Soybean CHX-type ion transport protein GmSALT3 confers leaf Na+ exclusion via a root derived mechanism, and Cl− exclusion via a shoot derived process
Funding information: Australian Research Council, Grant/Award Numbers: CE1400008, DE160100804, DE170100346, FT130100709; National Natural Science Foundation of China, Grant/Award Number: 31830066; Scientific Innovation Project of Chinese Academy of Agricultural Sciences
Abstract
Soybean (Glycine max) yields are threatened by multiple stresses including soil salinity. GmSALT3 (a cation-proton exchanger protein) confers net shoot exclusion for both Na+ and Cl− and improves salt tolerance of soybean; however, how the ER-localized GmSALT3 achieves this is unknown. Here, GmSALT3's function was investigated in heterologous systems and near isogenic lines that contained the full-length GmSALT3 (NIL-T; salt-tolerant) or a truncated transcript Gmsalt3 (NIL-S; salt-sensitive). GmSALT3 restored growth of K+-uptake-defective Escherichia coli and contributed towards net influx and accumulation of Na+, K+ and Cl− in Xenopus laevis oocytes, while Gmsalt3 was non-functional. Time-course analysis of NILs confirmed shoot Cl− exclusion occurs distinctly from Na+ exclusion. Grafting showed that shoot Na+ exclusion occurs via a root xylem-based mechanism; in contrast, NIL-T plants exhibited significantly greater Cl− content in both the stem xylem and phloem sap compared to NIL-S, indicating that shoot Cl− exclusion likely depends upon novel phloem-based Cl− recirculation. NIL-T shoots grafted on NIL-S roots contained low shoot Cl−, which confirmed that Cl− recirculation is dependent on the presence of GmSALT3 in shoots. Overall, these findings provide new insights on GmSALT3's impact on salinity tolerance and reveal a novel mechanism for shoot Cl− exclusion in plants.
1 INTRODUCTION
There is a large market demand for soybean (Glycine max) as a staple edible oil and food crop; soybean is also an important component of intercropping and sequential cropping systems (Singh, 2010). As such, soybean production demand has been projected to increase by nearly 80% to 390 Mt by 2050 (Alexandratos & Bruinsma, 2012). However, salinity stress threatens soybean production. A soil salinity of EC (electrical conductivity) 18–20 dS/m, which is approximately 180–200 mM NaCl, has been shown to reduce yield by 61% from 2,261 ± 438 kg/ha under control conditions to 881 ± 260 kg/ha (Chang, Chen, Shao, & Wan, 1994). In another study, Blanco, Folegatti, Gheyi, and Fernandes (2007) found that the emergence of soybean (cv. Conquista) was completely inhibited when EC was close to 8.0 dS/m. Yet, natural variation in soybean salinity tolerance has been observed, with some rare soybean germplasm found to be relatively salt-tolerant. In a large-scale evaluation of soybean salinity tolerance, less than 3% of the 10,128 evaluated soybean germplasm exhibited salt tolerance at both the germination and seedling stage; only 83 soybean cultivars were found to have significant tolerance to salinity at the vegetative stage (Shao, Wan, Chang, & Chen, 1993). It has been observed that salt-tolerant soybean cultivars often have superior agronomic performance to salt-sensitive cultivars (Phang, Shao, & Lam, 2008), indicating breeding for stress tolerance in soybean may improve overall yields even under standard conditions (Gilliham, et al., 2017).
In soybean, a major quantitative trait locus (QTL) for salinity tolerance (Ncl) has been consistently mapped to chromosome 3 (Abel, 1969; Ha et al., 2013; Hamwieh et al., 2011; Hamwieh & Xu, 2008; Lee et al., 2004). Since 2014, several studies have been published focusing on the same dominant gene within this QTL on chromosome 3, in both wild (GmCHX1) and cultivated soybean (GmNcl/GmSALT3) (Do et al., 2016; Guan et al., 2014; Qi et al., 2014). This gene, denoted as Glyma03g32900 in the reference soybean genome of the William 82 variety (William 82. a1.v1.1), is the premier candidate for conferring improved salt tolerance in the Ncl locus. It should be noted that in the William 82 genome a2.v1, Glyma03g32900 appears to be incorrectly annotated as two transcripts (Glyma.03 g171600 and Glyma.03 g171700) rather than the full-length coding sequence obtained consistently from cDNA and encoding a full-length CHX protein. Here, we refer to the full-length gene obtained as GmSALT3.
According to phylogenetic analyses, the full-length GmSALT3 protein is a member of the CPA2 (Cation/Proton Antiporter 2) family of transporters; the most closely related sequence in Arabidopsis thaliana being AtCHX20 (a Cation/Proton Exchanger) (with 59% identity and 72% similarity) (Guan et al., 2014; Padmanaban et al., 2007). Several Arabidopsis CHXs, including AtCHX20, have been functionally characterized in heterologous systems; however, a connection to their function to roles in plants has been challenging, as examples of phenotypes associated with loss of function mutations is sparse (Sze & Chanroj, 2018). Functional studies of AtCHX have indicated that they might play role in modulating cation and pH homeostasis within the endomembrane system (Chanroj et al., 2011; Czerny et al., 2016; Padmanaban et al., 2007). The endomembrane localized AtCHX20 was, for example, suggested to be a K+ transporter involved in the osmoregulation of guard cells (Padmanaban et al., 2007).
In soybean, the salt-tolerant soybean cultivar Tiefeng 8 contains a full-length GmSALT3, while the salt-sensitive cultivar 85 to 140 contains a 3.78-kb copia retrotransposon insertion in exon 3 of GmSALT3 that truncates the transcript with a premature stop codon (making the 85 to 140 essentially a Gmsalt3 knockout). This retrotransposon insertion resulted in the loss of the 10th predicted transmembrane domain and the C-terminus of GmSALT3 (Guan et al., 2014). GmSALT3 is predominantly expressed in cells associated with the phloem and xylem (Guan et al., 2014); and expression of the full-length GmSALT3, but not the truncated version, results in low shoot Na+ and Cl− accumulation in saline conditions (Guan et al., 2014; Liu et al., 2016; Qi et al., 2014). This low shoot NaCl accumulation is associated with increased crop yield (Do et al., 2016; Liu et al., 2016). The GmSALT3 protein has been localized to the endoplasmic reticulum (ER), sharing the membrane localization of AtCHX20 in guard cells. This differs from most other transport proteins known to be involved in shoot Na+ and Cl− exclusion, which are mainly localized on the plasma membrane or tonoplast (Munns & Gilliham, 2015).
