Stem emissions of monoterpenes, acetaldehyde and methanol from Scots pine (Pinus sylvestris L.) affected by tree–water relations and cambial growth
Funding information: Academy of Finland, Grant/Award Numbers: 307331, 312571, 323843; University of Helsinki Research Foundation
Abstract
Tree stems are an overlooked source of volatile organic compounds (VOCs). Their contribution to ecosystem processes and total VOC fluxes is not well studied, and assessing it requires better understanding of stem emission dynamics and their driving processes. To gain more mechanistic insight into stem emission patterns, we measured monoterpene, methanol and acetaldehyde emissions from the stems of mature Scots pines (Pinus sylvestris L.) in a boreal forest over three summers. We analysed the effects of temperature, soil water content, tree water status, transpiration and growth on the VOC emissions and used generalized linear models to test their relative importance in explaining the emissions. We show that Scots pine stems are considerable sources of monoterpenes, methanol and acetaldehyde, and their emissions are strongly regulated by temperature. However, even small changes in water availability affected the emission potentials: increased soil water content increased the monoterpene emissions within a day, whereas acetaldehyde and methanol emissions responded within 2–4 days. This lag corresponded to their transport time in the xylem sap from the roots to the stem. Moreover, the emissions of monoterpenes, methanol and acetaldehyde were influenced by the cambial growth rate of the stem with 6–10-day lags.
1 INTRODUCTION
Forests are major sources of volatile organic compounds (VOCs) that play important roles in tree defence, in plant–insect and plant-to-plant interactions and in atmospheric chemistry (Niinemets & Monson, 2013). However, VOC emissions have long been measured mainly from the foliage, while emissions from the woody parts of trees remain little explored. Concentrating on leaf emissions is reasonable in the case of VOCs that are tightly linked to photosynthesis, such as isoprene (see e.g., Kreuzwieser, Schnitzler, & Steinbrecher, 2008). However, other compounds, such as methanol and acetaldehyde, are also produced in the tree roots or stem (Kreuzwieser et al., 2008), and large pools of monoterpenes and sesquiterpenes are stored in the resin of conifer stems (Strömvall, 2000). Ignoring emissions from tree stems may bias both long-term stand-level emission estimates and our understanding of volatile signalling, for example, between trees and insects, as many VOCs act as chemical cues of a tree's stress. It is necessary to first understand the dynamics and drivers of the stem emissions in various conditions to assess their role in the stand-level emissions budget and in forest ecosystem processes.
Studies that have measured VOC emissions from tree stems have found that the stems may indeed be sources of monoterpenes (Amin et al., 2013, 2012; Ghimire et al., 2016; Lusebrink, Erbilgin, & Evenden, 2013; Kovalchuk et al., 2015; Rhoades, 1990; Staudt, Byron, Piquemal, & Williams, 2019; Vanhatalo et al., 2015) and sesquiterpenes (Ghimire et al., 2016; Kovalchuk et al., 2015) along with methanol, acetone and acetaldehyde (Rissanen, Hölttä, & Bäck, 2018; Vanhatalo et al., 2020). VOC emissions from stems may be small compared with the emissions from foliage when the trees are unstressed; for example, stem monoterpene emissions represent approximately 2% of the stand-level emissions in a Scots pine-dominated boreal forest (Vanhatalo et al., 2020). However, when biotic stresses are present, stem VOC emissions increase (Amin et al., 2013, 2012; Ghimire et al., 2016; Heijari, Blande, & Holopainen, 2011; Kovalchuk et al., 2015; Lusebrink et al., 2013), even in relation to foliage emissions (Amin et al., 2012; Heijari et al., 2011). In addition to stresses, phenology-driven changes, such as springtime onset of transpiration, may cause monoterpene emission peaks from the stems (Vanhatalo et al., 2015).
Variables that drive foliage VOC emissions may also be expected to affect stem emissions. Temperature is one of the strongest variables driving the foliage emissions (e.g., Guenther, Zimmerman, Harley, Monson, & Fall, 1993; Shao et al., 2001; Tingey, Turner, & Weber, 1991) and it also affects stem emissions (Rissanen et al., 2016; Staudt et al., 2019; Vanhatalo et al., 2015, 2020). Other important variables include light (e.g., Guenther et al., 1993; Shao et al., 2001; Tingey et al., 1991), air humidity (Croteau, 1977; Llusià & Peñuelas, 1999; Schade, Goldstein, & Lamanna, 1999; Tingey et al., 1991), transpiration rate (Cojocariu, Kreuzwieser, & Rennenberg, 2004; Kreuzwieser et al., 2001; Rissanen et al., 2018; Seco, Peñuelas, & Filella, 2007), flooding (Copolovici & Niinemets, 2010; Holzinger, Sandoval-Soto, Rottenberger, Crutzen, & Kesselmeier, 2000; Kreuzwieser, Kühnemann, Martis, Rennenberg, & Urban, 2000) and drought (Bertin & Staudt, 1996; Llusià & Peñuelas, 2002; Lüpke, Leuchner, Steinbrecher, & Menzel, 2017; Staudt, Rambal, Joffre, & Kesselmeier, 2002). However, the level at which these environmental variables and physiological processes affect the stem emissions is not known.
Provided that the connections between the measured stem VOC emissions and the affecting physiological processes are strong, VOC emissions could be used as a signal of the processes and conditions occurring within the stem. For example, acetaldehyde or ethanol emissions from the foliage are known to increase when the roots are flooded as a result of anaerobic metabolism that produces ethanol (Copolovici & Niinemets, 2010; Kreuzwieser et al., 2000). Anoxic conditions may also occur in the stem, for example, near the heartwood or because of limited oxygen diffusion due to high stem water content (Sorz & Hietz, 2006) or rapid use of oxygen during high metabolic activity (Kimmerer & Stringer, 1988). Stem anoxia may be difficult to measure, but it could potentially be detected by following changes in acetaldehyde and ethanol emissions from the stem. Moreover, methanol production in plants is strongly connected to tissue growth, particularly the demethylation of pectin in cell wall formation processes (Galbally & Kirstine, 2002; Hüve et al., 2007; MacDonald & Fall, 1993; Nemecek-Marshall, MacDonald, Franzen, Wojciechowski, & Fall, 1995). Non-invasive stem methanol emission measurements may prove useful for acquiring information on the timing of cell wall formation processes of the cambial growth.
