Transcriptome and translatome changes in germinated pollen under heat stress uncover roles of transporter genes involved in pollen tube growth
Funding information: Ministerio de Ciencia e Innovación, Grant/Award Number: BIO2015-70483-R
Abstract
Plant reproduction is one key biological process that is very sensitive to heat stress and, as a result, enhanced global warming becomes a serious threat to agriculture. In this work, we have studied the effects of heat on germinated pollen of Arabidopsis thaliana both at the transcriptional and translational level. We have used a high-resolution ribosome profiling technology to provide a comprehensive study of the transcriptome and the translatome of germinated pollen at permissive and restrictive temperatures. We have found significant down-regulation of key membrane transporters required for pollen tube growth by heat, thus uncovering heat-sensitive targets. A subset of the heat-repressed transporters showed coordinated up-regulation with canonical heat-shock genes at permissive conditions. We also found specific regulations at the translational level and we have uncovered the presence of ribosomes on sequences annotated as non-coding. Our results demonstrate that heat impacts mostly on membrane transporters thus explaining the deleterious effects of heat stress on pollen growth. The specific regulations at the translational level and the presence of ribosomes on non-coding RNAs highlights novel regulatory aspects on plant fertilization.
1 INTRODUCTION
The evidence of a rapid global warming since the beginning of the industrial epoch is very solid (Intergovernmental Panel on Climate Change, 2014) and its global impact seems to have an anthropogenic origin, as paleoclimate reconstructions found no evidence of worldwide coherent warm or cold periods in the preindustrial times (Neukom, Steiger, Gómez-Navarro, Wang, & Werner, 2019). According to the known drivers of climate change, global warming predictions have been made with pessimistic scenarios especially for agriculture in low latitudes (Rosenzweig et al., 2014). The negative impact of enhanced global warming on agriculture poses serious risks for future food security and demands smart food system management strategies (Vermeulen, Campbell, & Ingram, 2012). Under this scenario, understanding how increased temperature impacts plant biology is an important task.
Plant reproduction is considered the most sensitive stage of plant development by the effects of global warming (Hedhly, Hormaza, & Herrero, 2009) and, for many crop plants, pollen seems to be particularly vulnerable to high temperatures (Herrero & Johnson, 1980; Ledesma & Sugiyama, 2005; Sato, Peet, & Thomas, 2002; Zinn, Tunc-Ozdemir, & Harper, 2010). Pollen sensitivity to heat occurs not only throughout its development prior to anther dehiscence but also during germination and pollen tube growth (Kakani et al., 2005; Kakani, Prasad, Craufurd, & Wheeler, 2002). Upon landing on the stigmatic tissue of the pistil, pollen grains of flowering plants hydrate and germinate to immediately initiate pollen tube growth over long distances to deliver the male gametes to the egg and central cells in the embryo sac for the double fertilization process (Lord & Russell, 2002). Growth of the pollen tube from the stigma to the ovules occurs at extremely rapid rates and requires a fine regulation of subcellular processes such as: well-ordered vesicle transport, precise cytoskeleton arrangements, strict and local pH establishment, proper energy management with amyloplast and mitochondrial logistics and delicate regulation of regulatory ion fluxes for osmotic adjustment (K+ transport) and signalling (Ca2+ transport). This impressive machinery demands exquisite coordination among all these processes for the rapid transport of germinal sperm cells to the micropyle (Chebli, Kroeger, & Geitmann, 2013; Hepler, Rounds, & Winship, 2013; Michard, Simon, Tavares, Wudick, & Feijó, 2017). One important aspect of this optimized cellular growth is the presence of local pH gradients with an acidic apex region and an alkaline band in the clear zone followed by a subapical region of acidic pH (Feijó, Sainhas, Hackett, Kunkel, & Hepler, 1999; Hepler & Winship, 2015). This complex organization of events demands an optimized protein synthesis machinery (Cheung & Wu, 2007). Ultrastructural analysis of pollen tube growth at high temperature revealed alterations in rough-endoplasmic reticulum, Golgi apparatus and mitochondria (Kandasamy & Kristen, 1989), thus supporting the notion that protein translation, vesicle transport and energy depletion during pollen tube growth may be limiting steps in the adaptation to high-temperature conditions. Molecular studies have uncovered heat responses in pollen subjected to high temperature similar to other plant cell types (Rieu, Twell, & Firon, 2017). In addition, several “omics” studies have been performed to characterize the response of pollen under heat stress conditions (Begcy et al., 2019; Chaturvedi, Ischebeck, Egelhofer, Lichtscheidl, & Weckwerth, 2013; Jegadeesan et al., 2018; Keller et al., 2018). To complement these approaches, the use of next generation sequencing of nucleic acids (NGS) techniques (Reuter, Spacek, & Snyder, 2015), is expected to help gain deeper insight in the response of pollen to heat stress conditions.
A breakthrough in the studies of mRNA translation was achieved with the development of the ribosome profiling technique (also called Ribosome Footprinting, Riboprofiling or Ribo-Seq), a ribosome-centric approach to isolate and sequence ribosome-protected mRNA fragments (RPFs) after RNase treatment that provides a quantitative profile of translation across the transcriptome with sub-codon resolution (Ingolia, Ghaemmaghami, Newman, & Weissman, 2009). The advantage of Riboprofiling is that, in parallel with massive sequencing of ribosome footprints, total RNA is also subjected to high-throughput sequencing thus allowing the direct comparison of the transcriptome, the translatome and the calculations of translational efficiencies for the complete set of transcripts (Ingolia, 2016).
Here we have used Riboprofiling with Arabidopsis pollen germinated in vitro under both optimum and high-temperature conditions. We provide high-resolution transcriptome and translatome profiles of pollen tubes germinated at basal temperature, and show their changes in response to heat stress. We have found that in vitro germinated pollen responds to high temperature by up-regulating heat shock genes in a similar manner to the vegetative organs. Heat stress also down-regulated specifically many membrane transporters, including K+ and carbohydrate co-transporters, thus finding a suitable explanation to the deleterious effects of high temperature on pollination. A subset of these transporters is up-regulated during semi in vivo pollen development, when heat shock genes are also expressed, illustrating the importance of these heat-sensitive functions during plant fertilization. Moreover, we have found specific regulations at the translational level and the unexpected presence of ribosome footprints on non-coding RNAs. Thus, our analysis of combined transcriptome and translatome is revealing novel insights on pollen responses to heat stress, including a tight connection between heat shock gene expression and membrane transporters that promote pollen tube growth and male fertility.
