Role of melatonin in UV-B signaling pathway and UV-B stress resistance in Arabidopsis thaliana
Funding information: Fujian Provincial Key Laboratory of Haixia Applied Plant Systems Biology Open Fund (Fujian Agriculture and Forestry University); Ministry of Education, First-class University and Academic programs of Northwest University; National Natural Science Foundation of China, Grant/Award Numbers: 31300223, 31572665; Natural Science Foundation of Shaanxi Province, Grant/Award Number: 2016JM3001; Northwest University Graduate Innovation and Creativity Funds, Grant/Award Number: YZZ17152; Opening Foundation of Key Laboratory of Resource Biology and Biotechnology in Western China (Northwest University)
Abstract
Melatonin (N-acetyl-5-methoxytryptamine) plays important roles in plant defences against a variety of biotic and abiotic stresses, including UV-B stress. Molecular mechanisms underlying functions of melatonin in plant UV-B responses are poorly understood. Here, we show that melatonin effect on molecular signalling pathways, physiological changes and UV-B stress resistance in Arabidopsis. Both exogenous and endogenous melatonin affected expression of UV-B signal transduction pathway genes. Experiments using UV-B signalling component mutants cop1-4 and hy5-215 revealed that melatonin not only acts as an antioxidant to promote UV-B stress resistance, but also regulates expression of several key components of UV-B signalling pathway, including ubiquitin-degrading enzyme (COP1), transcription factors (HY5, HYH) and RUP1/2. Our findings indicate that melatonin delays and subsequently enhances expression of COP1, HY5, HYH and RUP1/2, which act as central effectors in UV-B signalling pathway, thus regulating their effects on antioxidant systems to protect the plant from UV-B stress.
1 INTRODUCTION
Plants routinely confront a variety of abiotic environmental stresses that tend to reduce productivity; these include cold, drought, freezing, flooding, wounding, heat and ultraviolet-B (UV-B) radiation. UV-B stress, which affects rates of plant growth and development, is of particular concern because the amount of UV-B reaching the Earth's surface has been steadily increasing (by 6–14% each year) since the 1980s, as a result of declining ozone level in the upper atmosphere (Lee, 2016; McKenzie et al., 2011). UV-B (wavelength range 280–315 nm) is one of three classes of UV radiation in the electromagnetic spectrum; the other two are UV-A (315–400 nm) and UV-C (100–280 nm; Tilbrook et al., 2013). UV-B has been shown to trigger differential gene expression in the well-studied model plant species Arabidopsis thaliana (Brassicaceae). Genetic and transcription analyses have identified several regulators involved in UV-B-specific responses; these include UV-B receptor UV resistance locus8 (UVR8; Brown et al., 2005), multifunctional E3 ubiquitin ligase constitutively photomorphogenic 1 (COP1; Oravecz et al., 2006) and bZIP transcription factor elongated hypocotyl5 (HY5; Ulm et al., 2004) as positive regulators, and repressor of UV-B photomorphogenesis 1/2 (RUP1/2) as negative regulator (Heijde & Ulm, 2012).
Melatonin (N-acetyl-5-methoxytryptamine) is a well-studied animal hormone synthesized and secreted by the pineal gland in vertebrates. It is also present in diatoms, fungi, algae, ferns and higher plants (Vriend & Reiter, 2015b), indicating its selection as a regulatory substance during early stages of evolution (Arnao, 2014; Majidinia, Reiter, Shakouri, & Yousefi, 2018; Reiter et al., 2017; Zhao et al., 2019). Numerous studies have documented the role of melatonin in ameliorating various common abiotic and biotic stresses, including cold, drought, heavy metals, salt and pathogen attack (Arnao & Hernández-Ruiz, 2019a, 2019c; Bose & Howlader, 2020; Moustafa-Farag et al., 2019; Sharif et al., 2018; Wang, Reiter, & Chan, 2018). Relatively few studies have addressed the role of melatonin in UV-B responses. Kozai's group reported in 2006 that melatonin level in Glycyrrhiza uralensis (Fabaceae) increased under both high- and low-intensity UV-B radiation (Afreen, Zobayed, & Kozai, 2006). Later studies in 2012 and 2019 showed that melatonin ameliorated DNA damage and ROS reduction caused by UV-B stress in Nicotiana sylvestris and Malus hupehensis (Arnao & Hernández-Ruiz, 2014; Fischer, Kleszczyński, Hardkop, Kruse, & Zillikens, 2013; Hernández-Ruiz & Arnao, 2008; Nazir et al., 2020; Ullah et al., 2019; Wei et al., 2019; Zhang et al., 2012). The molecular mechanism whereby melatonin affects the UV-B pathway was not addressed in these studies. The present study was designed to elucidate the role of melatonin in responses to UV-B stress, and the molecular mechanism involved. We measured transcription levels of key genes associated with UV-B signalling pathway in response to UV-B and melatonin treatment in Arabidopsis.
