PHYTOCHROME-INTERACTING FACTOR 3 mediates light-dependent induction of tocopherol biosynthesis during tomato fruit ripening
Abstract
Tocopherols are important antioxidants exclusively produced in plastids that protect the photosynthetic apparatus from oxidative stress. These compounds with vitamin E activity are also essential dietary nutrients for humans. Although the tocopherol biosynthetic pathway has been elucidated, the mechanisms that regulate tocopherol production and accumulation remain elusive. Here, we investigated the regulatory mechanism underlying tocopherol biosynthesis during ripening in tomato fruits, which are an important source of vitamin E. Our results show that ripening under light conditions increases tocopherol fruit content in a phytochrome-dependent manner by the transcriptional regulation of biosynthetic genes. Moreover, we show that light-controlled expression of the GERANYLGERANYL DIPHOSPHATE REDUCTASE (SlGGDR) gene, responsible for the synthesis of the central tocopherol precursor phytyl diphosphate, is mediated by PHYTOCHROME-INTERACTING FACTOR 3 (SlPIF3). In the absence of light, SlPIF3 physically interacts with the promoter of SlGGDR, down-regulating its expression. By contrast, light activation of phytochromes prevents the interaction between SlPIF3 and the SlGGDR promoter, leading to transcriptional derepression and higher availability of the PDP precursor for tocopherol biosynthesis. The unraveled mechanism provides a new strategy to manipulate fruit metabolism towards improving tomato nutritional quality.
1 INTRODUCTION
Tomato, Solanum lycopersicum, is an important crop species in terms of both economic and nutraceutical value of its fruits (FAOSTAT, 2014; WHO, 2005). It is also a model for the study of fleshy-fruit development and ripening (Carrari & Fernie, 2006). During ripening, the differentiation of chloroplasts into chromoplasts is accompanied by metabolic changes that include degreening due to chlorophyll (Chl) catabolism, cell wall degradation leading to softening, and the accumulation of pigments, sugars, acids, and volatiles for disperser attraction. These biochemical processes not only result in organoleptic changes in flavour, texture, and colour that influence the consumption appeal but also determine the nutritional composition of the edible fruits (Gapper, McQuinn, & Giovannoni, 2013). Regarding nutraceutical compounds, besides the extensively studied carotenoids (Liu, Shao, Zhang, & Wang, 2015), tomato fruits are relevant source of tocopherols (Quadrana et al., 2013). Tocopherols are nonenzymatic lipid-soluble antioxidants exclusively synthesized in the plastids of photosynthetic organisms. They exist in four forms named α-, β-, γ-, and δ-tocopherol, differing in the number and position of the methyl radicals in the polar head (Figure 1). α-Tocopherol is especially important from the nutritional perspective as it displays the highest vitamin E (VTE) activity in mammals (DellaPenna & Pogson, 2006). Tocopherols prevent neuromuscular, neurodegenerative, and cardiac disorders by avoiding oxidative damage to human cells (Bellizzi, Franklin, Duthie, & James, 1994; Copp et al., 1999; Guggenheim, Ringel, Silverman, & Grabert, 1982; Ouahchi et al., 1995). Besides its nutritional value, tocopherols are essential protective substances in the chloroplasts, participating in the scavenging of reactive oxygen species and inhibition of lipid peroxidation (Krieger-Liszkay & Trebst, 2006; Miret & Munné-Bosch, 2015). As such, these compounds are an essential part of the photosynthetic machinery, affecting plant adaptability to light conditions and tomato fruit productivity (Almeida et al., 2016; Munné-Bosch, 2005; Spicher et al., 2017).