2 MATERIALS AND METHODS
2.1 Large-scale pollen collection using vacuum filtration
About 20.000 A. thaliana (Col-0) plants were grown in greenhouse for 4 weeks in a mixture of 25% vermiculite, 25% perlite and 50% soil under long-day photoperiod cycles. Pollen collection was performed by vacuum filtration following described protocols (Johnson-Brousseau & McCormick, 2004). Pollen was then scrapped off the 6 μm nylon mesh, and dried overnight under a chemical hood in a 2 mL tube. After weighing, a silica bead was added to the pollen and the samples were placed at −20°C. Harvests were done every second day for about 2 weeks. Yields ranged from 10 to 55 mg of pollen per day. A small aliquot from each sample was kept in a second tube for pollen viability and contamination assays. The evaluation of contamination was performed by microscopic examination of the samples, where both bacteria and fungi are readily visible. The whole experiment was repeated 3 times to obtain 3 biological replicates.
2.2 Pollen growth assays
The pollen tube growth was assayed using published protocols (Boavida & McCormick, 2007). Three- to six-month-old frozen pollen grains were first rehydrated for 1 h in a humid chamber. Pollen was then germinated on an agar medium containing 10% sucrose, 0.01% boric acid, 1 mM MgSO4, 5 mM CaCl2, 5 mM KCl and 1.5% low-melting agarose, pH 7.5 (adjusted with 0.1 M NaOH). For microscopic pollen examination, 1 mL of melted germination medium was spread on a glass microscope slide to build a flat pad where pollen can be spread after the agarose is solidified. The slides were placed inside a moisture incubation chamber to avoid dehydration of the medium, and incubated in the dark at 24°C or 35°C, for the indicated time. Pictures were taken with a Leica DM5000 microscope. Quantifications of pollen germination and tube length were performed with the IMAGEJ Software (Schindelin et al., 2012) using the cell counter and NeuronJ plug-ins (Meijering et al., 2004). Germinated pollen was scored positively for grains with a pollen tube length of at least the pollen diameter. For the Riboprofiling experiments, the protocol was scaled up to 10 cm-square petri dishes containing 30 mL of pollen germination medium. 25 mg of pollen grains were spread per plate with a soft brush in a moisture incubation chamber, sealed, and placed in the dark at 24°C or 35°C for 5 h before being collected. Three plates per temperature were prepared from each of the biological replicates. For the RTqPCR experiments the protocol was the same, with two biological replicates of 6 month-old frozen pollen grown for 5 h at 24°C or 35°C, and one additional treatment for 3 h at 24°C and two additional hours at 35°C.
2.3 Preparation of RNA and RPF libraries for Riboprofiling
Pollen was scrapped from the plates and ground in liquid nitrogen in an optimized ice-cold extraction buffer for Riboprofiling experiments (Hsu et al., 2016) containing: 0.1% Tris–HCl pH 8, 1% sodium deoxycholate, 40 mM KCl, 20 mM MgCl2, 10% polyoxyethylene-10-tridecyl ether, 1 mM DTT,100 μg/mL cycloheximide, 10 U/mL DNAse I (Illumina, USA). After centrifugation, the supernatants were transferred into a pre-chilled tube and split into 100 μL (for RNA library) and 200 μL (for RPF library) aliquots. Estimation of RNA concentration was performed with NanoDrop ND1000 (Thermo Fisher Scientific, USA). The libraries were obtained using the Illumina® TruSeq® Ribo Profile Mammalian kit (Illumina), with slight modifications to the standard protocol. The 100 μL samples of total RNA were denatured by adding SDS to 1% final concentration and kept aside until needed. In parallel, generation of ribosome protected fragment consisted of digesting 200 μg of RNA with 100 U of TruSeq Ribo Profile Nuclease for 1 h at 21°C, with shaking at 300 rpm. Reaction was stopped by adding 1 U/μL of SUPERase In RNase Inhibitor (Thermo Fisher Scientific, USA), then ribosomes were purified by size exclusion columns using MicroSpin S-400 columns following provider's recommendations (GE Healthcare, UK). Recovered ribosomes were denatured by adding SDS to 1% final concentration, and then both ribosome-bound RNA and denatured total RNA samples were purified with RNA Clean & concentrator-25 kit (Zymo Research, USA). RPFs were size-fractionated on 15% urea polyacrylamide gel electrophoresis (PAGE), by recovering fragments of 28 to 30 nt after gel staining with SYBR Gold (Thermo Fisher Scientific, USA), using Truseq Ribo Profile RNA Control as reference. Purified RPFs and total RNAs were rRNA depleted using the Ribo-Zero Magnetic bead kit (Illumina, USA) by adding 50 μL or 100 μL of magnetic beads per RPF or RNA samples respectively, according to manufacturer's instructions. Heat fragmentation of total RNA, end repair of RNA and RPF as well as downstream steps for 3′ adapter ligation, adaptor removal, reverse transcription, PAGE purification of cDNA and cDNA circularization were done following strictly the Illumina kit protocol. The cDNA Libraries were amplified by 13 and 15 PCR cycles for RNA and RPF, respectively. Library fragments of expected size were purified by non-denaturing PAGE and, after purification, DNA integrity and concentration were checked using Bioanalyzer 2100 expert High Sensitivity DNA Assay (Agilent Technologies, Inc). Equimolar (5 nM) pools of libraries were prepared for 100 bp paired-end sequencing on Illumina HiSeq4000 platform at Macrogen (Korea). Raw sequences and table containing counts and normalized TPM values generated in this study have been deposited in the Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo/) with the accession number GSE145795.
2.4 RTqPCR analsysis
The protocol for RNA extraction was the same as for the RNA sequencing libraries until the step of RNA purification with RNA Clean & concentrator-25 kit (Zymo Research, USA). After the estimation of RNA concentration performed with NanoDrop ND1000 (Thermo Fisher Scientific, USA), 500 ng of total RNA were used for cDNA synthesis with the PrimeScript RT reagent kit (Takara Bio Inc. Otsu, Shiga, Japan) in a volume of 10 μL and finally diluted with sterile water to 50 μL. Real time PCR was performed in triplicates with 0.5 μL of the cDNA using the PyroTaqEvaGreen qPCR mix according to the manufacturer's instructions (Cultek Molecular Bioline, Spain). The primers used are shown in Table S7 with the indicated references (Chen & Brandizzi, 2012; Czechowski, Stitt, Altmann, Udvardi, & Scheible, 2005; Ohama et al., 2016; Sze et al., 2004). The housekeeping gene (YSL8) suggested in previous studies as normalization gene (Czechowski et al., 2005) was analysed in our RNA sequencing libraries to confirm stable expression in pollen at different temperatures. Primers designed in this study were designed with the web tool pcrEfficiency (Mallona, Weiss, & Egea-Cortines, 2011). The quantification and statistical analysis of relative mRNA expression was performed as described (Ahmed & Kim, 2018).