In Arabidopsis, melatonin is synthesized from tryptophan. The final two steps in the biosynthetic pathway are catalysed sequentially by serotonin N-acetyltransferase (SNAT) and N-acetylserotonin methyltransferase (ASMT; Byeon, Choi, Lee, & Back, 2015; Kang, Kim, Park, & Back, 2009; Kang, Lee, Park, Kim, & Back, 2010; Kang, Park, Kim, Lee, & Back, 2009). SNAT acts as the rate-limiting enzyme in the overall melatonin biosynthetic pathway. In the present study, we used a SNAT knockdown mutant and a transgenic SNAT-overexpressing cell line to demonstrate that UV-B signalling pathway is affected by alteration of endogenous melatonin level.
Our findings clearly show that melatonin plays a key role in reduction of UV-B-induced damage in Arabidopsis, by upregulating expression of UV-B-inducible transcriptional activators and UV-B-regulated target genes. Experiments with cop1-4 and hy5-215 mutants revealed that melatonin lost the ability to enhance UV-B response in the absence of these UV-B signal core components.
2 MATERIAL AND METHODS
2.1 Plant material and growth condition
A. thaliana ecotype Columbia-0 (Col-0) was used as wild-type (WT) background and basis for mutants. cop1-4 (McNellis, von Arnim, & Deng, 1994) and hy5-215 (Oyama, Shimura, & Okada, 1997) mutants were kindly provided by Deng (Peking University) and Huang (Xiamen University). SNAT knockdown mutant (SALK-032239) was selected from NASC (Nottingham Arabidopsis Stock Centre), and homozygous line was identified by genotyping assay. Arabidopsis seeds were surface-sterilized with 70% (vol/vol) ethanol for 1 min and 0.1% HgCl2 for 3 min, washed five times with sterile water and cultured in Petri dishes in 1/2 MS medium (Murashige & Skoog, 1962) with 3% sucrose. pH was adjusted to 5.8–6.2, agar (2.1 g/L) was added, and dishes were autoclaved for 20 min at 121°C. Dishes with seeds were stratified at 4°C for 2 days for synchronization. Plants were grown at 20 ± 2°C with photoperiod 16-hr light/8-hr dark and light intensity (from cool white fluorescent tubes) 100 μmol m−2 s−1.
2.2 Generation of AtSNAT transgenic lines
AtSNAT coding sequence was PCR amplified using primer pair containing BamHI site (5′-CGCGGATCCATGCTACTAATCCCAATTTCTTC-3′ F) and EcoRI site (5′-CGCCTTAAGCTACTTTGGGTACCAAAACATGC-3′ R) and ligated into pRI101-AN vector, constructed vector was introduced into Agrobacterium tumefaciens strain GV3101 and transferred into Arabidopsis by floral dip (Zhang, Henriques, Lin, Niu, & Chua, 2006). AtSNAT transgenic lines were obtained after selection of T3 seeds on MS medium containing kanamycin. The transcription level of AtSNAT was further confirmed using qRT-PCR.
2.3 UV-B and melatonin treatment groups
Plants were subjected to one of four treatments: control (CK), melatonin (MT), UV-B or MT + UV-B. Ten-days-old soil-grown seedlings were sprayed for 3 days, at dusk, with water containing 0 or 100 μM melatonin. Expression of genes involved in melatonin synthesis and UV-B pathway was investigated by radiating seedlings for 2 hr, using Philips TL20W/01RS narrowband UV-B tubes (0.7 μmol m−2 s−1) + 100 μmol m−2 s−1 white light (white fluorescent tubes). Control group (−UV-B) was exposed to 100 μmol m−2 s−1 white light (white fluorescent tubes). For antioxidant enzyme and oxidative metabolite assays, seedlings were radiated for 6 hr.
2.4 Analysis of H2O2, O2·−, malondialdehyde and iron leakage
H2O2 level was determined as described by Jana and Choudhuri (1982). A total of 0.2 g leaf samples were homogenized in 1 ml of 50 mM phosphate buffer (pH 6.8), and homogenate was centrifuged at 6,000g for 25 min at 4°C. One millilitre supernatant was mixed with 0.5 ml titanium chloride in 20% H2SO4, and the mixture was centrifuged at 6000g for 15 min at 4°C. H2O2 content was measured at 410 nm.
Level of lipid peroxidation end product malondialdehyde (MDA) was measured based on 2-thiobarbituric acid (TBA) reaction as described previously (Hodges, DeLong, Forney, & Prange, 1999). In brief, leaf samples (0.5 g) were homogenized in 5 ml of 5% (wt/vol) trichloroacetic acid (TCA), and homogenate was centrifuged at 3,000g for 10 min. Two millilitre supernatant was added with 2 ml of 0.67% TBA, heated at 95°C for 30 min, and cooled to stop reaction. Sample was centrifuged at 3000g for 10 min, and absorbance of supernatant was measured at 450 and 600 nm. H2O2 and MDA were assayed using a spectrophotometer (model T6; PERSEE, Beijing, China).
