
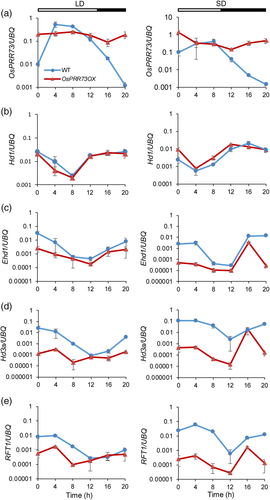
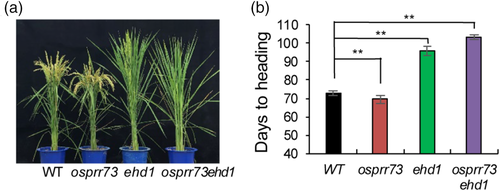
To elucidate the target of OsPRR73 in the photoperiodic flowering pathway, we measured the diurnal transcript levels of the key flowering genes Hd1, Ehd1, Hd3a and RFT1 in OsPRR73OX under both LD and SD conditions. The Hd1 expression pattern appeared to exhibit an advanced phase shift phenotype under SD conditions, but there were no alterations under LD conditions (Figure 3b). The expression levels of Ehd1, Hd3a and RFT1 were significantly decreased in OsPRR73OX compared with the wild type under both conditions (Figure 3c–e). We also detected the diurnal expression of Ghd7 and OsPRR37 under LD conditions in OsPRR73OX and the wild type. The expression levels of both Ghd7 and OsPRR37 in OsPRR73OX were lower than those in the wild type (Figure S5b,c). In addition, the osprr73ehd1 double mutant showed a delayed heading date, as did the ehd1 mutant, but a 30-day delayed heading than the wild type under NLD conditions (Figure 4). Thus, OsPRR73 acts upstream of Ehd1 to regulate flowering.
3.5 OsPRR73 binds to the promoter of Ehd1
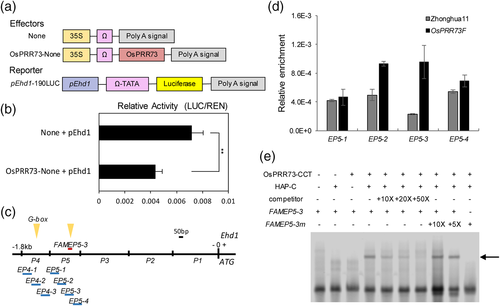
The LUC signal was weaker in the protoplasts co-transformed with OsPRR73-none and pEhd1-190LUC than in those co-transformed with pEhd1-190LUC alone as the control (Figure 5a,b), indicating that OsPRR73 reduced the LUC signal through association with the promoter of Ehd1 in vivo. Then, the 1.8 kb promoter sequence of Ehd1 was divided into five fragments (Figure 5c), which were used to perform a yeast one-hybrid assay with the OsPRR73 protein. The results showed that OsPRR73-AD had a weak ability to activate the P4 LacZ reporter, and P5 LacZ autoinduced in Yeast (Figure S6). Upon searching PlantCARE, a database of plant promoters and cis-acting regulatory elements, two G-boxes (CACGTC) were found in the promoter of Ehd1, one on P4 and one on P5 (Figure 5c). Furthermore, we produced Ubi::OsPRR73::FLAG lines, hereafter designated OsPRR73F, which exhibited heading date phenotypes similar to those of OsPRR73OX plants under NLD conditions (Figure S7). Several primer pairs on the P4 and P5 regions were designed for ChIP-PCR analysis of OsPRR73F leaves with anti-FLAG antibodies. The analyses showed that there was significant enrichment on P5 instead of P4 (Figures 5d and S8), suggesting that OsPRR73 bound to P5 of Ehd1 in vivo. It has been reported that the CCT domain of TOC1 or PRRs is sufficient to directly bind to the G-box (CACGTG) or T1ME (TGTG) motif (Gendron et al., 2012). The MBP-tagged CCT domain of OsPRR73 (OsPRR73-CCT) was purified (Figure S9), and a 58-bp fragment (FAMEP5-3) containing a G-box and a T1ME motif was used to perform an electrophoretic mobility shift assay (EMSA). The result showed that the shift band was detected when OsPRR73-CCT and HAP domains of OsHAPs proteins (Ghd8/OsHAP5C) were incubated together (Figure 5e). To check for specificity, an unlabelled oligonucleotide identical to the DNA probe and probe containing mutations in the G-box and T1ME site were used to be challenged the protein complex. These results indicate that OsPRR73 directly associates with the G-box or T1ME motif in the promoter of Ehd1.