We aimed to gain insight on stem VOC emission dynamics by testing the environmental variables and tree processes that potentially affect the stem emissions of a mature tree in field conditions. We used 3 years of summertime data on stem monoterpene, methanol and acetaldehyde emissions from mature Scots pine trees and analysed the emissions and temperature-normalized emissions against soil water availability and tree water relations along with stem growth rate and metabolic activity. We hypothesized that (a) increased soil water availability and xylem water potential would increase the monoterpene emissions because of potentially enhanced production and pressure changes within the stem, (b) an increased transpiration rate would increase the emissions of the water-soluble compounds methanol and acetaldehyde provided that they are transported in the xylem sap and (c) radial stem growth and stem carbon dioxide (CO2) efflux, used as a proxy for stem metabolic activity, would correlate with high emissions of all measured compounds, particularly methanol.
2 MATERIALS AND METHODS
2.1 Site
The measurements were conducted at the SMEAR II station (Station for Measuring Ecosystem–Atmosphere Relations) in Hyytiälä, Southern Finland (61°51′N, 24°17′E, 181 m above sea level) (Hari & Kulmala, 2005). The forest is dominated by approximately 55-year-old Scots pine trees, with undergrowth of Norway spruce [Picea abies (L.) H.Karst] and deciduous species. The dominant Scots pines are approximately 20 m tall. The site is of medium fertility and has an annual mean temperature of 3.5°C and an annual precipitation of 711 mm (Ilvesniemi et al., 2010; Pirinen et al., 2012). The soil is podzolic and rarely dry (mean soil water content [SWC] and soil water potential are presented in Table 1).
| Year | Tree no | Chamber location | Stem diameter under chamber (cm) | Chamber volume (L) | Chamber type | Mean temperature (°C) | Mean soil water content, horizons A–C (m3 m−3) | Min/max soil water potential, horizons A–B (kPa) |
|---|---|---|---|---|---|---|---|---|
| 2013 | Tree 1 | Inside living canopy, 12 m | 8.4 | 0.25 | Type 1 | 15.9 (3.9) | 0.21 (0.051) | −466/−47 |
| 2015 | Tree 2 | Inside living canopy, 12 m | 10.7 | 0.55 | Type 1 | 14.8 (3.8) | 0.22 (0.049) | −780/0 |
| 2017 | Tree 3 | Inside living canopy, 15 m | 6.4 | 1.1 | Type 2 | 13.6 (3.9) | 0.27 (0.017) | −31/0.5 |
2.2 Stem flux measurements
We measured monoterpene, methanol and acetaldehyde stem emissions during 3 years (2013, 2015 and 2017) and selected a time window from May to August because of the coherent availability of data from each year. A different tree was measured each year (Table 1) to see whether we could identify similar responses to environmental drivers when investigating individual trees.
The VOC emissions were captured using custom-made chambers built around tree stems. Two chamber types were used in the measurements: type 1 in 2013 and 2015, described in detail in Vanhatalo et al. (2015) and type 2 in 2017, described in Vanhatalo (2018) (Table 1, Figure S1, see description of the functional differences between the chamber types in Section 2.3 below). The chamber cores consisted of either a polyethylene-coated aluminium spiral (type 1) or aluminium pieces covered with FEP-tape (fluorinated ethylene propylene; type 2) that supported the FEP foil wrapped around the tree stem. The foil was tightened using elastic cable ties around the stem at the top and bottom of an approximately 20–35 cm long section of tree stem. The chambers were installed at a height of 12 m (2013 and 2015) or 15 m (2017), depending on accessibility from the measurement scaffold. We chose to measure the top of the stem to gain a strong VOC emission signal (Rissanen et al., 2018; Vanhatalo et al., 2020). The measured stem section was straight and had smooth bark, which ensured a tight fit of the chamber.
The stem chambers were measured in sequence with other chambers and sampling locations at the research site, including frequent measurements from ambient air. Every 3 hours in the measurement sequence, the stem chamber was measured three times, with an approximately 30-min pause between individual measurements. In total, depending on the small changes in the measurement sequence, the stem chambers were measured 24–32 times per day. During measurement, 1 L min−1 of sample air was drawn from the chamber to the analysers and replaced with 1 L min−1 of compressed ambient air. The measurement time in type 1 chambers was 2 min 45 s and 1 min 30 s in type 2 chamber. Between measurements, 0.4 L min−1 of compressed ambient air was fed through the chamber to flush the chamber air and tubing and to avoid a build-up of VOCs or humidity. Two additional lids on the type 2 chamber surface equipped with small fans were opened between measurements to improve flushing of the chamber. Temperatures in the stem chambers were constantly measured with copper–constantan thermocouples.
The concentrations of monoterpenes (m/z 137), methanol (m/z 33) and acetaldehyde (m/z 45) in the chamber sample air were analysed in a proton transfer reaction—quadrupole mass spectrometer (PTR-QMS; IONICON, Innsbruck, Austria). The PTR-QMS was calibrated two to three times per month with a standard gas (Apel–Riemer Environmental, Inc., Broomfield, CO, USA) containing the measured compounds (methanol, acetaldehyde, and α-pinene as the monoterpene) along with a number of other compounds, such as benzene (m/z 79), toluene (m/z 93) and 1,2,4-trichlorobenzene (m/z 182) to monitor instrument sensitivity. The E/N was set to approximately 106 Td. The calibration procedure and maintenance of the PTR-QMS and concentration calculations were conducted similarly over the whole measurement period, and they are reported in Taipale et al. (2008). The sample air was also measured with a Li-840 A analyser (Li-Cor, Lincoln, NE) to determine the CO2 and water (H2O) concentrations of the sample.
 (1)
(1)Due to instrument maintenance and a few system malfunctions, there were short periods of missing data. To ensure that the daily data were representative, the data from days that had less than 10 measurement points on VOC emissions out of 24–32 were omitted from the analysis.