2.5 Bioinformatic data analysis
To determine which of the paired files corresponded to reads from the coding strand, htseq-count v0.10 was used and the files were kept for downstream analysis. After quality analysis with FastQC v0.11.5, reads were processed for adaptor and low quality regions removal with cutadapt v1.16 (Martin, 2011). Clean, trimmed reads ranging between 20 to 40 nt for RNA samples and 20 to 30 nt for RPF samples were selected for downstream analysis. Reads corresponding to rRNA, tRNA, snRNA and snoRNA sequences were identified and removed with bowtie2 v2.3.2 (Langmead & Salzberg, 2012) using the set of rRNA, tRNA, snRNA and snoRNA TAIR10 sequences downloaded from Ensembl Plants (Cunningham et al., 2018). The remaining reads were mapped to the TAIR10 Arabidopsis reference genome using HISAT2 v2.1.0 (Kim, Paggi, Park, Bennett, & Salzberg, 2019), and entries for reads mapping to more than one locus were excluded with samtools v1.5 (H. Li et al., 2009). For RPF samples, 27 to 28 nt uniquely mapped reads were selected for downstream analysis using the Linux command line “awk.” The final sets of RNA 20 to 40 nt and RPF 27 to 28 nt uniquely mapped reads were assigned to specific genes, 5′UTRs, CDSs and 3′UTRs using htseq-count v0.10 (Anders, Pyl, & Huber, 2014) and the last Arabidopsis TAIR10 genome annotation from Araport11 (Cheng et al., 2017) containing 37,336 genes. Correlation heat-maps were obtained with multiBamSummary and plotCorrelation, from the deepTools v3.1.0 package (Ramírez et al., 2016). PCA analysis was performed in R v3.4.4, using the cluster v2.1.0 (Mächler, Rousseeuw, Struyf, Hubert, & Hornik, 2012), Biobase v2.38.0 (Huber et al., 2015), qvalue v2.10.0 (Storey, Bass, Dabney, & Robinson, 2019) and fastcluster v1.1.25 (Müllner, 2013) packages. Ribowave v1.0 (Xu et al., 2018) was used for the study of periodicity of RPF reads, and Xtail v1.1.5 (Xiao, Zou, Liu, & Yang, 2016) for the differential transcriptional/translational analysis and for the studies of differential translational efficiency. The fold change comparisons always refer to the 35°C data (treatment) versus the 24°C data (no treatment). The GO term enrichment analysis was performed using the web-based g:Profiler toolset (Raudvere et al., 2019) with the g:SCS threshold method and either annotated genes or custom list of genes when indicated. RStudio v1.2.5001 (TeamRStudio, 2019) was used with the ggplot2 library for correlation plots and periodicity graphs, and with the UpSetR library for the intersection set analysis. To obtain the heat-map viewer for gene expression under different pollen developmental stages we used the web-based tool “Arabidopsis Heat Tree Viewer” (http://arabidopsis-heat-tree.org/). Comparison of gene lists with Venn diagrams was performed with the web tool Venny 2.1 (Oliveros, 2015). Graphical visualization of the read coverage on mapped genes was performed with the Integrative Genomics Viewer (IGV) (Robinson et al., 2011).
3 RESULTS
3.1 Establishing the protocol for Riboprofiling studies of pollen response to elevated temperature
We first established a protocol for large-scale pollen collection and in vitro pollen germination necessary to perform the Riboprofiling studies. We grew around 60,000 A. thaliana Col-0 plants under greenhouse conditions to collect pollen by vacuum filtration with a modified vacuum cleaner as previously published (Johnson-Brousseau & McCormick, 2004). Pollen was collected and, after dehydration, stored in dry aliquots at −20°C to allow the synchronous processing of the samples. A small amount of every frozen aliquot, after proper rehydration, was tested for germination under in vitro conditions. Only non-contaminated samples showing similar pollen germination percentages were used to quantify germination and pollen tube growth under optimum temperature at 24°C and limiting high-temperature conditions. The effects of the high temperature on pollen growth have not been so well characterized for A. thaliana pollen germinated in vitro, so we performed preliminary tests in the range 32°C to 35°C and found that treatments at 35°C restricted pollen germination and growth without complete inhibition. Therefore we used 35°C as the limiting high-temperature condition. As shown in Figure 1A (left), large variations in pollen germination rates after cold-storage were found as it was shown in previous studies (Bou Daher, Chebli, & Geitmann, 2009) with a maximum germination percentage after 5 h of incubation at both temperatures, and germination was slightly affected by the elevated temperature. The pollen tube growth displayed more reproducible patterns allowing us to measure significant differences between both temperatures, showing a severe growth inhibition at 35°C from early growth stages (Figure 1A, right). We, therefore, selected a time point of 5 h of incubation to collect three biological replicates of germinated pollen both at 24°C and 35°C for Riboprofiling analysis. Once in vitro germinated pollen was collected by scrapping from the plates, every replicate was divided in two samples for parallel isolation of total RNA (RNAs) and ribosome protected fragments (RPFs), therefore, a total of 12 libraries were prepared for next-generation sequencing (Figure 1B).
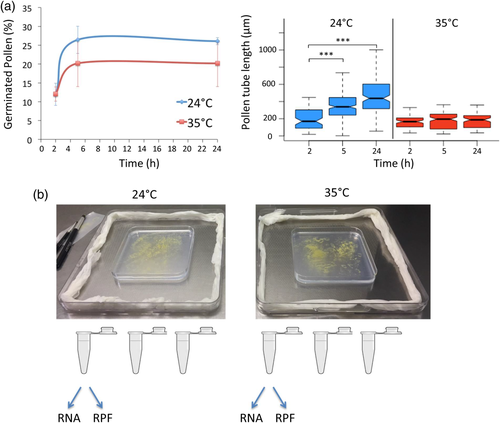
3.2 Riboprofiling shows enrichment of coding sequences in germinated pollen
High-throughput sequencing was performed using Illumina HiSeq4000, yielding a total of over 3,100 million paired-end 100 base reads for the 12 libraries. The correlation and PCA analyses among the different RNA and RPF libraries are shown in Figure S1. The data show very good cluster separations between the type of library (RNA and RPF) and between the temperature treatments (24°C and 35°C), with correlation coefficients above 0.95 among the triplicates. We then analysed the RNA and the RPF read length distributions (Figure S2A). Although RNA reads were spread in a large range size from 20 to 40 nucleotides, the RPFs showed a notable accumulation around 27 and 28 nucleotides as expected for ribosome footprint protection in A. thaliana (Hsu et al., 2016). After mapping to the A. thaliana reference genome, the read distributions were computed on 5′UTR, coding regions and 3′UTR, showing that ribosome footprints mapped to higher extent to coding sequences and to lesser extent to untranslated regions compared to RNA reads (Figure S2B). The bias of RPFs towards the translated frame can be used to infer the position of the ribosomal peptidyl-site (P-site) as a very good proxy to determine whether the ribosome is in frame with either start or stop codons. As an example, the meta-gene computing analysis of RPF distribution for 28 nucleotide fragments of one library is shown in Figure S2C,D. The data clearly show a well-defined trinucleotide (3-nt) periodicity with the enhanced peaks corresponding to the start and stop sites, and depict the expected coverage of −12 and −15 nucleotide position for the initiation P-site and termination A-site respectively, as it has been shown for high quality A. thaliana RPF libraries (Hsu et al., 2016). We found a very strong periodicity for the fragments of 27 and 28 nucleotides in all the libraries, therefore, we selected those reads for further analysis.