3.6 OsPRR73 works downstream of OsPhyB
OsPhyB is a flowering repressor (Takano et al., 2005; Weng et al., 2014). To examine whether OsPRR73 is regulated by phytochromes, we evaluated OsPRR73 expression in osphyb mutants (ZH11 background) in the field. The transcript abundance of OsPRR73 were significantly downregulated in osphyb mutants (Figure 6a), while the transcript levels of OsPhyB differed little among OsPRR73OX, osprr73 and the wild type under NLD conditions (Figure 6b), suggesting that OsPhyB is a positive regulator of OsPRR73. Osphyb mutants flowered 3 days earlier than wild-type plants under NLD conditions (Figure 6c,d). It was expected that OsPRR73 overexpression would complement the osphyb phenotype. We developed an osphybOsPRR73OX matrtial by crossing osphyb and OsPRR73OX and investigated the heading date. Flowering of osphybOsPRR73OX plants was delayed by approximately a week compared with that in the osphyb mutant, but the heading date in the double mutant was delayed by 3 days compared with that in the wild type under NLD conditions (Figure 6c,d). Taken together, the results indicate that OsPRR73 works downstream of OsPhyB in the photoperiodic flowering pathway.

3.7 OsPRR73 regulates circadian clock-associated genes
The expression pattern of OsPRR73 in OsPRR73OX was dramatically changed compared with that in the wild type. OsPRR73 expression increased nearly throughout the day, leading to an alteration of the oscillatory pattern compared with that in the wild type under both LD and SD conditions (Figure 3a). Native OsPRR73 mRNA levels were strongly reduced in OsPRR73OX (Figure S5a), indicating that OsPRR73 participates in a feedback loop. Compared with those in the wild type, the transcript levels of OsLHY, OsTOC1 and OsGI, rice circadian clock-associated genes, were significantly reduced in OsPRR73OX, leading to lower amplitudes of the oscillatory pattern under both conditions (Figure S10a–c). OsELF3 mRNA started to accumulate in the evening in the wild type under both LD and SD conditions (Figure S10d), while the transcript levels of OsELF3 were slightly decreased in OsPRR73OX under LD conditions (ZT = 0, 4, 8, 20) and slightly increased in OsPRR73OX under SD conditions (ZT = 16), respectively. Moreover, we detected diurnal expression patterns of other related circadian-associated genes, such as OsCAB1R, OsPCL1, OsPRR59, OsPRR95, OsZTL1 and OsZTL2. They showed significant alterations between OsPRR73OX and the wild type, especially in the morning (Figure S11).
In addition, we examined the free-running rhythms of OsLHY, OsTOC1, OsGI and OsELF3 in OsPRR73OX, osprr73 and the wild type under LL and DD conditions (Figure 7a–d). Under LL conditions, OsLHY maintained the same robust rhythmic patterns in OsPRR73OX, osprr73 and the wild type; it had the lowest amplitude in OsPRR73OX but the highest amplitude in the osprr73 mutant. The oscillatory pattern of OsTOC1 peaked mildly later in osprr73 than in the wild type, displaying a slightly delayed phase phenotype. OsTOC1 showed a prolonged period expression pattern (~28 hr) in OsPRR73OX compared with that in the wild type under LL conditions. The expression pattern of OsGI in OsPRR73OX was similar to that in the wild type, but it maintained robust rhythms and had a relatively higher expression at Zeitgeber Time 12 (ZT12; 12 hr after the lights turned on) and ZT36 in the osprr73 mutant than in both OsPRR73OX and the wild type. OsELF3 maintained rhythmic expression, and the range of fluctuation was much larger in the OsPRR73OX and osprr73 mutants than in the wild type, especially at ZT4 and ZT28. In general, the average expression level of OsELF3 was significantly higher in OsPRR73OX than in the wild type.
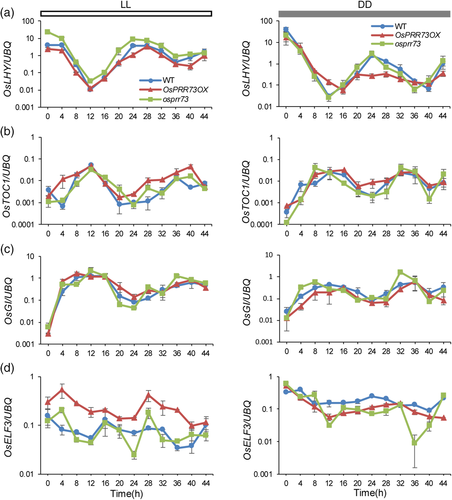
Under DD, it was hard to observe the expression pattern of OsLHY because the transcript level of OsLHY was dramatically decreased. However, the expression level of OsLHY in OsPRR73OX was much lower than that in the wild type. There was no significant difference in OsLHY expression between the osprr73 mutant and the wild type. The oscillatory patterns of both OsTOC1 and OsGI had an earlier phase phenotype in osprr73 and a later phase phenotype in OsPRR73OX than in the wild type. The expression patterns of OsELF3 in OsPRR73OX and osprr73 were arrhythmic under DD conditions; in contrast, those in the wild type maintained the same robust rhythm under DD as under the LD and SD conditions (Figure S10d). The average expression level of OsELF3 was relatively lower in the OsPRR73OX and osprr73 mutants than in the wild type. These results indicate that OsPRR73 affects the clock network and regulates other circadian clock genes at the transcriptional level.