Shoot salt exclusion requires the co-ordinated activity of many ion transporters that may: (a) limit the net entry of salt into the roots, (b) compartmentalize salt in the roots; and or (c) regulate vascular transport (most notably through root-based xylem exclusion) (Munns et al., 2020). Examples of transporters that have been implicated in salt tolerance in soybean include: the tonoplast localized GmNHX1 (Na+/H+ antiporter 1) (Li, Wong, et al., 2006), the plasma membrane localized GmSOS1 (salt overly sensitive 1, Na+/H+ antiporter) and the plasma membrane localized GmCAX1 (Ca2+/H+ antiporter 1) (Luo, Wang, et al., 2005; Luo, Yu, & Liu, 2005; Phang et al., 2008). These transporters are proposed to be involved in net exclusion of salt entry into roots (GmSOS1), salt compartmentation (GmNHX1) or signalling (GmCAX1). GmSALT3, was the first example of a transporter contributing to reduced net transfer of salt to the shoot in soybean (Cao, Li, Liu, Kong, & Tran, 2018). In other crop species, modulation of vascular ion load is commonly found to be one of the major salt tolerance mechanisms. For instance, in wheat, rice and Arabidopsis the HKT1;5-like (high-affinity K+ transport) proteins are expressed in xylem parenchyma cells and facilitate Na+ retrieval from xylem sap back into the root (Møller et al., 2009; Munns et al., 2012; Ren et al., 2005; Uozumi et al., 2000). GmSALT3 differs from these previous proteins; the ER-localized is unlikely to directly retrieve from or load ions into the xylem sap. Therefore, GmSALT3 confers a novel salt tolerance mechanism which is yet to be determined.
Here, we investigated the function of GmSALT3 in heterologous systems and in planta to gain insights into the mechanisms by which GmSALT3 contributes to salt tolerance. In heterologous systems, GmSALT3 complemented K+ uptake deficient Escherichia coli; while in Xenopus laevis oocytes, GmSALT3 expression resulted in increased net influx of Na+, K+ and Cl−. Phenotypes of soybean NILs differing in GmSALT3 alleles revealed that plants containing full-length GmSALT3, compared to truncated Gmsalt3, employ two distinct mechanisms to achieve shoot Na+ and Cl− exclusion by facilitating net xylem retrieval of Na+ and a novel mechanism of greater phloem Cl− retranslocation from shoots to roots. As such, we propose that the endomembrane GmSALT3 has a complex physiological role which ultimately impacts multiple ion fluxes across the plasma membrane.
2 MATERIALS AND METHODS
2.1 Plant materials and growth conditions
Soybean plants were grown in a greenhouse (28°C day, 16 hr light and 25°C night) at the Plant Research Centre, Waite Campus, University of Adelaide, Australia. Soybean seeds (Tiefeng 8, salt-tolerant parent; 85 to 140, salt-sensitive parent; NIL-T, near isogenic line with GmSALT3; NIL-S, near isogenic line with Gmsalt3) were imported from China through quarantine. Soybean seeds were germinated in pots containing a 1:1 mixture of perlite: vermiculite as described by Obermeyer and Tyerman (2005), with no nutrient solution added.
2.2 cDNA cloning and plasmid preparation
To synthesize GmSALT3 and Gmsalt3 cDNA (2,436 and 1,131 nucleotides, respectively), total RNA was isolated from roots of 4-week old soybean plants using the TRIzol method (Shi & Bressan, 2006). First-strand cDNA was synthesized using a Thermoscript RT III kit (Invitrogen). Gene specific primers (GmSALT3_gF and GmSALT3_gR, Gmsalt3_gF and Gmsalt3_gR; Table S1) were used to amplify GmSALT3 cDNA with Phusion High-Fidelity DNA Polymerase (New England Biolabs) using 35 cycles (98°C 30 s, 65°C 30 s and 72°C 150 s). Gel-purified PCR products were A-tailed using Taq polymerase (New England Biolabs) for 30 min at 72°C, and then recombined into Gateway entry vector PCR8/GW/TOPO (Invitrogen) for subsequent applications. Resulting clones were sequenced using internal primers (GmSALT3_F1, R1, F2, F3, F4 and F5, Gmsalt_F1, F2 and R1; Table S1). The sequence of GmSALT3-YFP was PCR amplified from a previously constructed plasmid pBS-35S::GmSALT3-YFP; the sequence of GmSALT3-TM10 (first 10 transmembrane domain of GmSALT3) was PCR amplified from PCR8-GmSALT3. Gel-purified PCR products of GmSALT3-YFP, GmSALT3-TM10 were recombined into entry vector PCR8 as described above.
GmSALT3 and Gmsalt3 CDS, GmSALT3-YFP, GmSALT3-TM10, AtCHX20 and AtKAT1 within entry vector PCR8 were cloned into the pGEM-HE vector and a modified version of pET-DEST42 vector using Gateway LR Clonase (Invitrogen), for X. laevis oocyte expression and E. coli expression, respectively. The E. coli strain TK2463 lacks the T7 RNA polymerase, therefore, the T7 promoter in pET-DEST42 was replaced with the TAC promoter. Inverse PCR was performed with 5 ng of plasmid, 50 nM of forward and reverse primer (SH_215 and SH_216, Table S1) and 0.25 units Phusion High-Fidelity Polymerase (New England Biolabs) in a final volume of 25 μL. Reactions were treated with Cloning Enhancer (Clontech Laboratories Inc.) to remove circular template, and the plasmid was re-circularized using In-Fusion HD enzyme (Clontech Laboratories Inc.).