H2O2 and O2− levels under UV-B stress were assayed by histochemical staining. Three-week-old plants were subjected to 0, 2, 4 or 6 hr UV-B radiation, and H2O2 was detected by 3,3′-diaminobenzinidine (DAB) assay. Samples were soaked in 1 mg/ml DAB solution (pH 3.8) for 8 hr, and decolorized by boiling in 95% ethanol for 10 min. For detection of O2−, samples were stained by 0.1% (wt/vol) NBT solution for 5.5 hr and decolorized by boiling in 95% ethanol for 40 min. Samples of both types were examined with a stereo microscope (Nikon C-LEDS; Nikon, Tokyo, Japan).
To measure the electrolyte leakage (Lee & Back, 2018), Arabidopsis leaves were immersed into 20 ml distilled H2O for 5 min to eliminate signals derived from wounded cells. And then were placed in 10 ml deionized water for 6 hr, solution conductivity was measured using a conductivity metre (DDBJ-350; INESA, Shanghai, China).
2.5 Hypocotyl length
Col-0, cop1-4 and hy5-215 seeds were surface sterilized, soaked with 0 or 1 μM melatonin solutions for 6 hr, cultured in 1/2 MS medium, exposed to white light for 6 hr, then kept in the dark for 2 days and then UV-B radiated by Philips TL20W/01RS narrowband UV-B tubes (0.7 μmol m−2 s−1) for 1 hr (UV-B treated group) or kept in dark (control group) and kept in the darkness for 7 days. Hypocotyl length was measured for ≥50 seedlings in each group, using ImageJ software programme (Cole & Chory, 2012).
2.6 Total RNA extraction and expression analysis of UV-B-inducible genes by quantitative real-time PCR
Total RNA was isolated from UV-B-treated and control seedlings using RNeasy Plant Mini Kit (Qiagen; Valencia, CA, USA). Transcription levels of UV-B related genes were analysed with a real-time PCR system (model CFX96; Bio-Rad), using gene-specific primers (Table S1). Quantitative real-time PCR (qRT-PCR) was performed with a 20-μl reaction volume containing 0.5 μl (10 pmol/μl) of each primer, 1 μl template cDNA and 10 μl SYBR Green (SYBR premix EX Taq; Takara). Fluorescent intensity data were acquired during the extension step. Transcription levels were estimated using a standard curve. Three biological replicates were performed for each treatment, and three technical replicates were performed for each biological replicate. Expression of each gene was normalized to that of Arabidopsis gene ACTIN8, and calculated relative to that of nontreated control.
Expression of UV-B-responsive genes as a function of time was analysed using 10-day-old seedlings grown under –UV-B and then transferred to +UV-B for various durations.
2.7 Melatonin extraction and assay
Melatonin was extracted by acetone/methanol method (Chen et al., 2003) and measured using an immunoassay kit (Shanghai Lanpai Biotechnology Co.; Shanghai) as per the manufacturer's instructions, and data were quantified using a standard curve. OD values were obtained using a Multimode Plate Reader (model M200 Pro; Tecan, Männedorf, Switzerland; Okazaki & Ezura, 2009).
2.8 Antioxidant enzyme extraction and activity assay
For extraction of antioxidant enzymes, leaves (0.2 g) were ground in a chilled mortar, and homogenized in 1 ml of 50 mM phosphate buffer (pH 7.0) containing 4% (wt/vol) polyvinylpolypyrrolidone and 1 mM EDTA. Homogenates were centrifuged at 12,000g for 15 min at 4°C, and supernatants were used for estimation of antioxidant enzyme activities.
Catalase (CAT) activity was estimated by monitoring absorbance decrease at 240 nm resulting from H2O2 reduction. Peroxidase (POD) activity was estimated based on increase of absorbance at 470 nm, and degree of guaiacol oxidation in a 90 s period.
2.9 Statistical analysis
Analyses were performed using Origin software programme, V. 8.0, with values expressed as mean ± SE. Differences between means were evaluated by one-way ANOVA, Fisher's LSD test or Tukey's HSD post hoc test and considered significant for p < .05.