3.8 OsPRR73 binds the promoter of OsLHY
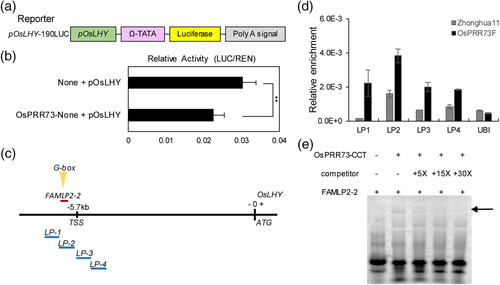
A Luciferase transcriptional activity assay showed that the LUC signal was weaker in the protoplasts co-transformed with OsPRR73-none and pOsLHY-190LUC than those in the protoplasts co-transformed with None and pOsLHY-190LUC as the control (Figure 8a,b), indicating that OsPRR73 repressed OsLHY through association with the promoter of OsLHY in vivo. With searching PlantCARE, a G-box motif was also found near the potential transcription start site (TSS) region of OsLHY, located 5.7 kb upstream of the start codon (Figure 8c). ChIP-PCR analysis of OsPRR73F leaves with anti-FLAG antibodies (Figure 8d) and EMSAs (Figure 8e) showed that OsPRR73 directly interacted with the promoter of OsLHY in vivo and in vitro.
4 DISCUSSION
4.1 OsPRR73 is involved in feedback loops of the circadian clock network in rice
The circadian clock genes are functionally conserved among higher plants (Murakami et al., 2007; Song et al., 2010). OsPRR73 is a homologous protein of PRR7 (Figure S1c). PRR7 is one component of the Arabidopsis clock and functions as a transcriptional repressor to directly repress the core clock genes CCA1 and LHY (Farré et al., 2005; Nakamichi et al., 2010). As expected, OsPRR73 maintained robust rhythmic expression under LL and DD conditions, with accumulation at midday (Figure 2b). Here, OsPRR73 functioned as a transcriptional repressor to bind the promoter of OsLHY, a homologous gene of CCA1 and LHY (Figure 8). In Arabidopsis, PRR7 is the direct target of EC and TOC1 (Gendron et al., 2012; Mizuno et al., 2014). CCA1 directly represses PRR7 in vivo (Kamioka et al., 2016). CCA1/LHY, ELF3, PRR9, and PRR7 form complex transcriptional feedback loops in Arabidopsis (Adams et al., 2015; Kamioka et al., 2016; Lu et al., 2012; Nakamichi et al., 2010). Compared with the wild-type line, the OsPRR73 overexpression and knockout lines displayed various alterations in the oscillatory patterns of OsLHY, OsTOC1, OsGI and OsELF3 under LL and DD conditions (Figure 7), indicating that OsPRR73 has a close relationship with these rice circadian clock-associated genes at the transcriptional and translational levels. OsPRR73 directly or indirectly repressed its own expression, leading to arrhythmic expression patterns of OsPRR73 in OsPRR73OX under LD and SD conditions (Figure 3a). In addition, the expression of OsPRR73 was increased in the oself3 mutant compared with the wild type, while the transcript abundance of OsLHY was reduced (Yang et al., 2013; Zhao et al., 2012; Zhu et al., 2018). Thus, OsPRR73 forms transcriptional feedback loops with other rice clock-related proteins, including OsLHY, with which it forms a morning loop.
4.2 OsPRR73 responses to light
Leaf is the main organ perceiving sunlight, and OsPRR73 is preferentially expressed in mesophyll (Figure 2a). OsPRR73 expression peaked in the daytime, and its expression level was tremendously reduced in DD compared with LL (Figure 2b), indicating that OsPRR73 is positively regulated by light. OsPhyB is a rice photoreceptor and negatively regulates rice flowering under LD conditions (Takano et al., 2005). In this study, OsPRR73 worked downstream of OsPhyB under LD conditions (Figure 8). PhyB physically interacts with ELF3 to provide direct light input to the clock in Arabidopsis (Kolmos et al., 2011; Liu, Covington, Fankhauser, Chory, & Wagner, 2001). OsELF3 promotes rice flowering and negatively regulates the transcript level of OsPRR73 under LD conditions (Yang et al., 2013; Zhu et al., 2018). Thus, OsPRR73 responds to light as a component of the clock to integrate light signalling into input pathways. However, these pathways remain largely unknown in rice.