2.3 Chamber type effect on measured VOC emissions
The main functional difference between stem chamber type 1, used in 2013 and 2015, and stem chamber type 2, used in 2017, was that type 2 chamber was dynamic because of the opening and closing ventilation holes, which allowed for the emission calculations with the mass balance equation. Using the mass balance equation allowed for a shorter measurement time, as the chamber did not need to reach a steady state for emission calculations. The ventilation holes and fans also made chamber flushing between measurements more efficient. Because of the more efficient flushing, the relative humidity limit of 75% for methanol and acetaldehyde emissions could potentially have been elevated for the type 2 chamber, but we decided to use the same limit for both chamber types for consistency.
Comparing the emission dynamics measured by the two chamber types, we did not have a reason to suspect that data from the two chamber types would have been incomparable for the purpose of this study: the emission dynamics measured by the two chamber types were similar both on a daily scale and over the measurement periods. It is possible that the type 2 chamber performed better in capturing the emissions, thereby contributing to the higher measured emissions in 2017, but the higher emissions may also have been caused by tree-to-tree variation and the measurement location being higher on the stem (Vanhatalo et al., 2020). Because we focused on dynamics rather than on absolute values in this study, we did not compare the chamber type effects on the absolute emissions further.
2.4 Auxiliary measurements
We compared the emission patterns of monoterpenes, methanol and acetaldehyde with the stem chamber temperature and a set of variables describing water status in the soil and within the tree: SWC, transpiration and xylem diameter, and another set describing stem growth and activity: cambial growth rate and stem CO2 efflux already described above.
Data on SWC in the A-, B1-, B2- and C-horizons were continuously measured at the SMEAR II station and are available at SmartSMEAR (https://avaa.tdata.fi/web/smart/smear/download). SWC was measured from horizons A, B1, B2 and C from five locations over the measurement site using a Campbell TDR100 Time-Domain reflectometer (Campbell Scientific, Logan, UT). We used the mean SWC over all the locations and soil horizons to obtain a general picture of the root system's humidity conditions. We also used ambient relative humidity data measured by a Rotronic MP102H relative humidity sensor (Rotronic Measurement Solutions, Basserdorf, Switzerland) at a height of 16 m, corresponding to humidity at the tree canopy height.
Shoot transpiration was measured from the water vapour concentrations in shoot chambers installed in the upper canopy of the trees equipped with stem chambers. The sample air from the shoot chambers was directed into a Li-840 A analyser (Li-Cor, Lincoln, NE, USA) to determine the water vapour concentration of the sample air, and the fluxes were calculated using the mass balance equation (Equation [1]). The shoot gas exchange measurement workflow and shoot chambers are described in detail by Kolari et al. (2009, 2012) and Aalto et al. (2014). We omitted the transpiration data when relative humidity in the chamber exceeded 75%, and the resulting gaps in data were filled by the optimal stomatal control model (Hari, Mäkelä, Korpilahti, & Holmberg, 1986). This model calculates stomatal conductance as a function of irradiation and vapour pressure deficit, and multiplying the obtained stomatal conductance by vapour pressure deficit gives an estimate of transpiration. The vapour pressure deficit was calculated from temperature and relative humidity inside the shoot chamber and irradiation was measured from the top of the shoot chamber with a Li-Cor Li-190 quantum sensor (Li-Cor; Lincoln, NE). No irradiation measurements were made next to the stem chambers. The measured irradiation may be unmeaningful for the measured stem VOC emissions because the light conditions vary greatly on the different sides of the chamber.
As a proxy for xylem water potential, we used xylem diameter changes measured by linear variable displacement transducers (point dendrometers, model AX/5.0/S; Solartron Inc., West Sussex, UK). Direct measurements with a psychrometer were not possible due to the high liquid resin content of the pine trees. The diameter change measurement describes the changes in xylem diameter due to changes in xylem water potential (Dietrich, Zweifel, & Kahmen, 2018; Irvine & Grace, 1997) but also includes changes in osmotic potential of the living cells (Lintunen, Lindfors, Nikinmaa, & Hölttä, 2017). Changes in whole-stem diameter were measured simultaneously.
Cambial growth of the stem was estimated from the changes in living bark dimensions, calculated from the difference between the whole-stem diameter and xylem diameter. From this value, we took the derivate over 10 days as a proxy for the growth rate. To describe stem metabolic activity related to growth and maintenance, we also used CO2 efflux from the stem. As the CO2 efflux is strongly connected to stem respiration and temperature, we normalized it to temperature by using the residuals from an exponential temperature fit made separately for each year.
2.5 Data analysis
 (2)
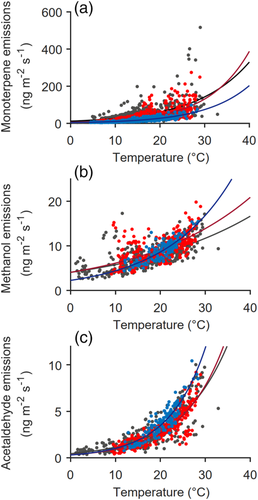
(2)In Equation (2), E is the measured emission rate (ng m−2 s−1), E0 is the temperature-normalized emission rate, also called emission potential (ng m−2 s−1), β is the empirical coefficient describing the temperature impact (K−1), T0 is the standard temperature (303 K or 30°C) and T is the temperature in the chamber (K). We omitted a window from the analysis if the coefficient of determination (R2) of the equation fit to the measured data was less than 0.40. We then smoothed the fitted β values by calculating the mean β for each day over the previous 30 days (including data from May into the data for early June; Figure 1a). We used the 30-day window because we detected the changes in the temperature sensitivity between months (Figure 2). In addition, any changes in temperature dependency of the emissions are probably not rapid but gradual processes driven, for example, by growth or enzyme activity (Vanhatalo et al., 2018). The smoothed β parameter, βs, was interpolated from daily values to the time stamps of the original data. With the βs, we returned to Equation (2) and calculated the final emission potential E0 over the studied periods (Figure 1b).