2.3 Growth assay in E. coli
Competent cell preparation and E. coli transformation were conducted as described by Chanroj et al. (2011). The resulting transformants were grown on KML media (10 g/L tryptone, 5 g/L yeast extract, 10 g/L KCl) supplemented with 100 μg/mL carbenicillin and incubated at 37°C overnight; positively transformed colonies were identified by colony PCR with gene specific primers (GmSALT3_gF and _gR; Gmsalt3_gF and gR). For E. coli growth experiments, freshly transformed cells were first grown overnight in 5 mL KML at pH 7.2. Cell cultures were replenished (OD600 = 0.5) and grown for 3 hr in KML, and then washed with low potassium media (10 g/L tryptone, 2 g/L yeast extract, 100 mM d-mannitol) three times. Cells were normalized to OD600 0.5 for a 96-well plate assay. In each well, 20 μL of normalized cell suspension was added to 180 μL growth solutions. All test media were supplemented with 100 μg/mL carbenicillin, with or without 0.5 mM isopropyl β-d-1-thiogalactopyranoside (IPTG). The 96-well plates were inserted into FLUOstar Omega Fluorescence microplate reader (BMG LABTECH) set to 37°C and A600 was measured every 15 min for 18 hr with continuous shaking at 200 rpm.
2.4 Characterization of GmSALT3 in X. laevis oocytes
pGEMHE-DEST containing GmSALT3 was linearized using SphI-HF (New England BioLabs); cRNA was synthesized using mMESSAGE mMACHINE T7 Kit (Ambion). A total of 46 nL/23 ng of cRNA or equal volumes of RNase-free water were injected into oocytes with a Nanoinject II microinjector (Drummond Scientific). Oocytes were incubated for 48 hr in Calcium Ringer's solution (96 mM NaCl, 2 mM KCl, 5 mM MgCl2, 5 mM HEPES, 0.6 mM CaCl2). All solution osmolalities were measured with a Vapor pressure osmometer (Wescor) and adjusted to 240–260 mOsmol/kg using mannitol. Measurement of ion profiles in oocytes followed Munns et al. (2012) with the following modifications. Six replications of three grouped oocytes were used for flame photometry (Sherwood 420), and Cl− was measured by manual titrimetric Cl− measurement using a chloride analyser (Sherwood® Chloridometer 926S). For YFP imaging, oocytes were analysed with a Zeiss LSM 5 Pascal Confocal Laser-Scanning Microscope equipped with an argon laser. Excitation/emission conditions for YFP were 514 nm/523 to 600 nm.
2.5 Ion-selective microelectrode flux measurements from oocytes
The MIFE (Microelectrode Ion Flux Estimation technique; University of Tasmania, Hobart, Australia) allows non-invasive concurrent quantification of net fluxes of several ions using protocols outlined by Shabala, Shabala, Bose, Cuin, and Newman (2013) with oocytes being used rather than plant tissues in our experiments. Potassium and chloride commercial ionophore cocktails (99,311 and 99,408, respectively; all from Sigma-Aldrich) were used as liquid ion exchanger (LIX) when measuring K+ and Cl− fluxes. The Na+ fluxes were measured using an improved Na+ LIX described in Jayakannan et al. (2011). Oocytes were washed in ND96 (96 mM NaCl, 1 mM KCl, 1 mM MgCl2, 5 mM HEPES, pH 7.5). After incubation in ND96 for 72 hr, fluxes were measured after being transferred to BSM (5 mM NaCl, 0.2 mM KCl, 0.2 mM CaCl2, 5 mM HEPES, pH 7.5). During measurements, the ion-selective electrodes were positioned using a 3D-micromanipulator (MMT-5, Narishige, Tokyo, Japan), 100 μm from the oocyte surface. A computer-controlled stepper motor moved the electrode between two positions (100 and 200 μm, respectively) from the oocyte surface in 6 s cycles. The CHART software (Newman, 2001) recorded the potential difference between the two positions and converted them into electrochemical potential differences using the calibrated Nernst slope of the electrode. Net ion fluxes were calculated using the MIFEFLUX software for spherical geometry (Newman, 2001).
2.6 Transmission electron microscopy
Fresh soybean NIL-T and NIL-S roots were sectioned to 1 mm (length), stems were cross sectioned (1 mm in length and 1 mm in radius). Root and stem samples were incubated overnight in 1.5 mL microcentrifuge tubes with fixative (2.5% Glutaraldehyde, 4% Formaldehyde, 4% Sucrose, 0.1 M Phosphate buffer). Samples were washed three times in 1X PBS and then washed with osmium (263,257, Sigma-Aldrich) for 4 hr. Sections were then washed three times in 1X PBS and soaked in 1X PBS for 20 min and then embedded in a 1% agarose gel. Agarose embedded soybean roots were cut into blocks (1 cm long). A series of dehydration steps were performed after embedding, using increasing concentrations of ethanol: 50, 70, 90 and 95% (each for 30 min) and 100% ethanol for overnight dehydration. Samples were then incubated in increasing concentrations of resin (Spurrs): 5, 10, 15, 20, 25, 30, 40 and 50% (each for 1 hr), 50% overnight, 75% (4 hr), 100% (4 hr) and 100% (overnight). Resin wells containing the agarose blocks were then incubated at 60°C for 3 days for polymerization. Polymerized resin capsules were cut to 70 nm thickness using a diamond knife and visualized under transmission electron microscopy (TEM; PHILIPS CM100).
2.7 NaCl treatment and ion accumulation
Soybean plants were treated with 100 mM NaCl every 2 days (1.5 L solution per tray) at 14 days after sowing; the second dose of 1.5 L 100 mM NaCl was applied 16 days after sowing; the same volume of reverse osmosis (RO) water was applied to the tray of the control plants. Plant tissues were harvested and dried in an oven overnight at 60°C or freeze-dried overnight. Dry weight was recorded, then dried samples were digested in 10 mL 1% (v/v) nitric acid overnight at 65°C or freeze-thawed in Milli-Q water three times. Oven-dried samples were utilized to test Na+ and K+ accumulation with flame photometry (Sherwood 420), Cl− was measured by titrimetric Cl− measurement by using a chloride analyser (Sherwood Chloridometer 926S).