3.3 The transcriptome and translatome landscapes of germinated pollen at optimum temperature
The gene-specific read counts for RNA and RPF were normalized using the transcript per kilobase million (TPM), as it shows better consistency than reads per million per kilobase (RPKM) normalization for comparisons among different samples (Li, Ruotti, Stewart, Thomson, & Dewey, 2010; Wagner, Kin, & Lynch, 2012). The complete set of raw counts and normalized TPM data for the A. thaliana genome have been deposited at GEO with the accession number GSE145795. Although a minimum value of 1 TPM has been established as a threshold to qualify a gene as expressed in other plant tissues (Hofmann, Schon, & Nodine, 2019) and metazoans (Hebenstreit et al., 2011), we chose the more conservative value of 2 TPM. We then defined the A. thaliana pollen transcriptome at 24°C as the set of genes having at least 2 TPM in the average of the 3 replicates of RNA samples at 24°C, with the condition that at least 2 of the 3 replicates should also score a minimum of 2 TPM values. The table of 6,098 genes corresponding to the germinated A. thaliana pollen transcriptome at 24°C can be found as Table S1_Tab1.
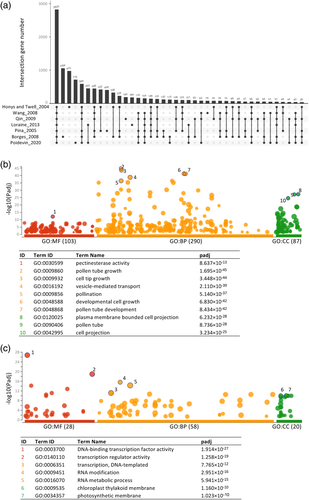
With the aim to validate our transcriptome list of germinated pollen at 24°C, we first checked the expected absence of photosynthetic genes in our samples of heterotrophic tissue by analysing the expression values of 30 light-harvesting complex genes of photosystem I and II (Umate, 2010) and we found all of them either not detectable or with TPM values close to zero. Next, we compared the transcriptome gene list with previously reported Arabidopsis pollen gene expression at different developmental stages (Borges et al., 2008; Honys & Twell, 2004; Loraine, McCormick, Estrada, Patel, & Qin, 2013; Pina, Pinto, Feijó, & Becker, 2005; Qin et al., 2009; Rutley & Twell, 2015; Wang et al., 2008). To find the common set of pollen transcriptome genes among the different gene lists, we used the UpSetR bioinformatics tool to visualize the intersecting sets (Conway, Lex, & Gehlenborg, 2017). Figure 2A shows the number of genes for all possible intersections among the different lists, with 2,825 genes common to all of them. In addition to the set of genes common to all datasets, our defined pollen transcriptome gene list also includes genes catalogued as pollen-expressed genes in the different published sets. The differences very likely account for variations among the pollen developmental stage, growth conditions, RNA extraction procedures and the technologies used (microarrays versus RNA-Seq), but also for the threshold values used to define a gene as being expressed. To further check whether the set of genes properly define the pollen transcriptome, we used the web-based g:Profiler toolset (Raudvere et al., 2019) to find enriched and underrepresented biological categories in our transcriptome gene list. The results shown in Figure 2B, display the presence of expected overrepresented terms such as “pectinesterase activity,” “pollen tube growth,” “vesicle-mediated transport” and “cell projection” among others. On the contrary, Figure 2C shows underrepresented GO terms including “transcription regulator activity,” “RNA metabolic process,” “chloroplast thylakoid membrane” and “photosynthetic membrane,” expected down-regulated terms during pollen development.
Once we confirmed that our transcriptome list adequately reflected the mRNA levels of genes involved in pollen germination and growth, we wanted to compare these data with those provided by RPF libraries. These libraries represent the quantitative translational status of the gene set or the translatome, defined as the ribosome-bound mRNA fragments considered to be actively translated in a given situation. We defined the A. thaliana pollen translatome at 24°C as the set of genes having at least 2 TPM in the average of the 3 replicates of both RNA and RPF samples at 24°C, with the condition that at least 2 of the 3 replicates should also score a minimum of 2 TPM values. The translatome gene list for germinated pollen at 24°C (shown in Table S1_Tab2), accommodates a total of 4,782 genes. We were intrigued by the large list of 1,316 genes absent in the translatome but present in the transcriptome, shown in Table S1_Tab3. Therefore, we performed a GO analysis with this gene list and identified several terms related to “DNA replication,” “DNA repair” and “chromosome organization,” which are typical GO terms for sperm cells as shown in Figure S3A. In fact, 40% of the 1,316 list of genes are present in the list of sperm genes defined by Borges and coworkers (Borges et al., 2008). Figure S3B shows one example of strong translational repression with the IGV visualization of RNA and RPF reads for the gene AT5G16020 encoding a gamete expressed protein GEX3 only functionally relevant in the female side (Alandete-Saez, Ron, & McCormick, 2008). This indicates that a strict translational regulation occurs during pollen germination to avoid the translation of unnecessary transcripts at this developmental stage.
The use of proteomic approaches has revealed only a modest correlation between mRNA transcript and protein levels (Baerenfaller et al., 2008; Maier, Güell, & Serrano, 2009; Vogel & Marcotte, 2012), therefore, we carried out a correlation plot analysis between the germinated pollen transcriptome and translatome (Figure S4A). The correlation R value of 0.88 suggests a rather high correspondence between the transcriptome and the translatome for germinated pollen at 24°C, although some exceptions can be detected. To visualize the differences in the translational profile revealed by the RPF values we used RiboWave (Xu et al., 2018), a pipeline able to denoise the original RPF signal and extract the 3-nt periodicity of the reads known as Periodic Footprint P-site or PF P-site. In Figure S4B we plotted the PF P-site values of two gene examples taken from Figure S4A with similar transcriptional value but obvious differences at the translational level (RPF), the main differences are visualized by alterations in PF P-site values throughout the coding region. The higher translated gene AT5G50030 displays an average of 1,332 PF P-site reads compared to the 124 average reads of AT3G42640. Altogether we have obtained representative gene lists of transcriptome and translatome of A. thaliana pollen (Table S1) germinated at optimum temperature (24°C).