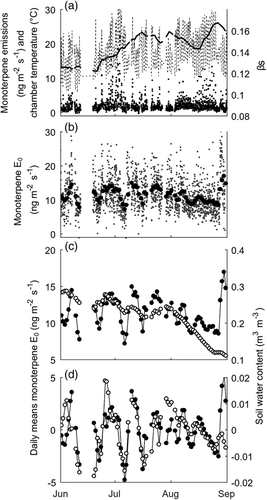
Finally, we calculated the daily means of the emission potentials E0 (Figure 1b) and compared them with the daily means of the explanatory variables separately for each year (Figure 1c). Because long-term VOC emission trends may be related to processes in the stem that were not considered in this study, we also tested detrending the daily means of the esmissions potential and explanatory variables by subtracting their 15-day moving means from the daily means (Figure 1d). We used the 15-day window for the moving average, as it removed the month-to-month trends but did not dampen the shorter-scale changes in the environment.
VOC emissions measured from the bark surface may have a delayed response to the environmental and physiological variables that is related to time lags of temperature changes in the stem or time lags of production and transport of the compounds. Thus, we used cross-correlation analysis for each year separately to analyse possible lags between the time series. This was done by shifting the emission potential time series 1 day at a time in relation to the explanatory variable and finding the lag that yielded the best correlation (Figure S2). For the lag to be considered relevant, it needed to be less than 11 days and it needed to occur in the same scale for at least two of the three trees. We chose 11 days as the limit because with longer time periods, the tested variable could possibly correlate with itself again, which made interpreting the lag and correlation estimates with other variables difficult. We chose the smaller lag in cases where two lags gave local maximum correlations within the 11 days. If we did not find a clear local maximum correlation or the correlation continuously increased with the added lag beyond the 11 days, we assumed no lag. To estimate the sensitivity of the correlation and lag to missing data, we repeated the analysis while removing one data point at a time (Figure S2), and to estimate the sensitivity of the correlation to the lag, we calculated the correlations also with ±1 days of lag (Table S2).
To combine the effect of each explanatory variable over the 3 years, all the variables were normalized by scaling them from zero to one, and we used repeated measures correlation Rmcorr (Bakdash & Marusich, 2017) in R studio (RStudio team, 2019) to calculate the correlations over the years. Rmcorr is an application of Analysis of covariance (ANCOVA) that calculates a correlation over repeated measures from several subjects considering that there are individual differences between the measured subjects (Bakdash & Marusich, 2017). The relevant time lags found in the cross-correlation analysis were included in this calculation. We repeated these correlation analysis steps also for E0 calculated with the β parameter that was fixed to one value over the summer, separately for each year. In the following, we will primarily report the results obtained by the flexible β (fitted in three-day windows and smoothed) but discuss the differences between the two approaches.
2.6 Modelling emissions
To test the relative importance of the explanatory variables in explaining the VOC emissions, we combined them with temperature to explain the measured non-normalized stem VOC emissions. We used a generalized linear model (GLM from R Stats v3.6.1, Vienna, Austria) with gamma distribution and log link because the VOC emissions data were closer to gamma than normal distribution and the relation between temperature and the VOC emissions was log-linear, as has been reported before (e.g., Guenther et al., 1993). To be able to use gamma distribution, we had to remove any below-zero emission values. Firstly, we added each explanatory variable separately to a GLM explaining the VOC emissions of each year only with chamber temperature and tested time lags from zero to 11 days between the explanatory variable and the VOC emissions. To avoid the confounding effect of the inverse daily dynamics between certain explanatory variables and the VOC emissions, we used the daily mean values of the explanatory variables. Secondly, for further modelling, we chose the variables that had significant effects with a lag smaller than 11 days and combined these variables and temperature to the full GLM explaining VOC emissions over the three summers. We added year as a factor and interactions between the year and model variables into the model. To allow changes in temperature effect during the growing season, we also divided the summers into 15-day periods and added an interaction between the period and temperature into the full model.
We tested the significance and relative importance of each variable in the model by extracting them from the model and calculating the Akaike information criterion (AIC) and a proxy for R2 for GLM (1-[residual deviation/null deviation]) of the model when the variable or interaction was removed. To analyse how much the explanatory variables could explain the VOC emissions in a case where the temperature effect was fixed over the summer, we tested the significance and relative importance of the variables also in a reduced model, where the period–temperature interaction was removed. The calculations were performed using Matlab (version R; R Core Team, 2017; The MathWorks, Inc., Natick, MA) and Rmcorr analysis and GLM modelling using R studio (version 1.1.463; RStudio Team, 2019, Boston, MA).
3 RESULTS
3.1 Measured emissions
The measured stem emissions of all compounds, that is, methanol, acetaldehyde and monoterpenes approximately followed the diurnal pattern of temperature (Figure 3) and correlated positively with temperature also over the growing seasons (Figure 2, Table S1). However, the temperature sensitivity of the emissions appeared to change slightly from month to month (Figure 2) and the emission dynamics sometimes deviated from the temperature pattern over the growing season (Figure S3). The correlations of the emissions with the other environmental or physiological variables were typically smaller in relation to temperature (Table S1).
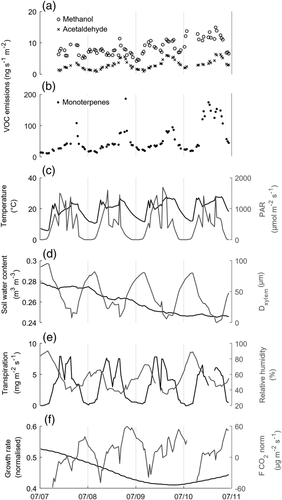
We detected occasional below zero values of methanol, acetaldehyde and monoterpene emissions indicating potential uptake of these compounds, but measuring such small fluxes may be imprecise. Even with the occasional below zero values, the daily mean emissions were positive.
3.2 Emission potentials
The emission potentials of monoterpenes, methanol and acetaldehyde—normalized to temperature using the flexible β parameter—varied both during the growing seasons 2013, 2015 and 2017 and between the trees and years (Figure S4). Tree 3 in 2017 showed the largest emission potentials of all compounds, monoterpenes in particular (Figure S4).