Freeze-dried samples were used to measure NO3 accumulation. The measurement method was modified according to Qiu, Henderson, Tester, Roy, and Gilliham (2016). Supernatant (50 μL) of freeze-dried samples in water was added into 200 μL of 5% (W/V) salicylic acid/H2SO4 and incubated at room temperature for 20 min, then 50 μL of this mixture was transferred into 950 μL of 2 M NaOH and incubated at room temperature for at least 20 min (to cool down to room temperature). Of this, 250 μL was loaded into separate wells of a flat-bottom transparent 96-well plate (Greiner Bio-One, Austria). Absorbance was measured at 410 nm. Standards measured included 0, 0.5, 1, 2, 2.5, 5, 7.5 and 10 mM KNO3.
2.8 Phloem and xylem sap extraction
Phloem sap was extracted according to the method of Rupassara (2008) and Ren et al. (2005). Soybean stems were cut approximately 2 cm above the ground level with the upper part immediately dipped in 1.5 mL of 0.1 mM EDTA solution (pH adjusted to 8 with NaOH) in a 2 mL microcentrifuge tube for 20 min, then the tube was immediately dipped in liquid nitrogen and stored in −80°C until analysis. The glutamine concentration in the extract was measured using a Glutamine Assay Kit (EGLN-100, EnzyChrom, BioAssay Systems). Xylem sap was extracted using a pressure chamber. Lower parts of the cut stems with roots were transferred into the pressure chamber. Pressure was increased gradually (0.05 MPa increments) until the xylem sap presented. The first two drops emerging were discarded using a micropipette to reduce contamination from damaged cells or phloem sap (Berthomieu et al., 2003). Xylem sap was then collected during the following 5 min, and stored at −20°C until analysis. Ion contents were measured as stated above.
2.9 Grafting of soybeans
NIL-T and NIL-S were grown in a growth chamber with a 16 hr light (28°C)/8 hr dark (25°C) cycle at 60% humidity. The grafting protocol followed Guan et al. (2014) with the following modifications. After grafting, when the first trifoliate leaf fully expanded (about 10 days after grafting), healthy plants were selected and treated with 100 mM NaCl for 8 days. Na+, K+ and Cl− contents were measured in soybean leaves and roots.
3 RESULTS
3.1 GmSALT3 expression impacts Na+, K+ and Cl− fluxes in heterologous systems
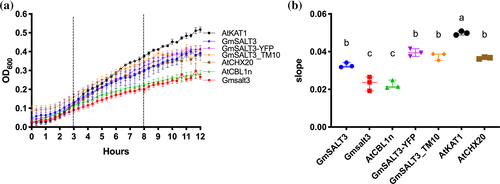
We functionally characterized GmSALT3 in heterologous systems, including E. coli and X. laevis oocytes. The E. coli strain TK2463 (trkAΔ, kup1Δ kdpABCDEΔ) is defective in its native K+ uptake mechanisms (Epstein, Buurman, McLaggan, & Naprstek, 1993; Figure 1) and shows a reduced growth trajectory in low K+ media (Epstein et al., 1993). We transformed TK2463 bacteria with our constructs of interest and controls; expression of the proteins was driven by the IPTG-inducible tac-promoter. In low K+-containing medium (~2 mM K+), expression of full-length GmSALT3 improved growth compared to expression of truncated Gmsalt3, and the negative control (AtCBL1n, a 12 aa peptide with the sequence MGCFHSKAAKEF; Batistič, Waadt, Steinhorst, Held, & Kudla, 2010; Figure 1a,b). Arabidopsis AtCHX20 (Chanroj et al., 2011) and AtKAT1 (Anderson, Huprikar, Kochian, Lucas, & Gaber, 1992), a known plasma membrane potassium channel from Arabidopsis, were included as positive controls and restored bacteria growth as expected (Figure 1a,b). We had previously used GmSALT3-YFP for determining the subcellular localization of GmSALT3 (Guan et al., 2014), and therefore, investigated if this translational fusion is functional and could complement TK2463 E. coli. In addition, we were interested whether the last transmembrane domain of GmSALT3 is important for functionality - truncated Gmsalt3 from NIL-S contains only 9 out of the 10 predicted alpha-helices. We, therefore, constructed GmSALT3-TM10, that has all 10 TM alpha-helices with only the large (383 aa) hydrophilic C-terminus removed. Both, YFP-tagged GmSALT3 and GmSALT3-TM10 increased E. coli growth to that of GmSALT3, and significantly greater than that of Gmsalt3 (Figure 1a,b). None of the test constructs or controls had a significant impact on bacterial growth rates on high K+ media (Figure S1c,d); or showed significant differences on low K+ media without IPTG-induction (Figure S1a,b). In addition, we measured K+ content of E. coli cells, to determine the impact of GmSALT3 expression on K+ accumulation. Bacteria were grown for 12 hr in low K+ following IPTG-induction, and then harvested. Results indicated that expression of full-length GmSALT3 leads to higher K+ content in bacteria, as does expression of GmSALT3-YFP, GmSALT3_TM10, AtKAT1 and AtCHX20 (Figure S1e). Expression of truncated Gmsalt3 resulted in similar low K+ content as in the negative control.
We chose a second heterologous expression system to investigate if the ER-localized GmSALT3 impacts the fluxes of ions additional to K+. We expressed YFP-tagged and untagged GmSALT3, as well as Gmsalt3, in X. laevis oocytes and detected fluorescence at the PM in GmSALT3-YFP expressing oocytes, similar to the PM-localized positive control TmHKT1;5-A-YFP (Figure 2a) (Triticum monococcum High-Affinity K+ Transporter 1; Munns et al., 2012). This indicates that GmSALT3-YFP localizes to the PM in oocyte, which is different from its ER-localization in plant cells (Guan et al., 2014). It is not uncommon for plant endomembrane proteins to at least partially localize to the PM in frog oocytes (Henderson et al., 2015; Maurel, Reizer, Schroeder, & Chrispeels, 1993); however, this restricts the interpretation of the data regarding the impact of ER-localized transporters on PM ion fluxes.