3 RESULTS
3.1 UV-B radiation alters endogenous melatonin level
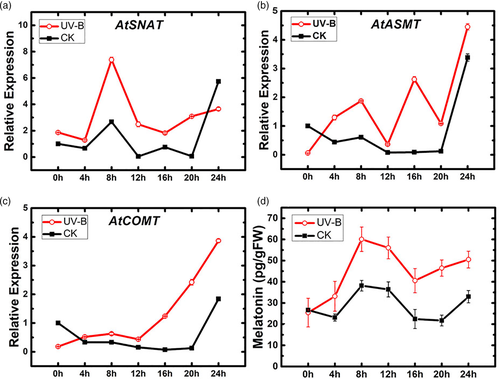
Endogenous melatonin level in A. thaliana was altered by UV-B radiation treatment. We used fluorogenic qRT-PCR to measure expression of three synthase genes following 2 hr UV-B treatment: AtSNAT, AtCOMT and AtASMT Each of the three genes showed increased expression relative to control following UV-B treatment (Figure 1a–c). AtSNAT showed the greatest (~5-fold) increase, with a peak at 8 hr. AtASMT and AtCOMT had peaks at 24 hr. Endogenous melatonin level had a peak at 8 hr following UV-B treatment (Figure 1d). These findings all indicate that UV-B radiation enhances melatonin content in A. thaliana.
3.2 Effects of UV-B and melatonin treatment on antioxidant enzyme and MDA levels
In response to various abiotic stresses (e.g., salt, cold), melatonin acts as a defender to help internal antioxidant systems protect plants from damage (Bajwa, Shukla, Sherif, Murch, & Saxena, 2014; Li et al., 2018; Zheng et al., 2017). We pretreated plants with 0.1 mM melatonin prior to UV-B treatment, and then assayed various physiological indexes and antioxidant enzymes. Melatonin pretreatment inhibited UV-B-induced increase of H2O2 and MDA (Figure 2a,b), reduced H2O2 and MDA level in UV-B-treated plants respectively to 57.1 and 50.9% of control (non-UV-B-treated) value.
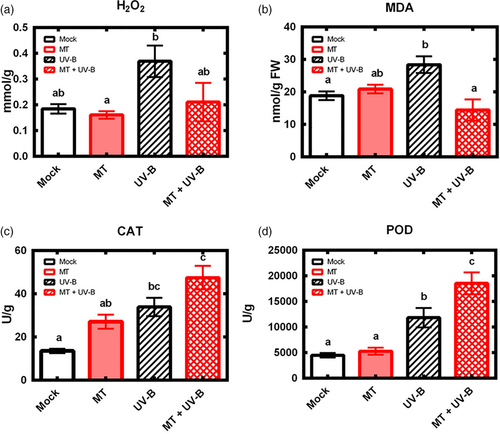
Under normal circumstances, antioxidant enzymes maintain steady activities in vivo. Both POD and CAT activity were induced in UV-B treatment group and were further elevated to a higher level in MT + UV-B double treatment group (Figure 2c,d). These findings, taken together, clearly indicate that melatonin affects the Arabidopsis antioxidant system and suggest that melatonin plays a role in signalling pathway to regulate gene transcription in addition to its role as antioxidant and free radical scavenger.
3.3 Exogenous melatonin alters expression of genes involved in UV-B signalling pathway
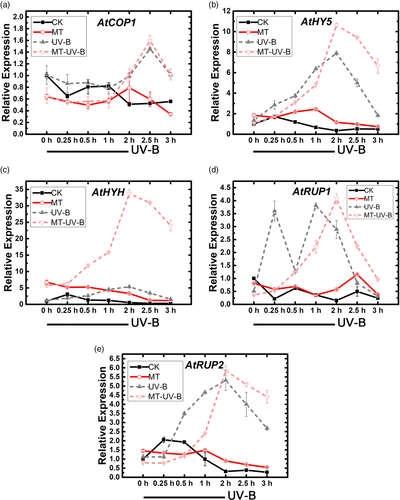
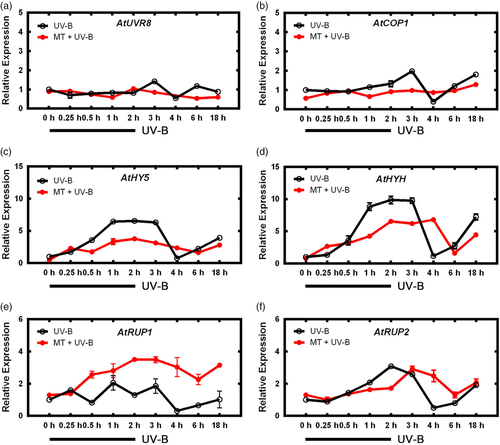
To evaluate the above hypothesis, we used qRT-PCR to assay effects of exogenous melatonin and subsequent UV-B treatment on transcription levels of genes involved in UV-B signalling pathway (UVR-8, COP1, HY5, HYH, RUP1, RUP2). COP1 in the MT + UV-B group showed an initial decline at 1 hr (COP1 in the UV-B group did not), and had peaks at 0.25 and 2 hr (UV-B group had its peak at 2 hr; Figure 3). Two basic leucine zipper (bZIP) transcription factors, HY5 and HYH, showed large increases in the MT + UV-B group – particularly HYH, whose expression was nearly sixfold higher than that of UV-B group at 2 hr and remained high until 4 hr, before declining to normalized level. Similar trends were observed for RUP1/2, downstream of HY5, which act as feedback inhibitors; peaks were delayed but slightly higher in MT + UV-B group. These findings suggest that melatonin affects UV-B signalling pathway by delaying activation of RUP1/2, to enhance transcription of COP1, HY5 and HYH.