3.4 Transcriptional and translational alterations of germinated pollen by high temperature
The RNA and RPF libraries of germinated pollen at 35°C were computed the same way as it was done previously with the 24°C libraries, allowing us to generate both the transcriptome and translatome gene lists of germinated pollen at 35°C shown in Table S2_Tab1 and Tab2, respectively. Similar to the libraries at optimum temperature, the gene numbers at the restrictive temperature of 35°C were 6,089 for the transcriptome and 4,742 for the translatome. We compared the intersection sets among the 4 gene lists generated (transcriptome 24, translatome 24, transcriptome 35 and translatome 35) using Venn diagrams (Figure 3A). A total of 4,386 genes were common to all 4 sets of genes; 1,415 genes present in the transcriptomes were absent in the translatome lists and 919 genes were exclusively expressed at either 24°C (464 genes) or 35°C (455 genes).
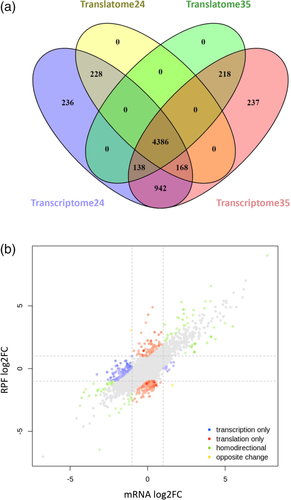
To perform a thorough statistically-based analysis of differential expression for the transcriptomes and translatomes at 24°C and 35°C, we used the Xtail pipeline, a DESeq2-based protocol with improved sensitivity and accuracy to quantify differential translations with ribosome profiling data (Xiao et al., 2016). To apply the Xtail analysis, we first generated a valid list of genes using only those present in either the translatome 24 or the translatome 35 to avoid invalid data processing for those genes present in transcriptomes but absent in the translatome gene lists. The valid list of genes for Xtail analysis contains 5,138 genes (Table S3). Then we run the Xtail pipeline with the valid list of genes to obtain a table (Table S4) that summarizes the quantitative differences at the transcriptional (log2FC mRNA) and translational level (log2FC RPF). Figure 3B displays the plot of transcriptional and translational differences of A. thaliana pollen germinated in vitro upon heat shock. We found that the vast majority of genes with differential expression (log2FC > 1 or log2FC < −1) display homodirectional changes for the transcriptome and the translatome, with a low number of genes displaying unique transcriptional or translational changes (blue and red coloured dots in Figure 3B). We initially picked those up- or down-regulated genes, both at the translational and transcriptional level, as a proxy for the major increases and reductions of protein levels. According to the restrictive selection criteria, we found 355 up-regulated and 300 down-regulated genes (Table S4). Both lists of genes were analysed with the g:Profiler tool for the presence of enriched terms compared in this case to the valid list of 5,138 genes used for the Xtail analysis.
3.4.1 Heat up-regulated HSP and ER stress genes.
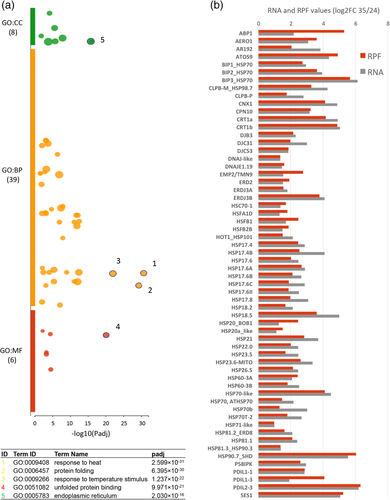
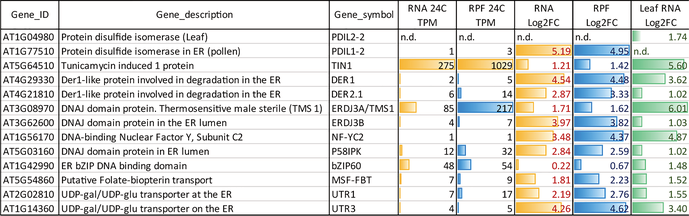
As it is shown in Figure 4A among the up-regulated genes we could find enriched terms related to “response to heat,” “protein folding,” “response to temperature stimulus,” “unfolded protein binding” and “endoplasmic reticulum.” Figure 4B shows the comparison of RNA and RPF log2FC values >1 for a list of representative genes of the most significant GO terms identified, including members of the large heat-shock protein family mostly the small HSP20-like, DNAJ chaperones, ER-related proteins and other members of the unfolded-protein-response (UPR) and the cytosolic-protein-response (CPR) pathways (Table S5).
In a more detailed analysis, Table 1 shows the transcriptional and translational expression levels in germinated pollen for the list of 13 genes co-induced by the three stressor pathways (heat shock, UPR and CPR), previously reported for A. thaliana leaves (Sugio et al., 2009). It is remarkable that all the genes are also expressed and induced in germinated pollen upon heat-stress, with the only exception of AtbZIP60, a key gene regulator of the UPR pathway (Iwata & Koizumi, 2005) present in germinated pollen although neither transcriptionally nor translationally induced by heat. Altogether the data show that the highly conserved and protective cellular response to heat stress in terms of protein folding seems to be activated in pollen as in vegetative tissues, sharing transcriptional signatures with the related UPR and CPR pathways.
|
3.4.2 Heat down-regulated mainly transport genes
On the other hand, we examined the down-regulated list of genes with the aim to identify heat-sensitive pathways. We found strong enrichment of terms defined as “secondary active transmembrane transporter,” “active ion transmembrane transporter,” or “carbohydrate:proton symporter” all linked to key pathways related to ion homeostasis and secondary transport of carbohydrates coupled to H+ gradient (Figure 5A). Figure 5B shows the comparison of RNA and RPF log2FC values <1 for transporter genes detailed in Table S6. One paradigmatic case is the enrichment of the cation/H+ exchangers (CHX) down-regulated upon heat stress. The CHX gene family contains 28 members in A. thaliana which have been postulated to participate in diverse pollen developmental activities such as ion and metabolite transport, osmotic adjustments, vacuole formation and vesicular trafficking among others (Sze et al., 2004). Although some functional redundancy has been found between CHX transporters (Chanroj, Padmanaban, Czerny, Jauh, & Sze, 2013; Evans, Hall, Pritchard, & Newbury, 2011; Lu et al., 2011), 23 of them displayed >2 TPM values in both 24°C and 35°C transcriptomes and, therefore, can be considered as bona fide members of the germinated pollen transcriptome.