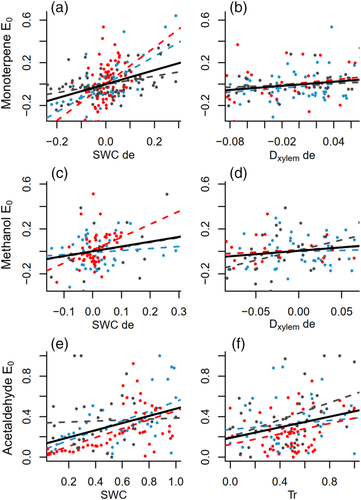
Through all the years, the emission potentials of monoterpenes and acetaldehyde, and to a lesser extent the emission potential of methanol, were related to changes in SWC (Figure 4a,c,e; Table 2). The detrended values of monoterpene emission potential and SWC correlated consistently over all the years without lags or with a lag of 1 day, whereas the acetaldehyde emission potential without detrending followed SWC with a lag of 2–4 days (Table 2). The detrended methanol emission potential correlated with SWC with lags of 3–5 days (Table 2). These time lags between SWC and acetaldehyde and methanol emission potentials corresponded approximately to the time of water transport from the stem base to the top of the stem, where the emissions were measured (12 or 15 m). The transport times, calculated based on the mean transpiration rate over the measurement period and dimensions of each tree, ranged from 3–5 days depending on the tree (see Methods S1, Table S3).
| Years (Pearson's correlation) | Combined years (Rmcorr) | |||
|---|---|---|---|---|
| 2013 | 2015 | 2017 | ||
| Monoterpene E0 | ||||
| RH (%) | 0.21 (2 d) | 0.49 (2 d) | −0.11 (1 d) | 0.17 |
| Detrended | 0.14 (1 d) | 0.38 (3 d) | 0.34 (1 d) | 0.31 |
| SWC (m3 m−3) | 0.35 (1 d) | 0.19 | 0.54 | 0.33 |
| Detrended | 0.67 | 0.41 (1 d) | 0.47 | 0.42 |
| Dxylem (μm) | 0.16 (1 d) | 0.44 (3 d) | 0.19 | 0.26 |
| Detrended | 0.17 (1 d) | 0.56 (2 d) | 0.21 (1 d) | 0.42 |
| Tr (mg m−2 s−1) | 0.10 | −0.12 | 0.08 | 0.02 |
| Detrended | 0.16 | −0.07 | −0.02 | 0.02 |
| F CO2 norm (μg m−2 s−1) | 0.03 (1 d) | 0.28 | 0.44 (3 d) | 0.28 |
| Detrended | 0.19 (1 d) | 0.17 | 0.05 (2 d) | 0.11 |
| Growth rate (mm day−1) | NA | 0.36 (7 d) | 0.82 (10 d) | 0.56 |
| Detrended | NA | 0.28 (7 d) | 0.19 (6 d) | 0.24 |
| Methanol E0 | ||||
| RH (%) | −0.01 (1 d) | 0.59 (6 d) | 0.66 | 0.22 |
| Detrended | 0.20 (1 d) | 0.30 (4 d) | 0.60 | 0.38 |
| SWC (m3 m−3) | −0.55 | 0.45 (4 d) | −0.03 | −0.13 |
| Detrended | 0.11 (3 d) | 0.38 (3 d) | 0.32 (5 d) | 0.27 |
| Dxylem (μm) | −0.73 | 0.51 (4 d) | −0.24 | −0.27 |
| Detrended | 0.06 (1 d) | 0.42 (4 d) | 0.54 | 0.35 |
| Tr (mg m−2 s−1) | 0.29 (8 d) | −0.01 | 0.62 (4 d) | 0.22 |
| Detrended | 0.28 (8 d) | 0.13 | 0.56 (4 d) | 0.27 |
| F CO2 norm (μg m−2 s−1) | 0.52 (9 d) | 0.08 | 0.11 (3 d) | 0.22 |
| Detrended | 0.25 (8 d) | 0.08 (10 d) | 0.50 (3 d) | 0.34 |
| Growth rate (mm day−1) | NA | 0.67 (7 d) | −0.52 | 0.18 |
| Detrended | NA | 0.66 (7 d) | 0.30 (7 d) | 0.49 |
| Acetaldehyde E 0 | ||||
| RH (%) | 0.27 (2 d) | 0.26 (4 d) | 0.36 (7 d) | 0.28 |
| Detrended | 0.19 (2 d) | 0.22 (4 d) | 0.34 (7 d) | 0.23 |
| SWC (m3 m−3) | 0.58 (4 d) | 0.57 (2 d) | 0.04 (4 d) | 0.45 |
| Detrended | 0.56 (7 d) | 0.27 (4 d) | 0.56 (4 d) | 0.42 |
| Dxylem (μm) | 0.71 (4 d) | 0.45 (4 d) | −0.59 | 0.11 |
| Detrended | 0.42 (2 d) | 0.30 (4 d) | 0.36 (7 d) | 0.26 |
| Tr (mg m−2 s−1) | 0.13 (1 d) | 0.28 (1 d) | 0.44 (1 d) | 0.30 |
| Detrended | 0.28 (1 d) | 0.28 (1 d) | 0.34 (2 d) | 0.29 |
| F CO2 norm (μg m−2 s−1) | −0.35 | 0.65 (2 d) | 0.02 | 0.08 |
| Detrended | 0.11 (1 d) | 0.26 | 0.43 | 0.31 |
| Growth rate (mm day−1) | NA | 0.67 (7 d) | −0.52 | 0.05 |
| Detrended | NA | 0.62 (10 d) | 0.22 (8 d) | 0.40 |
- Note: When cross-correlation analysis indicated a significant, consistent lag in the emission response, the correlation coefficients are reported including the lag (the time lag in days in parentheses). Emissions were measured from three different trees during three summers, 2013, 2015 and 2017 at the SMEAR II station. Bold values are significant at p < .05. NA indicates missing data. RH: ambient relative humidity, SWC: soil water content mean over the A–C horizons, Dxylem: xylem diameter, proxy for water potential, Tr: transpiration, F CO2 norm: temperature-normalized stem CO2 efflux, growth rate: derivate of stem diameter over 10 days.
In addition to the correlation with SWC, the detrended monoterpene emission potential correlated positively with xylem diameter (proxy for water potential) with a lag of 1–2 days (Figure 4b, Table 2) and with relative humidity with a lag of 1–3 days (Table 2). Acetaldehyde emission potential correlated positively with transpiration, both with and without detrending, with a consistent lag of 1–2 days (Figure 4f, Table 2). Similarly to the monoterpene emission potential, acetaldehyde emission potential also correlated with xylem diameter and ambient relative humidity, but the time lags associated with the correlation varied between trees, ranging from 2 to 7 days (Table 2). The detrended methanol emission potential was also connected to xylem diameter and ambient relative humidity with lags of up to 4 days (Table 2). The correlations and lags involved in the correlations between the methanol emission potential and transpiration varied largely between the trees (Table 2).