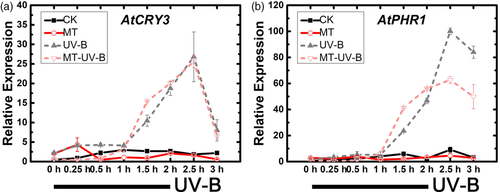
Expression of downstream genes CRY3 and PHR1, involved in repair of UV-B-induced DNA damage, was also assayed. Expression of these genes was activated earlier in MT + UV-B group than in UV-B group, and maintained at a lower level (Figure 4), suggesting that melatonin helps plants start defence earlier and reduces damage from UV-B stress.
3.4 Exogenous melatonin alters expression of hy5 mutant gene under UV-B radiation
Effects of melatonin on UV-B signalling pathway were further investigated by examining gene expression of hy5 mutant (hy5-215) under the treatments described in Section 3.3. COP1 expression in hy5 mutant was not enhanced in MT + UV-B group (Figure 5). The trend of increased HY5 transcription in hy5-215 was not notably different from findings for Col-0 (Figure 3), and was maintained for MT + UV-B group. HYH, which was greatly increased for Col-0, was only slightly enhanced in UV-B group and had a more gradual increase in MT group. In UV-B group, increases of RUP1/2 were ~50% of those for Col-0. In MT group, only expression of RUP1 was notably higher than in UV-B group and its increase was irregular. Thus, when HY5 in UV-B signalling pathway was knocked down, melatonin did not upregulate genes downstream of HY5, suggesting that HY5 is a crucial gene governing the effect of melatonin on UV-B signalling pathway.
3.5 Alteration of melatonin-mediated UV-B signalling is dependent on cop1
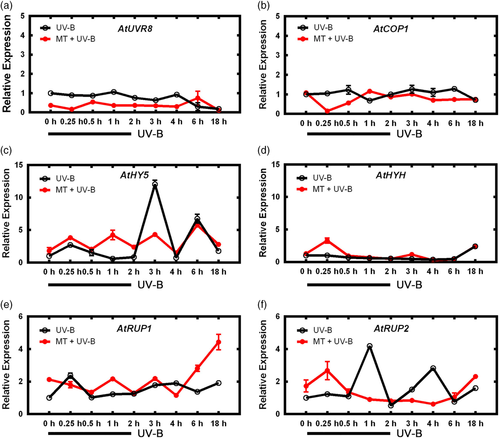
HY5 was shown to play an important role in UV-B signalling pathway response to melatonin, however, the role of its upstream gene, COP1, remained unclear. We used cop1-4 mutant to evaluate the effect of melatonin on COP1 expression. In mutant lines in which COP1 expression was knocked down, UV-B treatment had no effect on COP1 expression, regardless of melatonin pretreatment (Figure 6), in contrast to clearly increased COP1 expression in WT (Figure 3). Expression of HY5 was also affected, without notable increase in MT group. Transcription of RUP1 remained fairly constant in cop1-4 lines, with only small peaks at 15 min (UV-B group) or 1 hr (MT + UV-B group). RUP2 expression showed similar trends, except that the peak times of UV-B and MT + UV-B groups were reversed. These findings indicate that melatonin no longer had a notable effect downstream of UV-B signalling pathway when COP1 was knocked down.
3.6 Effect of melatonin on hypocotyl elongation
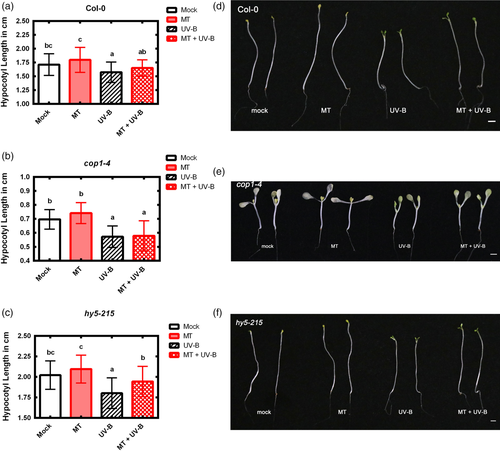
COP1 is a key skotomorphogenesis inducer that generally causes hypocotyl elongation under dark growth condition (Osterlund & Deng, 1998). Effect of melatonin on COP1 was further evaluated by measuring hypocotyl length of cop1-4, hy5-215 and WT lines following soaking treatment with 1 μM melatonin. A pilot experiment showed that melatonin at concentration 1 μM significantly increased hypocotyl length of Col-0 (Figure S1), regardless of UV-B treatment. Subsequent full experiment showed that melatonin treatment induced notable hypocotyl elongation in WT and hy5-215, whereas such elongation in cop1-4 mutant was not significant (Figure 7), supporting the concept that cop1 is the key gene for effect of melatonin.