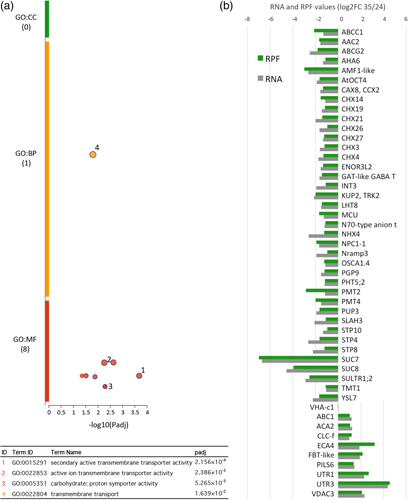
Most of the CHX gene family members display a down-regulation both at the transcriptome and translatome levels although only seven members display values below our stringent cutoff (log2FC < 1) as shown in Figure 5B. In addition to the CHXs, another gene family negatively affected by elevated temperature at the transcriptional and translational level during pollen germination is the sucrose/H+ transporter family. Arabidopsis has 9 genes encoding sucrose/H+ symporters (AtSUC1-AtSUC9) mostly expressed in sink tissues and phylogenetically distributed in three subtype groups (Sauer, 2007). Our transcriptome and translatome data indicate that 5 out of the 9 gene family members are transcribed and 4 of them properly translated at optimum temperature, but most of the members display negative log2FC values for transcriptome and translatome. In fact, two of them (AtSUC7 and AtSUC8) show the top-ranking values of down-regulated genes by heat stress according to the results of the Xtail analysis (Table S4). In a similar manner, the related family of hexose transporters STP is negatively affected by temperature with three members displaying negative log2FC values <1. In addition to these predominant gene families, the list of membrane transporters down-regulated at high temperature is remarkable including primary membrane-energizing transporters such as AHA 6 which may be highly deleterious for pollen tube growth considering the importance of H+ homeostasis. In contrast, the vacuolar H+ ATPase VHA, and many diverse membrane transporters showed little to no significant changes or even induction after the heat shock (Figure 5B). Overall, these data suggest that a failure in the orchestrated presence of at least 39 membrane transporters under heat stress could be detrimental for proper germination and growth of the pollen tube.
To gain further insight into the roles of the heat-sensitive membrane transporters, we checked their expression during pollen development, as previous studies showed specific expression patterns during pollen maturation (Bock et al., 2006). We used transcriptome data published by others (Borges et al., 2008; Qin et al., 2009) showing expression of A. thaliana pollen in different stages: dry pollen, pollen germinated in vitro at two different times (0.5 and 4 h), pollen germinated semi in vivo (SIV) and sperm cells. Germinated pollen includes a vegetative tube cell and two sperm cells, and when germinated in SIV condition refers to pollen tubes emerged from a cut style on nutrient medium after germinated on a stigma (Qin et al., 2009). As shown in Figure 6A, we found a striking relationship between a subset of heat-down regulated transporters in our analysis and their up-regulation in SIV germinated tubes at basal temperature compared to dry pollen.
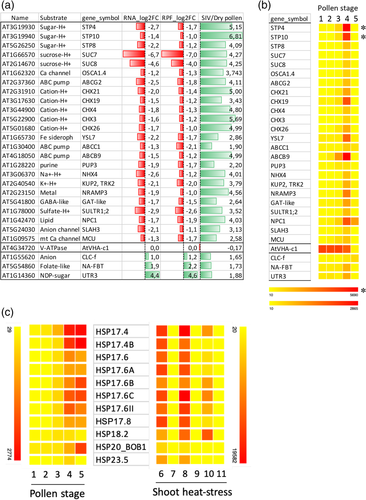
To further illustrate this finding, we used the web-based tool “Arabidopsis Heat Tree Viewer” to compare five different pollen developmental stages. As it can be seen in Figure 6B, there is a notable up-regulation of gene expression for at least 23 transporters after 4 h in vitro growth and especially in tubes grown under SIV. We also checked the expression during these five pollen stages of the heat-induced genes identified previously in our dataset, to find co-regulated patterns, and we focused on the 17 pollen-expressed genes induced by heat belonging to the conserved family of the small heat shock proteins (sHSPs) in plants (Scharf, Siddique, & Vierling, 2001; Waters, 2012). We found that 11 of them displayed a similar pollen gene expression pattern to the transporters, with increased expression during in vitro germination further enhanced in SIV conditions, whereas in shoots, although highly induced by heat, their basal expression is null (Figure 6C). These data suggest that during pollen germination there is a strong requirement for expression of key ion and carbohydrate transporters together with proteins involved in folding and refolding, and, during heat stress conditions, in spite of a proper induction of the folding machinery, a lack of gene expression response for the transporters may lead to pollen tube growth defects.
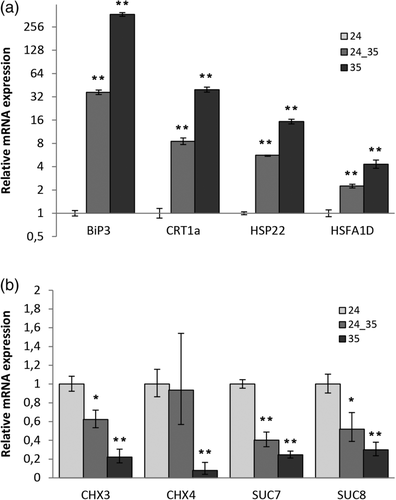
To further verify the gene expression data obtained by deep sequencing, we selected 4 up- and 4 down-regulated genes to validate the data with RTqPCR analysis. After rehydration, pollen was grown for 5 h both at 24°C and 35°C. In addition, to differentiate the effect of heat on germinated pollen, we run in parallel another in vitro test where pollen was grown for 3 h at 24°C and then shifted to further 2 h at 35°C (sample 24_35). The data obtained are shown in Figure 7. We could reproduce the sequencing data with the RTqPCR analysis when comparing the 24 and 35 samples for the up- and down-regulated genes. In the case of the shifted sample (24_35) the data also confirm the results for the up-regulated genes indicating that germinated pollen at optimum temperature does respond to the heat treatment. The data obtained for the down-regulated genes also confirm the down-regulation by heat although the differences are not so dramatic. A plausible explanation for these cases is that the mRNA half-life also accounts for the amount of mRNA present in the sample in particular for short heat treatments.