4.3 Ehd1 is a direct output gene of the rice circadian clock
Photoperiodic flowering control is one physical output of the circadian clock (Johansson & Köster, 2019). PRR7 is a flowering promoter in the long-day plant Arabidopsis (Nakamichi et al., 2005). Mutations in other PRR genes delay Arabidopsis flowering under LDs, and double or triple mutants exhibit much stronger phenotypes, suggesting functional redundancy among the PRR genes (Farré et al., 2005; Nakamichi et al., 2005, 2007). Here, OsPRR73 was demonstrated to be a flowering suppressor in the short-day plant rice. OsPRR73 directly bound to the Ehd1 promoter and repressed its expression, ultimately resulting in delayed heading. The expression patterns of 5 OsPRR genes form a quintet in the daytime in rice showed in Figure S12 (Murakami et al., 2003). Both OsPRR37/Ghd7.1 and OsPRR73 are cumulatively expressed from morning until noon, OsPRR59 and OsPRR95 peak in the afternoon, and OsTOC1 peaks at dusk. OsPRR37 delays heading date under LDs by reducing Ehd1 mRNA levels (Koo et al., 2013; Liu et al., 2018; Yan et al., 2013). The expression of OsPRR37 was reduced in OsPRR73OX under LD conditions (Figure S5c). OsPRR73 and OsPRR37 likely share functional redundancy in regulating flowering under LDs through the Ehd1-Hd3a/RFT1 pathway. Hd1 promotes rice heading under SD conditions (Kojima et al., 2002; Yano et al., 2000). OsPRR73 slightly increased Hd1 expression under SD conditions (Figure 3b). In contrast, OsPRR73 overexpression caused later flowering under SD condition. Thus, OsPRR73 likely regulates heading mainly through the Ehd1-mediated pathway under SD conditions.
The Hd1-mediated and Ehd1-mediated photoperiod pathways under LD conditions are integrated by interactions among Ghd7, DTH8/Ghd8/Hd5 and Hd1, the upstream regulators of Ehd1 (Goretti et al., 2017; Nemoto et al., 2016; Wei et al., 2010; Yan et al., 2011; Zhang et al., 2017). The transcript abundance of Ghd7 was reduced in OsPRR73OX under LD conditions (Figure S5b). However, Ehd1 expression was sharply decreased. Thus, OsPRR73 overexpression delayed heading date. Moreover, the late-heading date phenotype of the osprr73ehd1 double mutant revealed that Ehd1 functions downstream of OsPRR73 in flowering control (Figure 4). The finding that the osprr73ehd1 double mutant showed a later heading date than that of the ehd1 mutant under LD conditions (Figures 4b and S13) implies that there is another downstream pathway of OsPRR73 in rice heading control. In addition, the CCT domain of Arabidopsis CO, which shares a similar structure with HAP2, interacts with HAP3 and HAP5 (Wenkel et al., 2006). Arabidopsis TOC1 has DNA-binding activity relying on the CCT domain that binds to the G-box (CACGTG) or T1ME (TGTG) motif (Gendron et al., 2012). The binding ability of OsPRR73 also relies on the CCT domain in the presence of HAP proteins, and OsPRR73 directly associates with the G-box or T1ME motif of the Ehd1 promoter (Figure 5e,f). Ehd1 is a cereal-specific gene that has no homologous gene in Arabidopsis. Ehd1 is a direct output gene of the rice clock involved in photoperiodic flowering control, which regulates plant flowering in a much more elegant way than the photoperiodic flowering pathways in Arabidopsis (Golembeski, Kinmonth-Schultz, Song, & Imaizumi, 2014).
In addition to regulating flowering time, OsPRR73 positively regulates plant height and grain yield in rice (Tables 1 and 2). These findings help to better elucidate the mechanism of the circadian clock in crops and suggest that circadian genes may be scientifically utilized to breed high-yield crop varieties for different cropping zones worldwide.
ACKNOWLEDGEMENTS
We thank Mr. JB Wang for his excellent work in field. And great thanks for the comments and suggestions from Professor C. Robertson McClung in Dartmouth College for this manuscript. This work is supported by grants from the National Basic Research Program of China (2016YFD0100802), the National Natural Science Foundation of China (31601283, 31701054).
CONFLICT OF INTEREST
The authors declare there is no conflict of interest.
DATA AVAILABILITY STATEMENT
All raw data associated with this manuscript will be made available on request.