3.7 Alteration of melatonin biosynthesis affects melatonin-mediated UV-B response
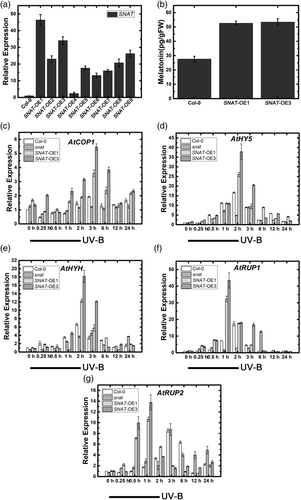
To further elucidate the role of endogenous melatonin in UV-B signalling pathway, we generated a SNAT-overexpressing line through Agrobacterium-mediated transformation (Figure 8a,b), and selected two independent transgenic lines showing high SNAT expression for subsequent analysis. SALK_032239 line was selected as SNAT transcription-repressed mutant. Previous experiments showed that findings from altered biosynthesis of endogenous melatonin were consistent with findings from application of exogenous melatonin. Positive regulatory genes in UV-B signalling pathway (COP1, HY5, HYH) showed early, large increases in SNAT-overexpressing lines and later responses in SNAT knockdown mutant. This trend was even more pronounced in negative regulatory genes (RUP1/2; Figure 8c–g), indicating that promotion of endogenous melatonin synthesis positively regulates UV-B signalling pathway.
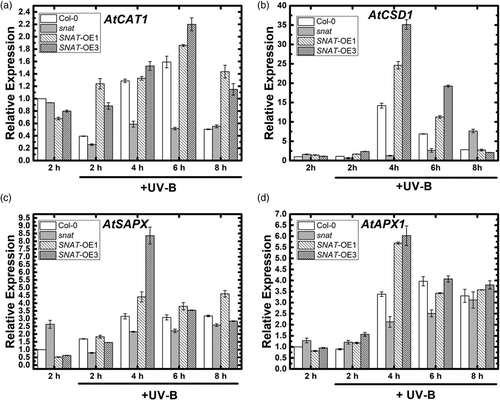
Expression of four genes (CAT1, APX1, SAPX, CSD1) that play key roles in anti-oxidation system downstream of UV-B signalling pathway was examined under high and prolonged UV-B radiation treatment. UV-B was applied for 8 hr, and samples were taken every 2 hr. CAT1 increased 2 hr earlier and to a greater degree in MT-treated group than in non-treated group (Figure 9). Expression of APX1 and SAPX increased at hour 4 under UV-B stress in WT and SNAT-OE lines; the increase in SNAT-OE was twice that in WT. Time of increase of CSD1 was the same in WT and SNAT-OE, whereas CSD1 transcription was much higher in SNAT-overexpressing lines. Expression of these four genes was clearly reduced in the SNAT mutants. These findings indicate that alteration of melatonin biosynthesis affects transcription of genes in UV-B signalling pathway, and promotes transcription of downstream genes responsive to oxidative stress when SNAT is overexpressed.
3.8 Alteration of endogenous melatonin biosynthesis affects plant physiological indexes under UV-B stress
To evaluate effects of endogenous melatonin on SNAT transgenic lines, we assayed melatonin levels in SNAT-OE lines and SALK_032239 under UV-B stress. In the absence of UV-B treatment, melatonin level was higher in SNAT-overexpressing lines than in Col-0 or SNAT knockdown mutant (Figure 10a). Under UV-B stress, melatonin synthesis was induced in all four lines. snat mutant had a peak at hour 2, and SNAT overexpression was associated with significant increases of melatonin content (200.29 and 209.28 pg/gFW) at hour 4.