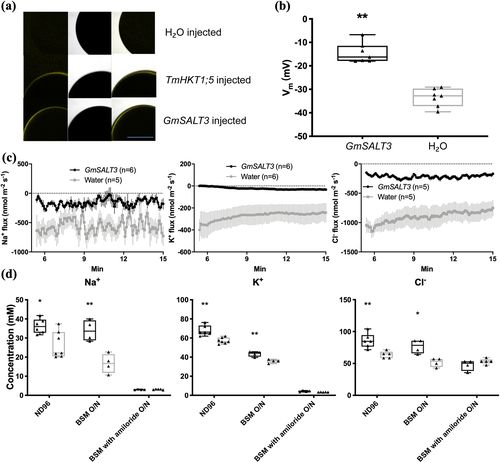
Two electrode voltage-clamp electrophysiology revealed that the resting membrane potential of GmSALT3-injected oocytes was more positive compared to H2O-injected oocytes when incubated in ND96 media (Figure 2b), but no consistent current differences were detected. This suggests that transport through GmSALT3 might be electroneutral, which is consistent with the CPA2 signature sequence for electroneutral ion transport (Masrati et al., 2018). Therefore, we chose to conduct microelectrode ion flux estimation (MIFE) experiments to measure the net fluxes of specific ions across the plasma membrane of oocytes non-invasively and so does not rely upon net charge movement in a particular direction.
Flux analysis indicated that in GmSALT3-injected oocytes net efflux of Na+, K+ and Cl− was reduced compared to H2O-injected oocytes (Figure 2c). Subsequent ion content analysis of oocytes revealed that GmSALT3-injected oocytes contained significantly more K+, Na+ and Cl− compared to H2O-injected oocytes, in agreement with the reduced net ion efflux in oocytes (Figure 2d). To test whether the GmSALT3 induced differences in ion concentration could be blocked, we used the Na+ channel and Na+/H+ exchanger inhibitor amiloride hydrochloride (Darley, Van Wuytswinkel, Van der woude, & Mager, 2000). Oocytes were transferred from ND96 to BSM (low Na+, K+ and Cl−) and incubated overnight with or without the inhibitor. GmSALT3-injected oocytes incubated without amiloride, showed a smaller relative K+, Na+ and Cl− decrease in compared to H2O-injected oocytes as expected (Figure 2d). In the presence of amiloride, however, this difference was not observed (Figure 2d), strongly suggesting that GmSALT3 affects the transport of K+, Na+ and Cl− across the PM in oocytes. Collectively, the heterologous data has advanced our knowledge by confirming that GmSALT3 encodes a competent transport protein, certainly capable of transporting K+, and affects the flux of Na+, K+ and Cl− across a plasma membrane. However, as it is unclear whether the net fluxes of Na+, K+ and Cl− across the plasma membrane of oocytes were all carried directly by GmSALT3, rather than endogenous X. laevis transport proteins we focused our efforts on further characterizing the impact of GmSALT3 on salt transport in planta.
3.2 GmSALT3 modulates Na+, K+ and Cl− accumulation in soybean
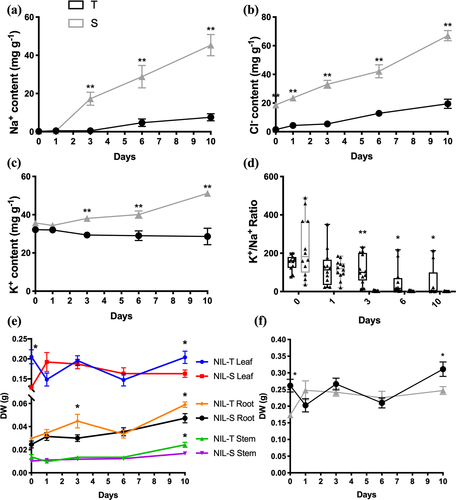
Liu et al. (2016) previously used an extreme salt treatment (200 mM NaCl) screen that typically results in the death of even the more salt-tolerant germplasm after a prolonged exposure. Using this method, it was observed that full-length GmSALT3 improved shoot Na+ and Cl− exclusion over 10 days, with higher shoot Cl− accumulation occurring prior to shoot Na+ accumulation in NIL-S (Liu et al., 2016). Here, we used a lower salt concentration (100 mM) that soybean plants are more likely to encounter in the field, and which typically does not result in death of the plants prior to seed production. Furthermore, we studied the time course of Na+, Cl− and K+ accumulation in NIL-T and NIL-S for different leaf types, stems and hypocotyls (Figure 3). We confirmed that Cl− accumulates to higher concentrations in NIL-S shoots compared to those of NIL-T (Figure 3b). Even without a salt treatment Cl− concentration was found to be greater in NIL-S aerial tissues (leaves and stems), whereas significantly higher Na+ accumulation was detected in aerial tissues only after 3 days of NaCl treatment in NIL-S, with Cl− being even more pronounced as time after salt treatment progressed (Figures 3a,b, S2 and S3). We further observed that the K+/Na+ ratio was significantly higher in NIL-T leaves compared to NIL-S when measured after day 3 (Figure 3d). Total K+ content was increased in NIL-S compared to NIL-T (Figures 3c and S3), but K+ accumulation increased to lesser extent compared to Na+ accumulation. K+ increased from 35.7 ± 1.2 mg/g (day 0) to 51.2 ± 1.2 mg/g (day 10), compared to 0.3 ± 0.1 mg/g and 45.3 ± 5.6 mg/g for Na+ in the same period (Figure 3a,c). It is likely that K+ accumulated in shoots of NIL-S, as a response to increasing shoot Na+ content—which did not occur in NIL-T. However, although shoot K+ increased in NIL-S, the K+/Na+ ratio was lower. In addition, salt treatment affected biomass production and after 10 days NaCl treatment the total dry weight of NIL-T was significantly higher, as were individual dry weights of leaves, roots and stem (Figure 3e,f).