We also found connections between the emission potentials of monoterpenes, methanol and acetaldehyde and metabolic activity of the stem. Cambial growth rate of the stem, measured in 2015 and 2017, positively related to the emission potential of monoterpenes and to the detrended emission potentials of methanol and acetaldehyde, with lags of 6–10 days (Figure 5a,c,e; Table 2). Positive correlations also existed between stem CO2 efflux and the monoterpene emission potential with a lag of up to 3 days (Figure 5b, Table 2) and between stem CO2 efflux and the detrended acetaldehyde emission potential with a lag of up to 1 day (Figure 5f, Table 2). The detrended methanol emission potential correlated with stem CO2 efflux with inconsistent lags of 3–10 days (Figure 5d, Table 2).
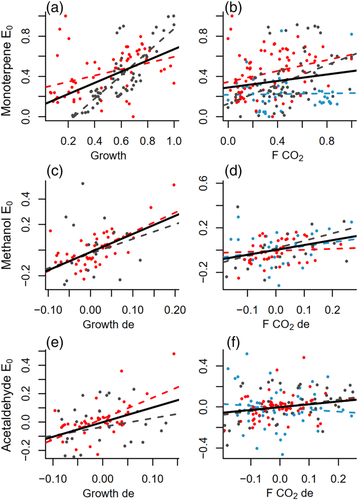
The main results of the correlation analysis were similar when using either the flexible or fixed β, both highlighting the correlations with soil water potential and growth (Table S4). The relations between monoterpene emission potential and transpiration, methanol emission potential and relative humidity and acetaldehyde emission potential and xylem diameter were clearer when using the fixed β, whereas using the flexible β generally provided similar and slightly stronger correlations over the trees regarding the other relations (Table 2, Table S4). This was clear especially in the correlations between emission potentials and stem CO2 efflux and growth (Table 2, Table S4).
3.3 Modelling VOC emissions
To determine the relative importance of the tree and soil water status and variables describing the stem metabolic activity in explaining the measured, non-normalized emissions of monoterpenes, methanol and acetaldehyde from the stem, we combined them with temperature using GLM. In addition to temperature, SWC, relative humidity and temperature-normalized stem CO2 efflux explained the monoterpene emissions; relative humidity, transpiration and temperature-normalized stem CO2 efflux explained the methanol emissions and SWC, relative humidity and transpiration explained the acetaldehyde emissions (Table 3). Xylem diameter could not be used in the monoterpene or acetaldehyde models because of its strong correlation with SWC. It was also omitted from the final methanol model despite its correlations with detrended methanol emissions because it did not have a significant effect. Although growth rate correlated with the emission potentials, the missing 2013 data prevented it from being used in the models.
| Removed variable or interaction | Reduced model | Full model | ||
|---|---|---|---|---|
| AIC | R2 | AIC | R2 | |
| Monoterpenes (all variables included) | 18,660 | 0.912 | 17,450 | 0.934 |
| Tcuv | 22,048 | 0.812 | 22,048 | 0.812 |
| SWC | 19,484 | 0.894 | 17,871 | 0.927 |
| RH | 18,765 | 0.910 | 17,608 | 0.931 |
| F CO2 | 19,104 | 0.902 | 17,558 | 0.932 |
| SWC, RH and F CO2 | 19,928 | 0.882 | 18,178 | 0.922 |
| Methanol (all variables included) | 5,104 | 0.894 | 3,912 | 0.945 |
| Tcuv | 5,914 | 0.837 | 5,914 | 0.837 |
| RH | 5,147 | 0.892 | 3,982 | 0.943 |
| Tr | 5,224 | 0.887 | 3,954 | 0.944 |
| F CO2 | 5,436 | 0.874 | 3,942 | 0.944 |
| RH, Tr and F CO2 | 5,537 | 0.866 | 4,097 | 0.939 |
| Acetaldehyde (all variables included) | −316 | 0.903 | −695 | 0.920 |
| Tcuv | 1,670 | 0.752 | 1,670 | 0.752 |
| SWC | 109 | 0.881 | −643 | 0.918 |
| RH | −281 | 0.901 | −687 | 0.920 |
| Tr | −191 | 0.896 | −655 | 0.918 |
| SWC, RH and Tr | 323 | 0.867 | −561 | 0.914 |
- Abbreviations: F CO2: temperature-normalized CO2 efflux from the stem, RH: relative humidity, SWC: soil water content mean over the A–C horizons, Tcuv: temperature inside the chamber, Tr: transpiration.
- Note: In the full model, a temperature interaction with 15-day periods is added; in the reduced model, all parameters have interaction only with year. The model coefficients are presented in Tables S4–S10. Full models as used in R GLM:
- EMmonoterpene = GLM(Emonoterpene ~ year + Tcuv + SWC + RH + F CO2 norm + Tcuv:year + SWC:year + RH:year + F CO2 norm: year).
- EMmethanol = GLM(Emethanol ~ year + Tcuv + RH + Tr + F CO2 norm + Tcuv:year:period + RH:year + Tr:year + F CO2 norm: year).
- EMacetaldehyde = GLM(Eacetaldehyde ~ year + Tcuv + SWC + RH + Tr + Tcuv:year:period + SWC:year + RH:year + Tr:year).
In all three models, temperature was clearly the most important variable explaining the emissions. Removing temperature caused the greatest increases in AIC and the greatest reductions in R2 in both the full models, where the temperature effect was allowed to change over the growing seasons using an interaction with 15-day periods, and in the reduced models, where the period interaction was removed (Table 3). Removing temperature decreased the R2 of the full monoterpene model from 0.93 to 0.81, the methanol model R2 from 0.95 to 0.84 and the acetaldehyde model R2 from 0.92 to 0.75. Removing the period interaction of the temperature effect had a smaller impact on the R2 of the full models (Table 3).