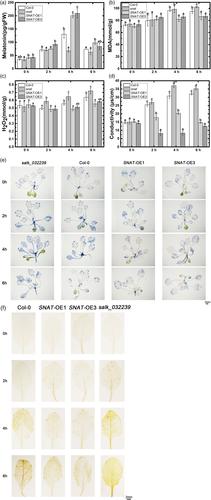
ROS generation and other physiological indexes (e.g., ion leakage) were examined to evaluate the UV-B stress resistance of various endogenous melatonin levels (Figure 10b–d). MDA content generated by UV-B stress was reduced in SNAT-overexpressing lines, whereas membrane lipid peroxidation was greater in Col-0 and snat mutant. Ion leakage data also showed that increase of endogenous melatonin enhanced UV-B stress resistance. Conductivity values in the absence of UV-B treatment did not differ significantly among the four lines. Under UV-B stress, conductivity increased notably in WT and low-MT lines, increased slightly in SNAT-OE1 and decreased in SNAT-OE3. Results of H2O2 detection experiments were essentially consistent with the above findings. DAB and NBT staining experiments revealed an inhibitory effect of melatonin on H2O2 and O2− generation induced by UV-B stress (Figure 10e,f). That is, under UV-B stress, H2O2 and O2− generation were low in high-MT lines, but high in low-MT lines. These findings for physiological indexes, taken together, demonstrate that changes of SNAT transcription alter endogenous melatonin content, and that high melatonin content enhances UV-B stress resistance and reduces UV-B-induced cell damage.
4 DISCUSSION
Melatonin, a small molecule widespread in organisms ranging from microbes to plants to higher vertebrates, is involved in numerous physiological processes, including redox reactions, biosynthesis, circadian clock and stress defences (Arnao, 2014; Arnao & Hernández-Ruiz, 2020). Many studies have demonstrated function of melatonin in enhancing plant defences against various abiotic stresses, including cold, salt and drought (Li et al., 2012, 2018; Turk et al., 2014; Wang et al., 2013; Zheng et al., 2017; Zuo et al., 2014). It was reported in 2006 that high-intensity UV-B-induced melatonin synthesis in G. uralensis (Afreen et al., 2006). A 2012 study of melatonin function in responses to UV-B stress focused on its effect on antioxidant system (Zhang et al., 2012). The present study revealed for the first time that melatonin affects the UV-B signalling pathway to enhance UV-B stress resistance in a model plant system (Figure S2). Physiological experiments showed that exogenous melatonin helped reduce UV-B-induced cell damage and DNA damage, and several physiological indexes had higher values in the UV-B-stressed group pretreated with melatonin than in the non-pretreated group. Removal of excessive accumulated ROS within cells through increased antioxidant system activity is a crucial protective mechanism in stress-exposed plants. Previous researches demonstrated that under stress condition, melatonin acts as an antioxidant at cellular level to alleviate ROS and indirectly activates the gene expression of antioxidant enzymes (Arnao & Hernández-Ruiz, 2019a, 2019b; Khan et al., 2020; Moustafa-Farag et al., 2019). We also hypothesized that melatonin functions at the gene level to enhance expression of antioxidant enzymes, and their protective effect. This concept was supported by our experiments on melatonin synthesis under UV-B radiation, in contrast to previous reports that long-term UV-B radiation reduces melatonin synthesis (Afreen et al., 2006). It appears that melatonin synthesis is induced by UV-B radiation (Figure 10a).
COP1 plays an inhibitory role in visible light signalling. HY5 is degraded through COP1 ubiquitination in darkness, but can be accumulated in cop1 seedlings, resulting in a constitutive photomorphogenic mutant phenotype that includes shortened hypocotyl (Osterlund, Hardtke, Wei, & Deng, 2000). cop1-4 is an ethyl methane sulfonate (EMS)-mutagenized line having a less severe phenotype in which hypocotyl is shortened under light condition and slightly elongated under constant dark (Deng & Quail, 1992). Treatment with melatonin through soaking of seeds significantly increased hypocotyl elongation during skotomorphogenesis (Arnao & Hernández-Ruiz, 2017; Hernández-Ruiz, Cano, & Arnao, 2004, 2005). Such increase was greatly suppressed in cop1-4 mutant, and cop1 mutation per se also inhibited hypocotyl elongation. We presume that melatonin effect is reduced by loss of cop1 function. COP1 act as the central effector of UV-B pathway through its WD40 domain, directly binding to UVR8 and downstream genes HY5 and HYH. The mechanism whereby binding of UVR8 to COP1 causes COP1 to activate downstream transcription factors remains unclear (Heijde & Ulm, 2012; Yin & Ulm, 2017). In particular, HYH was induced in Col-0 and HY5 mutant by MT + UV-B, but was downregulated in cop1-4 by the same treatment, indicating that absence of COP1 interferes with the regulatory effect of melatonin on UV-B signalling pathway. Melatonin was considered as a potential regulator of COP1, since they exist together and interact in mammals, and are cooperatively involved in tumorigenesis, metabolism and light regulation (Sanchez-Barcelo, Mediavilla, Vriend, & Reiter, 2016). Melatonin was also reported to affect ubiquitin-proteasome system in mammals (Vriend & Reiter, 2014, 2015a) and plants (Vriend & Reiter, 2015b). However, the detailed mechanism whereby melatonin affects COP1 function remains to be clarified.