3.4.3 Changes in translational efficiency during heat stress
We analysed whether Riboprofiling revealed any alteration in translational efficiency (TE), referred to the changes in the ratio of RPF to RNA counts for a given gene between two different conditions. The Xtail algorithm uses two parallel pipelines to quantify the TE changes, first as the difference between the log2FC of RPF and RNA across the two temperature conditions, and second as the difference between the log2 ratios of RPF to RNA in both conditions (log2Rs). Both calculations should yield a similar result, and the more conserved pipeline with better p-value is selected for the final assessment (log2FC_TE) of differential translation (Xiao et al., 2016). Figure S5A shows the correlation plot of log2Rs at 24°C and 35°C where a notable homodirectional pattern, similar to the transcriptome versus translatome shown in Figure 3B, can be observed. In spite of some exceptions (coloured dots in Figure S5A), most of the alterations in TE occur for RNA and RPF in a correlated manner, therefore we see no evidence of wide effects of high temperature on translation dynamics. According to acceptable p-value < .05 we found 77 and 120 genes with down-regulated or up-regulated log2FC_TE values, respectively (Table S4). In spite of the low number of genes affected (3.8%), we investigated the nature of those changes by considering all possible regulatory scenarios. Table 2 shows a summary of all the 9 possible changes at either the RNA or RPF between both temperatures that may lead to final TE changes.
| log2FC RNA | log2FC RPF | TE Up (# genes) | TE Up (%) | TE Down (# genes) | TE Down (%) |
|---|---|---|---|---|---|
| − | − | 16 | 13.3 | 2 | 2.6 |
| + | − | 0 | 0 | 1 | 1.3 |
| = | − | 0 | 0 | 45 | 58.4 |
| − | + | 2 | 1.7 | 0 | 0 |
| + | + | 9 | 7.5 | 9 | 11.7 |
| = | + | 28 | 23.3 | 0 | 0 |
| − | = | 52 | 43.3 | 0 | 0 |
| + | = | 0 | 0 | 1 | 1.3 |
| = | = | 13 | 10.8 | 19 | 24.7 |
| Sum (% pollen translatome) | 120 | 2.3 | 77 | 1.5 | |
As it can be seen, among the down-regulated TE genes, a large number (58.4%) show changes caused by RPF with no RNA alterations, suggesting specific translational inhibition mechanisms. Intriguingly, the case is the opposite for a large proportion (43.3%) of the up-regulated TE genes with most of the changes affecting at the RNA level. The graphical representation of log2FC_TE as a function of the p-value is shown in the volcano plot of Figure S5B. Some exceptions to the homodirectional pattern are the ATP-dependent caseinolytic (Clp) protease (AT1G09130) which suffers a strong down-regulation at the translational level with the increase in temperature, or the annotated as non-coding RNAs AT5G06845 and AT1G05853 with surprisingly enhanced ribosome footprints at 35°C. In fact, the top three genes with up regulated log2FC_TE values are non-coding RNAs with RPF values unexpectedly increased at 35°C (Table S4). The functional interpretation of RPF reads on annotated non-coding RNAs remains to be clarified and is considered in the next section.
3.5 Revisiting predicted functions and annotations for the translatome of germinated pollen
In spite of experimental and bioinformatics removal of rRNAs, tRNAs, snRNAs and snoRNAs, our transcriptome and translatome lists contained genes annotated as non-coding RNAs. These include mostly long-non-coding RNAs, transposable elements and a few small-nucleolar RNAs that probably escaped the filtering due to annotation issues. Apart from their possible roles as regulatory transcripts with transcriptional and post-transcriptional impact, some of these RNAs show significant RPF reads suggesting the presence of ribosomes according to the very strict RPF size used in the analysis (only 27 and 28 nt long). One paradigmatic example is the gene AT2G41310 with very high transcriptome values both at 24°C and 35°C, but very low RPF values (Tables S3 and S4). This gene, annotated as ARR8/ATRR3, encodes an A-type response regulator involved in cytokinin-mediated signalling, and it caught our interest as it was not expected to be expressed in pollen. Figure S6A shows the IGV coverage data showing that the reads fall into the 3′ untranslated region overlapping with a non-coding RNA (AT2G09250) in the opposite orientation. Therefore, there is no doubt that read coverage in that region does not correspond to the encoded ARR8 protein. We then used the Ribowave pipeline to examine the periodicity of PF P-sites in that region. The results shown in Figure S6B demonstrate the presence of periodic P-site footprints at both temperatures matching different putative open reading frames, thus suggesting that this gene could be translated in germinated pollen. Similar observations were found for other non-coding-RNAs showing detectable RPF reads in the genes AT5G06845 and AT1G05853, however, the limiting PF P-site density and low coverage values in most of these cases precludes the achievement of any definitive conclusion as to whether those genes are indeed translated.
4 DISCUSSION
In this work we have used a high-resolution ribosome profiling technology to achieve a comprehensive study of how heat affects both the transcriptome and the translatome of A. thaliana in vitro germinated pollen. In the course of this work, we found some limitations of using in vitro germinated pollen after cold-storage, needed to accomplish the large-scale pollen collection required for the Riboprofiling analysis. For instance, a large number of pollen grains do not complete germination even under optimum growth conditions after cold-storage, but still the percentage of germinated pollen grains (with highly reproducible tube growth behaviour) is sufficient to evaluate gene expression according to the data obtained. Another example illustrating the validity of our approach is that the transcriptome and translatome changes shown are largely affecting the pollen vegetative cell, and in fact, we have observed strong translational repression of GO terms specific for sperm cells that under those conditions would be inactive as the case example gene GEX3 (Figure 3B). The gene GEX3, known to be transcriptionally active in both male and female gametophytes, showed no phenotypic alteration for pollen development when down-regulated, and the analysis of gamete-specific GEX3 antisense and overexpression transgenic lines had reduced seed set caused exclusively by defects on the female gametophyte (Alandete-Saez et al., 2008). Other strategies to characterize the Arabidopsis pollen translatome in vivo followed different experimental approaches, like the use of transgenic plants expressing tagged ribosomal genes from heterologous pollen promoters and the isolation of mRNAs co-precipitating with ribosomal subunits; leading to a rather different dataset (Lin et al., 2014) as one would expected for too many differences in the nature of the biological samples, the processing and the downstream technologies implemented.
Our results provide novel insights on the effects of high temperature on pollen gene expression after obtaining a complete dataset of the transcriptome and the translatome of in vitro germinated pollen at basal (24°C) and restrictive temperature (35°C). The differential expression analysis uncovered new findings that can be summarized as follows: (i) the conserved cellular machinery to cope with heat stress is well functioning in germinated pollen, as we found up-regulated many genes of the heat-response, the UPR and the CPR pathways; (ii) the enrichment of membrane transporters, involved in ion and carbohydrate transport among the down-regulated genes, identified heat-sensitive targets; (iii) we detected a co-regulation between heat-sensitive membrane transporters and heat-induced proteins during specific stages of pollen germination, namely in vitro and SIV, illustrating new co-regulatory aspects of pollen germination; (iv) although we found a high correlation between transcription and translation, specific translational efficiency alterations could also be uncovered in germinated pollen; and (v) we could detect the presence of ribosome footprints on non-coding RNAs, illustrating the need of further refinement for gene expression on germinated pollen.