After establishing the timeline for shoot ion accumulation in 100 mM NaCl, we selected a 4-day time point for further experiments, as the differences in both Na+ and Cl− accumulation in NIL-T and NIL-S tissues can be clearly discerned (Figures 4 and S5), and performed a more detailed analysis of ion concentrations in a range of tissue types, and in the vascular sap. After 4 days, Na+ accumulated to a greater extent in all aerial tissues in NIL-S, including leaves (both first trifoliate leaf, FL and youngest trifoliate leaf, YL), petioles of the first trifoliate leaf (FLP) and youngest trifoliate leaf (YLP), higher stem (HS), lower stem (LS) and hypocotyl (Hy). This is consistent with what we observed for whole shoot ion content analysis. No significant differences were observed between the NILs for Na+ in roots under salt treatment (primary root, PR and secondary root, SR; Figure 4a). Under control conditions (4 days), Na+ content was very low, but significantly higher in secondary root (SR) of NIL-T compared to NIL-S, with no significant differences in other tissues (Figure S5). No significant differences between NIL-T and NIL-S were observed for K+ under control conditions (Figure S5). And except for the higher K+ accumulation in leaves, no differences were found in any other of the investigated aerial tissues or roots (Figure 4b).
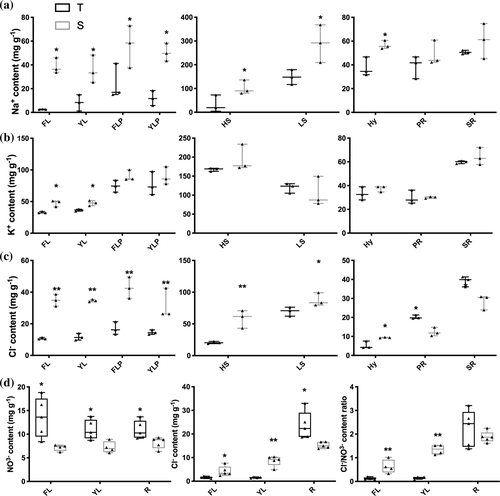
Cl− accumulation pattern showed similarities but also differences to that of Na+ accumulation, under saline conditions. In aerial parts and hypocotyls more Cl− accumulated in leaves, stems and hypocotyls of NIL-S compared to NIL-T (Figure 4c), similar to Na+. While we found that in roots (PR and SR), NIL-T accumulated significantly more Cl− than NIL-S (Figure 4c). Under control conditions, Cl− content was significantly elevated in leaves and stem of NIL-S, with no differences for hypocotyl and roots (Figure S5). We also measured NO3− content and found that NIL-S accumulated less NO3− in leaves (both FL and YL) and roots compared to NIL-T, which lead to a high Cl− to NO3− ratio in leaves of NIL-S, but not in roots (Figure 4d). In summary, significantly less Na+, K+ and Cl− accumulated in NIL-T shoots than NIL-S under saline conditions. Leaf ion exclusion is in many plant species achieved by retention of ions in the root, primarily by reducing net ion concentration in the shoot-ward direction solute flow. To investigate if this is also the case in soybean, we determined xylem and phloem sap ion concentrations.
Ion concentrations within soybean stem phloem and xylem sap were examined at the 4-day time point following NaCl treatment. Glutamine contents within the phloem sap was used to normalize ion measurements to account for volume differences measurements, as glutamine concentration within the phloem remains constant throughout the day (Corbesier, Havelange, Lejeune, Bernier, & Périlleux, 2001). It was found that our estimates of Na+ and K+ contents were not significantly different between the phloem sap of NIL-T and -S plants. As expected, the Na+ concentration in the xylem sap in NIL-S was significantly greater compared to NIL-T, indicating less Na+ is loaded into the xylem, or more is retrieved. Surprisingly, and in apparent contrast to the leaf accumulation data, the Cl− concentration in xylem sap was lower in NIL-S than NIL-T (Figure 5b). In addition to the higher xylem sap concentrations, Cl− concentration was also significantly higher in the phloem sap in NIL-T (Figure 5a). Phloem sap was harvested from a position where the flow direction is root-wards, close to the junction of root and shoot. The higher Cl− in NIL-T phloem sap, therefore, indicated an increased flow of Cl− from the shoot to the roots in NIL-T plants, this would be consistent with Cl− being recirculated from the stem to the roots via the phloem, prior to its accumulation in shoot tissue vacuoles. Under control conditions (4 days with water treatment), no significant differences were observed between NIL-T and NIL-S for the ion concentrations within stem phloem and xylem sap (Figure S5d), which may be related to the detection levels of the assays.