bZIP transcription factor HY5 plays a key role in UV-B signalling (Brown et al., 2005; Brown & Jenkins, 2008; Huang et al., 2012; Stracke et al., 2010; Ulm et al., 2004). As with COP1, HY5 involvement places UV-B into a larger network of plant light-responsive signalling, in which HY5 plays a well-characterized, central role in modulation of light-responsive gene expression. Genes encoding various transcription factors are induced by UV-B, and potentially control a broad transcriptional network that can amplify UV-B response (Tilbrook et al., 2013). Under UV-B exposure, COP1 is required for HY5 expression induction (Oravecz et al., 2006). Once expression is induced, HY5 becomes involved in a positive feedback loop that enhances COP1 expression, by binding to one of three specific ACGT-containing elements (ACEs) within the COP1 promoter (Huang et al., 2012). In hy5-215, nucleotide g-1117 is replaced by a. Because it occurs in the first intron, this change does not have a major impact on hy5 transcription. However, melatonin effect is still impaired in hy5-215 because HY5 transcription is induced after 2.5 hr. Expression of downstream genes (HYH, RUP1, RUP2) induced by exogenous melatonin is also altered in hy5-215 (although to a lesser degree than in cop1 mutant), with reduced transcription at later stages. HYH encoded protein has 49% similarity to HY5 (Holm, Ma, Qu, & Deng, 2002), and appears to have overlapping function. We found that melatonin strongly alters expression of these two genes (particularly HYH). Melatonin presumably has complex effects on this signalling pathway, but detailed mechanisms of such effects await further study.
Early studies regarding effects of melatonin on abiotic stress in plants involved application of exogenous melatonin. Recent studies increasingly use transgenic lines to elucidate the roles of endogenous melatonin. In Arabidopsis, SNAT acts in the penultimate step of melatonin biosynthesis to catalyse conversion of serotonin into N-acetyl-serotonin (Back, Tan, & Reiter, 2016), or as the final-step (and rate-limiting) enzyme to convert 5-methoxytryptamine to melatonin. We generated a SNAT (At1g32070)-overexpressing line and a SNAT-knockdown mutant (SALK_032239) to investigate functions of endogenous melatonin in UV-B pathway. Our findings on alteration of melatonin biosynthesis were consistent with those in previous studies based on application of exogenous melatonin. Melatonin content differences among SNAT-overexpressing and -knockdown lines were small under normal conditions, but became much more pronounced under UV-B treatment, suggesting that overexpression SNAT could further stimulate the intrinsic melatonin biosynthesis under UV-B radiation. The minor melatonin difference in the three lines may be due to the presence in Arabidopsis of various biosynthetic pathways and SNAT homologues. Along the same line, OsSNAT1 and OsSNAT2 have similar functions in Oryza sativa (Asian rice; Byeon, Lee, & Back, 2016; Lee & Back, 2017). At1g32070, At4g19985 and At1g26220 all have N-acetyltransferase activity, allowing compensation for loss of AT1g32070 function to reduce overall change. On the other hand, alteration of melatonin biosynthetic pathway through manipulation of SNAT leads to striking downstream changes in response to UV-B stress. Upregulation of SNAT transcription accelerates responses to UV-B, enhances UV-B stress resistance and reduces DNA and cell damage.
In conclusion, this study is the first to demonstrate that melatonin regulates UV-B signalling pathway and alters gene expression to affect antioxidant systems during defensive responses to UV-B exposure. Melatonin delays and subsequently enhances expression of COP1, HY5, HYH and RUP1/2, which act as central effectors in UV-B signalling pathway, thus regulating their effects on antioxidant systems to protect the plant from UV-B stress.
ACKNOWLEDGMENTS
This study was supported by grants from the National Natural Science Foundation of China (31300223, 31572665), Natural Science Foundation of Shaanxi Province (2016JM3001), Opening Foundation of Key Laboratory of Resource Biology and Biotechnology in Western China (Northwest University), Ministry of Education, First-class University and Academic programs of Northwest University, Northwest University Graduate Innovation and Creativity Funds (YZZ17152) and Fujian Provincial Key Laboratory of Haixia Applied Plant Systems Biology Open Fund (Fujian Agriculture and Forestry University). The authors are grateful to Xingwang Deng (Peking University) and Xi Huang (Xiamen University) for providing the cop1-4 and hy5-215 mutant lines. The authors are grateful to Dr. S. Anderson for English editing of the manuscript.
CONFLICT OF INTEREST
No conflict of interest exists in the submission of this manuscript, and manuscript is approved by all authors for publication. I would like to declare on behalf of my co-authors that the work described was original research that has not been published previously, and not under consideration for publication elsewhere, in whole or in part. All the authors listed have approved the manuscript that is enclosed.