An important question concerns the nature of the up- and down-regulation gene expression patterns. As we have monitored RNA and RPF levels in response to heat, it is unclear whether their alterations are due to changes in the synthesis or degradation, or both. Pollen grains contain stored mRNA, and it is assumed the mRNAs can be released and immediately translated after pollination to support rapid tube growth (Mascarenhas, 1993). Recent data have shown the presence of cytoplasmic mRNA granules, namely stress granules (SGs), in mature pollen grains co-localizing with processing body (PB) proteins, thus suggesting the presence of PBs during pollen maturation (Scarpin et al., 2017). Both SGs and PBs are intimately connected with each other and with the translational machinery, to modulate the mRNA turnover during stress conditions (Chantarachot & Bailey-Serres, 2018). Thus, an increase in transcript could reflect a combination of newly synthesized transcripts and released mRNAs from storage granules, and a down-regulation could be caused by reduced transcription or enhanced degradation in PBs, or both. The experiments shown in Figure 7 with germinated pollen at 24°C for 3 h and shifted for additional 2 h at 35°C suggest that mRNA turnover may impact at higher extent for down-regulation of gene expression, although this will require future experiments to quantify mRNA half-lives.
The differential gene expression analysis provided clues on the cellular pathways up-regulated and down-regulated by heat stress. Among the up-regulated pathways, we could find a significant enrichment of GO terms related to heat shock response, protein folding and quality control ER-dependent pathways demonstrating that A. thaliana pollen tube is able to sense and respond to heat stress in a similar manner to ER-dependent stress responses in leaves (Sugio et al., 2009). As a step to identify the molecular basis of heat sensitivity, we studied enriched GO terms of down-regulated genes. We focused on membrane transporters, as they were enriched among the 300 genes down-regulated by heat. Over 260 transporter genes expressed in germinated pollen we found 39 of them (14.8%) down-regulated, so for the majority of transporters, including the vacuolar H+ pump ATPase VHA, heat had little effect. However, down-regulation of those 39 genes affected to transporters with key functions during pollen tube growth, including the primary pump H+-ATPase AHA6, calcium channels like OsCA1.4, ABC pumps and H+-coupled secondary transporters for K+, anions (sulfate, phosphate), metals and amino acid transporters like LHT8. Most prominent is the large number of H+-coupled sugar transporters (SUC and STP genes) and members of the cation/H+ exchanger family (CHXs) with most of their family members affected. Altogether, the massive down-regulation of membrane transporters by heat may lead to devastating consequences due to the strong requirements of highly active vesicular transport during pollen tube growth.
We also discovered that about two thirds of these down-regulated transporters by high temperature are also up-regulated during in vitro germination and especially when pollen tubes were grown under semi in vivo (SIV) conditions (Qin et al., 2009). Increased expression of sucrose and hexose transporters likely provides carbon source for energy and for synthesizing pollen walls, but also signalling roles have been proposed (Rottmann, Fritz, Sauer, & Stadler, 2018a). So, is there any relationship between down-regulation of transporters by heat and their up-regulation by germination conditions? We have shown that, as in leaves, germinated pollen stressed by heat also triggers up-regulation of folding (chaperone HSPs) and quality control pathways, and many of those up-regulated genes are also induced under in vitro and in SIV germination conditions. One prevalent example is the family of cytoplasmic small HSPs (sHSPs), abundantly expressed in germinated pollen under heat stress as they are required to prevent protein aggregation. We have found that 13 out of 17 pollen expressed sHSPs are also induced during SIV, thus uncovering potential roles of these chaperones in the germination process. One of these chaperones, the sHSP BOB1 protein, was found incorporated into heat shock granules (HSGs) at high temperature (Perez et al., 2009). Therefore, we cannot discard that assembly of HSGs, stress granules and processing bodies may lead to either sequestration or targeted degradation of specific mRNAs instead of inhibition of transcription, to explain the gene down-regulation of transporters upon heat stress in germinated pollen.
An advantage of the Riboprofiling technology is that simultaneous identification of mRNA and RPFs allows the quantification of changes in translational efficiency (TE). The differential analysis of TE upon heat stress revealed that only a minor subset of genes displayed significant TE alterations. The sources of TE alterations are very diverse since they may affect to either RNA or RPF levels both at 24°C and 35°C. It is remarkable that, among the TE down-regulated genes, most of them include changes exclusively in the RPF, as an indication of translation-specific repression. On the other side, among the TE up-regulated genes, almost half of them are caused by changes in RNA levels. The nature of the translational regulations remains to be elucidated.
In addition to detecting gene expression changes, Riboprofiling analysis allows the direct observation of the precise mapping positions of the reads, using integrated visualization programs such as IGV. For instance, a questionable annotation could lead to erroneous hypothesis in the case of the ARR8 encoded gene (AT2G41310). Another example is the case of AtSUC7, initially considered as a pseudogene (Sauer et al., 2004), and recently shown to be translated in heterologous systems with functional restrictions on sucrose analogue transport (Rottmann et al., 2018b). Here we show that AtSUC7 is transcribed and translated in germinated pollen and it suffers a dramatic down regulation at both transcriptional and translational levels upon heat stress. The Riboprofiling of A. thaliana germinated pollen has also uncovered the unexpected presence of RPF reads on several non-coding-RNAs, such as long-non-coding-RNAs and small-nucleolar-RNAs. Additional experimental and functional data will be required to unequivocally show that those non-coding-RNAs are indeed subjected to translation during pollen germination and growth.
As a conclusion, we can affirm that the use of in vitro germinated pollen is a useful system to understand the molecular basis of heat-induced responses. Riboprofiling has provided many answers to better understand pollen gene expression responses, as for instance the particular sensitivity of transporters to the heat insult. Our gene expression data, combined with previously reported information on SIV germinated pollen, suggests that pollen tube growth is fully armed, with the co-induction of heat-chaperone proteins and transporters, to cope with a high demand of vesicle transport and resilience to environmental and/or female cues during its journey to the ovule. However, it has also raised many questions that remain to be addressed like the nature of the regulatory molecular mechanisms involved and the translation of annotated genes as non-coding RNAs. The implementation of this powerful technology to the diverse pollen developmental stages and environmental cues in future studies, will provide a more detailed picture of this key aspect of plant biology as it is the fertilization process in a constantly changing environment.
ACKNOWLEDGMENTS
The authors thank the Bioinformatics Core Service of the IBMCP for technical assistance. We also thank José M. Alonso, Anna Stepanova, René Toribio and Mar Castellano for sharing protocols with helpful advices on ribosome profiling, and for critical reading of the manuscript. We also thank Gad Miller and Nick Rutley for sharing data and stimulating discussions. We are indebted to Heven Sze (University of Maryland) for giving extraordinary input with transporter gene nomenclature and expression and very useful ideas to improve the quality of the manuscript. We also thank Mark A. Johnson (Brown University) for the development of the tool “Arabidopsis Heat Tree Viewer.”
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be considered as a potential conflict of interest.