In all models, the variables other than temperature had very small effects on R2, but they were not negligible because removing them increased the model AIC (Table 3). The relative importance of these variables was smaller in the full models than in the reduced models, where the temperature effect was fixed. For example, removing all variables except temperature reduced the R2 of the monoterpene model from 0.93 to 0.92 in the full model and from 0.91 to 0.88 in the reduced model (Table 3). Comparing the effects of tree and soil water status and stem metabolic activity, we observed that SWC was most important in the monoterpene model, causing the greatest AIC increases when removed. In the acetaldehyde model, SWC was the most important in the reduced model, but equally important with transpiration in the full model (Table 3). In the methanol model, CO2 efflux was the most important in the reduced model, but the effects of all the variables other than temperature were equally miniscule in the full model (Table 3).
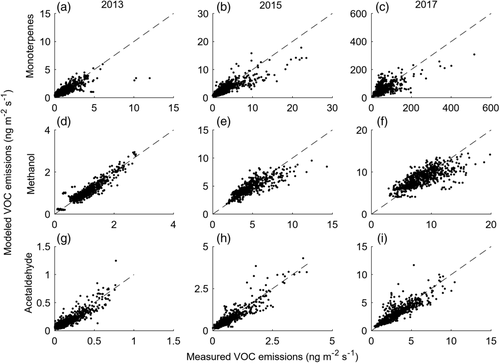
The full models generally captured the variation in the emissions of monoterpenes, methanol and acetaldehyde over the growing season (Figure 6, Table 3). However, in 2017, when emissions of monoterpenes were large, the monoterpene model did not capture the high emission peaks in mid-June in particular, but produced a patch of overestimations afterwards (Figure 6c, Figure S5c). This and other unexplained patterns in 2017 caused some residual autocorrelation that was not fixed by adding other explanatory variables or interactions.
4 DISCUSSION
4.1 Effect of temperature on stem VOC emissions
We found that emissions of monoterpenes, methanol and acetaldehyde from the three Scots pine stems over the three growing seasons followed the diurnal dynamics of temperature but varied over the growing season following changes in soil water potential and growth. Compared to earlier reports of monoterpene emissions from Scots pine stems: 0–30 ng m−2 s−1 (Rissanen et al., 2016) and 0–50 ng m−2 s−1 (Vanhatalo et al., 2020), the monoterpene emissions measured here were of the same magnitude in 2013 and 2015: 0–30 ng m−2 s−1 or higher in 2017: 0–600 ng m−2 s−1. Thus, the contribution of the stem monoterpene emissions to the ecosystem emissions was probably close to the 2% reported by Vanhatalo et al. (2020) or higher in 2017. Based on the resin composition of the measured pines (Rissanen et al., 2019) and earlier point measurements of monoterpene composition of the stem emissions (data not shown), we assume that the monoterpenes emitted from the stem mainly consist of α-pinene, Δ3-carene, myrcene and terpinolene.
The daily emission dynamics followed temperature, as reported previously (Rissanen et al., 2016; Staudt et al., 2019; Vanhatalo et al., 2015, 2020). The role of temperature was highlighted in the GLM: the other tested variables—SWC, relative humidity, transpiration and stem CO2 efflux—had small although not negligible effects on the full model fit. Based on the temperature relations fitted separately for each month of a growing season (Figure 3), it seemed probable that the temperature sensitivity of the emissions measured from the stem surface changed over the summer. Some of this apparent change in temperature sensitivity could be replaced by the other variables in the GLMs, but the full models allowing for flexibility in the temperature effect still explained the emission patterns better.
Even with the important role of temperature in explaining the emissions, temperature-normalized emission potentials of monoterpenes, methanol and acetaldehyde varied considerably over the growing season. These variations are potentially responses to the environmental or physiological variables that until now have remained little studied.
4.2 Effects of SWC and water potential on stem VOC emission potential
The detrended day-to-day variation in monoterpene emission potential was strongly related to changes in SWC and xylem diameter or relative humidity with a lag of 1–2 days. These relations correspond to results by Staudt et al. (2019), reporting highest emissions of α- and β-pinene from maritime pine (Pinus pinaster Aiton) stems during humid days. In contrast, Lusebrink et al. (2013) reported higher monoterpene emissions from the stems of drying lodgepole × jack pine (Pinus contorta Douglas ex Loudon × Pinus banksiana Lamb.) stems compared to well-watered controls. SWC surprisingly seemed to explain the monoterpene emission potential changes more directly compared to xylem diameter. This discrepancy between SWC and xylem diameter effects may be partly explained by the effects of osmotic regulation of living cells in the stems, which affects xylem diameter (Lintunen et al., 2017), at times deviating the diameter change signal from the water potential in the stem.
The positive relations between water availability, water potential, humidity and monoterpene emission potential may result from three overlapping effects. Firstly, changes in water availability change turgor pressures within the tree stem, which may enhance the diffusion of monoterpenes from unspecific, temporary storage pools that have been suggested to affect VOC emissions from leaves (Niinemets & Reichstein, 2002; Noe, Ciccioli, Brancaleoni, Loreto, & Niinemets, 2006). High water availability and water potential also increase the resin pressure in pines (see Helseth & Brown, 1970; Neher, 1993; Vité, 1961), although at a time scale of several days in boreal trees (Rissanen et al., 2019), which may increase the release of monoterpenes from the resin ducts (Rissanen et al., 2016). Secondly, high SWC and water potential in the stem probably support monoterpene production and emissions, as drought has been detected to reduce monoterpene emissions from leaves (Bertin & Staudt, 1996; Lüpke et al., 2017). Thirdly, as a more direct effect, changes in humidity and water availability may cause changes in bark conductance, for example, through wetting and swelling of bark tissues, as suggested by Staudt et al. (2019). Humidity may also change leaf cuticular conductance as suggested by Croteau (1977), Llusià and Peñuelas (1999) and Tingey et al. (1991). Interestingly, higher monoterpene emissions during high humidity have also been observed from freshly exuded resin (Pio & Valente, 1998).