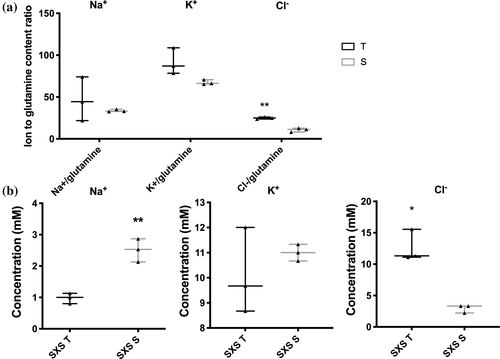
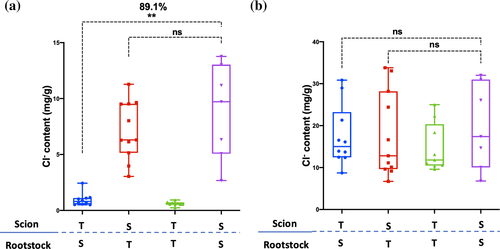
The observed higher Cl− concentration in phloem sap suggested that the leaf Cl− exclusion mechanism in NIL-T might be derived from the shoot, instead of the root. To investigate this, a grafting experiment was performed. Reciprocally grafted and self-grafted control plants were salt-treated (100 mM NaCl) for 8 days, as previously described (Guan et al., 2014). The differences in Na+ that we observed were consistent with previous grafting experiments, confirming that Na+ exclusion depends on a root derived mechanism (Figure S6; Guan et al., 2014). In contrast to the situation with Na+, grafting demonstrated that the scion, not the rootstock, is important for leaf Cl− exclusion. When a NIL-T scion was grafted on a NIL-S rootstock, the Cl− content in leaves was 89.1% lower when compared to leaves of self-grafted NIL-S (Figure 6a). Conversely, when the NIL-S scion was grafted on the NIL-T rootstock, the Cl− content in leaves was not significantly different compared to self-grafted NIL-S (Figure 6a). Controls confirmed that self-grafted NIL-S had a considerably higher Cl− content compared to self-grafted NIL-T (Figure 6a), as expected. In the roots, no significant difference were detected between grafted plants (Figure 6b). No difference was detected in root or shoot K+ content between the reciprocal grafted NILs (Figure S6).
As we identified the phloem as an important component of the GmSALT3-derived salt exclusion mechanism, we investigated the ultrastructure of the phloem to identify possible differences between NIL-T and NIL-S. In the phloem, the ER of companion cells reaches into the sieve elements and is directly exposed to the phloem sap (Turgeon & Wolf, 2009). This could theoretically enable a direct ion transport from and into the sap of phloem cells, through the ER-localized GmSALT3, and/or loss of GmSALT3 could lead to morphological changes of the ER ultrastructure. TEM imaging did not reveal obvious morphological differences between salt-treated NIL-T and NIL-S in root phloem cells (Figure S7), indicating lack of full-length GmSALT3 does not result in a disruption to subcellular morphology and that changes in ion flux are more likely directly responsible.
4 DISCUSSION
4.1 Transport function of GmSALT3
Plant CPA2 (Cation-Proton Antiporter 2)/CHX (Cation/H+ Exchangers) have traditionally been characterized using knockout strains of yeast. A close homolog of GmSALT3, AtCHX20 in Arabidopsis thaliana, is preferentially expressed in stomatal guard cells, and shows a localization pattern consistent with ER-localization, like GmSALT3 (Padmanaban et al., 2007). Similar to GmSALT3, expression of AtCHX20 was shown to enhance growth of (a different) E. coli strain deficient in K+ uptake (LB2003) (Chanroj et al., 2011), indicating that the two proteins catalyse the transport of K+. This assay also showed that YFP-tagged GmSALT3 and GmSALT3-TM10 were also functional for K+ transport, but not the truncated Gmsalt3, which lacks the last membrane embedded TM10. This suggests that the entire set of transmembrane domains are required for ion transport, and that Gmsalt3 is non-transport competent. Our results further suggest that the large C-terminus, found in all plant CHX proteins, is not required for CHX to conduct ion transport.
We then attempted a transport assay with greater resolution, that is, X. laevis oocytes, which has not been previously reported for plant CHX proteins. Using oocytes, two Drosophila CPA2 transporters, NHA1 and NHA2, were speculated to act as a Na +/H+ exchanger and an H+/Cl− cotransporter, respectively (Chintapalli et al., 2015), indicating that proteins from this family can putatively act as either, cation or anion transporters. We observed that GmSALT3 targeted to the PM (Figure 2a), unlike in planta, and led to a lower net ion efflux of all three ions (K+, Na+ and Cl−) across the PM (Figure 2c). Interestingly, the reduced efflux effect was inhibited by the Na+ channel and Na+/H+ exchanger inhibitor amiloride hydrochloride. Whilst this inhibitor is thought to be selective for Na+ transporters, it had an impact on the efflux rate of all three ions measured. It is possible that observed differences in ion flux and accumulation in heterologous systems are the result of changing cytosolic ion concentration that then impacts ion gradients across the PM and influences the activity of native ion transporters. In such a scenario the fluxes detected across the PM of oocytes would be the result of a combination of GmSALT3 and native transporters; and the inhibition of one component leads to the inhibition of the entire mechanism. To conclusively characterize the transport properties of GmSALT3, including stoichiometry, alternative techniques will need to be employed such as the incorporation of pure protein into liposomes devoid of other transport proteins (Nagarajan et al., 2016).
4.2 The effect of GmSALT3 on ion accumulation in planta following salt treatment
Salt tolerance includes tolerance to elevated levels of both Cl− and Na+ in the soil solution. In many plant species, one of the two ions is understood to be more deleterious to the plant when accumulated in the tissue; however, the situation for soybean is currently unclear with evidence for both ions being more toxic than the other (Läuchli, 1984). Whilst our work does not answer which ion is the most toxic, it does clearly demonstrate that Na+ and Cl− long-distance transport, and its accumulation in shoots, can be connected to a single gene, which renders the tolerance mechanisms currently inseparable. That said, although leaf Na+ and Cl− exclusion are connected to the same gene, GmSALT3, confers two distinct mechanisms that are uncoupled in planta. Initial evidence suggested that two separate leaf ion exclusion mechanisms exist in soybean comes from our experiments using plants treated with 100 mM NaCl. This salt concentration mimics a more physiologically relevant salt treatment than has previously been used on NIL plants (i.e. one that would be found in the field). Our data suggests, that in the conditions used here, soybean is more effective in shoot exclusion of Na+ than of Cl− in either NIL-T or NIL-S plants (Figure 3). Furthermore, we detected that Na+ accumulation occurred later in NIL-T than NIL-S, whereas Cl− accumulation occurs steadily in both genotypes after NaCl application, albeit at a reduced total concentration in NIL-T (Figure 3). The mechanisms regulating Cl− and Na+ transport are therefore likely to be distinct. More conclusive evidence was highlighted by the reciprocal grafting of NIL-T and NIL-S shoots and roots. These data indicate GmSALT3 is likely to function in the shoot and stem to limit the accumulation of Cl− in the shoot (Figure 6a). In contrast, Na+ was accumulated to greater concentrations in the aerial parts of NIL-S but no difference was observed in the roots compared to NIL-T (Figure 4a); in stem xylem sap, there was a lower Na+ concentration in NIL-T compared to NIL-S but no significant difference in stem phloem sap estimations (Figure 5). This indicates more Na+ is transported in the NIL-S xylem stream. Na+ measurements in leaves, xylem sap and the grafting experiment are consistent with a reduced net loading of Na+ xylem in NIL-T roots. Root-derived Na+ shoot exclusion mechanisms are well known from other plant species, as described in the introduction.