Unlike monoterpenes, acetaldehyde and methanol are not stored in specific structures within the Scots pine stem, but as water-soluble compounds, they can be transported dissolved in the xylem sap (Fall, 2003; Folkers et al., 2008; Grabmer et al., 2006; Kreuzwieser et al., 2000). Large acetaldehyde emissions are often measured in flooded and anoxic conditions because acetaldehyde is oxidized from ethanol that is a product of anaerobic metabolism (Fall, 2003; Kreuzwieser et al., 2000, 2001; Kreuzwieser, Scheerer, & Rennenberg, 1999). Both acetaldehyde and methanol are also released during the decomposition of dead plant materials, which is enhanced by moist soil conditions (Warneke et al., 1999). Released methanol and acetaldehyde may be taken up into the roots and xylem sap with soil water, and transported to the stem, contributing to the measured stem emissions.
We found that even in non-flooded conditions, acetaldehyde emissions from stems follow soil moisture dynamics with a lag of 2–4 days. The time lag corresponded to the time of water transport from the base of the tree to the stem top, which was estimated based on the mean transpiration rate. This suggests that even a moderate increase in soil moisture triggered acetaldehyde production in the soil or roots and, with a lag caused by the transport in the xylem sap, increased acetaldehyde emissions from the stem. The increase in acetaldehyde production may be caused by local water logging of small parts of the rooting system. The consistent correlations between the acetaldehyde emission potential and the transpiration rate with an approximately one-day lag give support to this hypothesis. Up to 1.5 days of discrepancies between the estimated water transport times and the lags between SWC peaks and emissions probably result from the fact that both estimates integrate 3 months. Controlled experiments would be interesting for analysing the effect of transpiration rate changes on the time lag of emissions. Coherently, shorter lags between acetaldehyde emissions and soil moisture have been detected in flooding experiments with potted seedlings, for example, ethanol and acetaldehyde emissions increased approximately 24 hr after flooding (Copolovici & Niinemets, 2010; Holzinger et al., 2000; Kreuzwieser et al., 2000), although the largest emissions may occur later (Copolovici & Niinemets, 2010).
High water availability and high water content in the stem may increase the local acetaldehyde emission potential also because of slow oxygen diffusion (Sorz & Hietz, 2006) and subsequent anaerobic conditions and ethanol production (Kimmerer & Stringer, 1988). Indeed, we observed positive correlations between acetaldehyde emission potential and xylem diameter—a proxy for the water content and water potential in stem—with a lag of 2–7 days. However, because xylem diameter and SWC also correlated with each other, we could not separate the effects of transported and possibly locally produced acetaldehyde.
In addition to acetaldehyde emissions, we also observed peaks in the methanol emission potential 2–5 days after anomalies of high SWC, in agreement with methanol emission peaks detected in flooded conditions (Copolovici & Niinemets, 2010; Holzinger et al., 2000). This effect may be connected to increased methanol release from decaying plant material (Warneke et al., 1999) and methanol transport from the soil similarly as in the case of acetaldehyde. However, the relations between methanol emission potential and SWC or transpiration were not strong and varied between trees, suggesting that the transport of methanol plays a minor role in explaining methanol emissions, as reported by Folkers et al. (2008) for methanol leaf emissions.
4.3 Effects of growth and stem respiration on stem VOC emission potential
The role of growth in methanol emissions is well known at the leaf level, and demethylation of pectin during cell wall development processes is considered one of the main methanol sources in plant metabolism (Galbally & Kirstine, 2002; Hüve et al., 2007). We observed a clear effect of stem growth on the methanol emission potentials in 2015 and a weaker effect in 2017, both with a lag of approximately 1 week. This lag was probably connected to the offset between the measured signal of stem radial growth and cell wall development (Chan et al., 2016; Cuny et al., 2015; Cuny, Rathgeber, Frank, Fonti, & Fournier, 2014). Such a clear connection between methanol emission potential and stem growth in 2015 was surprising because several sources and sinks affect stem methanol emissions. Cell wall formation occurs in all growing tree parts, which means that methanol emissions measured in one stem compartment partially originate from local production and partially from methanol that may have been transported from all the lower tree parts or soil. Moreover, part of the locally produced methanol may enter the xylem sap and be transported away, and methanol may also be actively metabolized in tree tissues (Jardine et al., 2017). Thus, the clear effect of stem growth on the methanol emission potential suggests that local methanol production is more important in relation to methanol transport or metabolism.
The reasons for the lagged growth effect on monoterpene and acetaldehyde emission potentials are less clear. Part of this effect may be explained by their increased production during higher metabolic activity in the stem. Acetaldehyde emissions may be increased by rapid growth and high metabolic activity in the cambium because the intense oxygen use may lead to anaerobic conditions and ethanol production (Kimmerer & Stringer, 1988). Growth and subsequent formation of new resin ducts and production (Li, Wang, & Wu, 2009; Schmidt et al., 2010) may increase monoterpene leakage from the stem. Elongation of radial resin ducts occurs simultaneously with cambial growth, potentially contributing to the increased monoterpene emission potential during or slightly after the most rapid growth. However, whether monoterpene production in the stem is limited to the production of new resin remains unclear.
In conclusion, we found that emissions of monoterpenes, methanol and acetaldehyde are strongly controlled by temperature, but their temperature-normalized emission potentials are also affected by relatively small changes in SWC and increased following stem cambial growth. These results imply that in the short term, temperature is sufficient for explaining and modelling stem emissions, but large variations in soil moisture and phenology should be taken into account if aiming to quantify emissions over longer periods. To describe the emissions with more accuracy, it is essential to gain more process-based understanding of the emission dynamics and the time scales of the various effects.
ACKNOWLEDGMENTS
The authors wish to acknowledge the work of staff at the SMEAR II station for creating and seeing to the infrastructure and instruments and providing good quality data to work with. We wish to thank Juho Aalto, especially for his work with PTR-MS and the chamber system and his advice at various stages of this manuscript, and Pasi Kolari for making the calculations manageable. This study was funded by The Academy of Finland (Center of Excellence program grant 307331) and the University of Helsinki Research Foundation. Y.S. was also supported by the Academy of Finland, project numbers 312571 and 323843.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Kaisa Rissanen: research design, data analyses and interpretation, writing the manuscript; Anni Vanhatalo: research design and performance, commenting on the manuscript; Yann Salmon: data analyses, commenting on the manuscript; Jaana Bäck: research design research, data interpretation, commenting on the manuscript and Teemu Hölttä: research design, data interpretation, commenting on the manuscript.