Known control mechanisms for shoot Cl− accumulation are also predominantly associated with the control of loading to the root xylem, with the role of the phloem in salt tolerance being overlooked (Li, Tester, & Gilliham, 2017; Wege, Gilliham, & Henderson, 2017). Our new finding that shoot Cl− exclusion is a phenotype conferred by the presence of GmSALT3 in the shoots (through grafting) is consistent with our estimations of phloem Cl− content, and we propose it occurs due to phloem recirculation of Cl− back to the roots. Phloem retranslocation of NaCl in saline conditions has been observed before in maize, where approximately 13–36% of the Na+ and Cl− imported to leaves through the xylem was retranslocated to the root again in the phloem (Lohaus et al., 2000); but no transport protein could be linked to this phenomenon. GmSALT3 might be involved in the transfer of Cl− from the xylem into the phloem sap, which is consistent with its expression in vascular parenchyma cells in the root and shoot (Guan et al., 2014). Further investigation is needed to uncover all the components involved in this newly identified salt tolerance mechanism and the interconnection between xylem and phloem loading for Cl− transport. Another finding that warrants further investigation is that NIL-T had a lower shoot Cl−/NO3− ratio than NIL-S plants, driven in part by the higher NO3− content found in NIL-T compared to NIL-S. The relationship between Cl− and NO3− content may not be a simple trade-off between Cl− and NO3− uptake as it was recently found that Cl− was found to stimulate nitrogen assimilation (Rosales et al., 2020). Investigation of the role of GmSALT3 on nitrogen transport and assimilation, and its impact on metabolic and photosynthetic activity with and without salinity might shed further light on this.
4.3 Putative in planta mechanisms for GmSALT3 regulating long-distance ion transport
Coupled to the complexities of identifying the transport properties of GmSALT3 in its native membrane (the endoplasmic reticulum; Guan et al., 2014), and the complex and distinct processes underlying the different salt ion accumulation phenotypes of the two NILs, we propose that GmSALT3 affects processes at the ER and is not directly mediating ion transport across the PM or tonoplast itself—this makes it unlike most other transport proteins identified as important for salt tolerance. Instead, we propose that, in planta, the endomembrane localized GmSALT3 influences ion gradients across the PM through currently unknown downstream effects. This might be through impacting the activity of PM-localized transporters, by changing their PM-abundance or changing cytosolic ion supply, or even more complex effects. For example, if the ionic milieu within the ER is affected this could impact the function or sorting of other membrane transporters or regulatory proteins (Bayer, Sparkes, Vanneste, & Rosado, 2017), which then impacts PM fluxes; similarly, a difference in the ER-membrane potential could lead to such effects. How GmSALT3 differentially affects the long-distance transport pathways in shoots and roots will now need to be prioritized. Further experiments will need to be carried out to decipher the novel mechanisms imparted by GmSALT3 that are important for salt tolerance. This might enable us to identify similar mechanisms in other (crop) plants.
5 CONCLUSION
To summarize, this work has provided further insights into the salinity tolerance mechanisms imparted by GmSALT3 in planta and in heterologous systems. We propose that, in NIL-T, the presence of full-length GmSALT3 mediates Na+ and Cl− exclusion from shoots through restricting net xylem loading of Na+, while Cl− is retranslocated from the shoots back into the roots via the phloem. This is the first time that a protein has been shown to confer phloem recirculation of Cl−, and that this confers trait improved salt tolerance in planta. Our data also demonstrates that GmSALT3 is a transport competent endomembrane localized protein, but fall short of describing the exact cellular mechanisms through which GmSALT3 alters differential transport processes across different cell-types, which is an area that requires further study. RNA-sequencing using NIL-T and NIL-S plants may be beneficial in investigating if GmSALT3 confers salinity tolerance in soybean via influencing transcription, and what distinctive pathways and genes might be significantly changed in NIL-T and NIL-S roots under saline conditions.
ACKNOWLEDGMENTS
We thank Dr Yue (Crystal) Wu for generating the AtCBL1n entry vector; Dr Sunita Ramesh and Assoc. Prof. Zhonghua Chen for providing AtKAT1 construct used in this study; CSIRO, Australia for donating E. coli strain TK2463. We thank Adelaide Microscopy, especially Gwenda Mayo for the use of TEM and other imaging facilities. This work was funded by the Natural Science Foundation of China, grant number 31830066 (Rongxia Guan) and Scientific Innovation Project of Chinese Academy of Agricultural Sciences (Lijuan Qiu). Yue Qu and Sam W. Henderson were supported by ARC Centre of Excellence funding awarded to Matthew Gilliham (CE140100008). ARC Fellowships supported Matthew Gilliham (FT130100709), Stefanie Wege (DE160100804) and Jayakumar Bose (DE170100346).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Matthew Gilliham, Yue Qu and Stefanie Wege designed experiments, with input from Lijuan Qiu and Rongxia Guan. Lijuan Qiu and Matthew Gilliham directed the project. Yue Qu performed ion accumulation measurement, TEM, IRGA, E. coli and oocyte expression, analysed the data and drafted the manuscript. Jayakumar Bose performed MIFE in oocytes. Sam W. Henderson assisted with experimental design and provided the E. coli expression vectors. Yue Qu, Matthew Gilliham and Stefanie Wege wrote the manuscript. All authors commented on the manuscript.