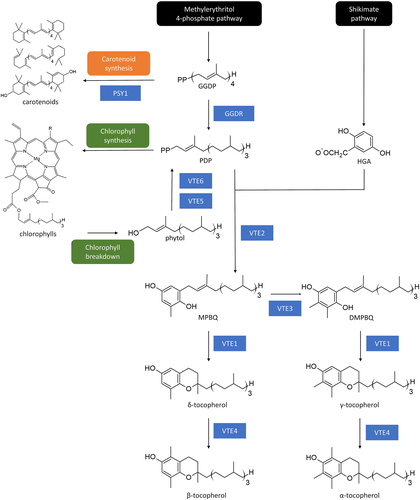
Tocopherols are formed by the condensation of two precursors: homogentisic acid, donor of a chromanol ring derived from the shikimate pathway, and phytyl diphosphate (PDP), a prenyl side chain originated from the methylerythritol-4-phosphate (MEP) pathway (Figure 1; Mène-Saffrané, 2018). The enzyme responsible for the first committed step is the HOMOGENTISATE PHYTYL TRANSFERASE, encoded by the VTE2 gene. Further, TOCOPHEROL CYCLASE (VTE1), DIMETHYL-PHYTYLQUINOL METHYL TRANSFERASE (VTE3), and γ-TOCOPHEROL C-METHYL TRANSFERASE (VTE4) are responsible for the balance between α, β, γ, and δ forms. Tocopherol biosynthesis is tightly linked to Chl and carotenoid metabolisms. The MEP intermediate geranylgeranyl diphosphate (GGDP) is the precursor for the production of carotenoids and PDP, which is further used for both, Chl and tocopherol biosynthesis (Figure 1). Conversion of GGDP into PDP is catalysed by the enzyme GERANYLGERANYL DIPHOSPHATE REDUCTASE (GGDR; Almeida et al., 2015; DellaPenna & Pogson, 2006; Quadrana et al., 2013). Additionally, besides the de novo synthesis from GGDP, PDP can be produced from the recycling of Chl degradation-derived phytol catalysed by PHYTOL KINASE (VTE5) and PHYTYL PHOSPHATE KINASE (VTE6; Ischebeck, Zbierzak, Kanwischer, & Dörmann, 2006; vom Dorp et al., 2015; Figure 1).
Tocopherol biosynthesis has been well described during tomato fruit development and ripening (Almeida et al., 2011, 2015). A network analysis based on a dedicated transcriptional and metabolite data showed that tocopherol biosynthesis is transcriptionally regulated, pinpointed the link between Chl and tocopherol metabolism, and revealed that the supply of the prenyl donor is limiting for VTE accumulation at later stages of fruit development (Quadrana et al., 2013). These hypotheses were further confirmed by metabolic and transcript profiling of fruits from Chl breakdown and ripening-impaired mutants (Almeida et al., 2015). During green stages, tomato GGDR (SlGGDR) produces de novo PDP for Chl biosynthesis and Chl turnover feeds tocopherol pathway via phytol recycling (Almeida et al., 2016). From the onset of ripening onwards, Chl synthesis ceases and the precursor GGDP is channeled towards carotenoid production by the transcriptional inhibition of the SlGGDR gene (Quadrana et al., 2013) and activation of the PHYTOENE SYNTHASE 1 (SlPSY1) gene, which encodes a fruit-specific enzyme diverting GGDP into the carotenoid pathway (Llorente et al., 2016). Chl degradation releases phytol, which is, in part, incorporated into tocopherol, leading to the increment of VTE content during ripening.
Although several advances have been achieved in characterizing VTE biosynthesis and accumulation pattern in tomato fruits, little is known about the molecular mechanisms directly regulating the enzyme-encoding genes. The network analysis mentioned above, including mRNA and metabolites quantification data in different organs and developmental stages, revealed a spatio-temporal coordination in the expression of some tocopherol biosynthetic genes. Moreover, the identification of common cis-regulatory elements in VTE biosynthetic pathway gene promoters suggested that these genes could be controlled by the same transcription factors (Quadrana et al., 2013).
Recently, WRINKLED1 (WRI1) was characterized in Arabidopsis thaliana as a protein that directly interacts with the promoter of the ACETYL COA CARBOXYLASE (ACC), the first committed step in plastidial fatty acid biosynthesis, inducing its transcription (Pellaud et al., 2018). Although wri1 mutant displayed conspicuous accumulation in total VTE content in seeds, this effect is not due to the direct regulation of VTE-related genes by WRI1 but caused by competition between VTE and lipid metabolisms for MEP pathway precursors (Mène-Saffrané, 2018; Pellaud et al., 2018). Hence, no transcription factors directly targeting the promoters of the tocopherol biosynthetic enzyme-encoding gene have been reported, so far.
Considering that light has a central role in regulating chloroplast activity and differentiation during tomato fruit ripening (Cocaliadis, Fernández-Muñoz, Pons, Orzaez, & Granell, 2014) and that tocopherols are photosynthesis-related compounds synthesized in the plastids, it is expected that light also affects the accumulation of fruit tocopherols, as already demonstrated for carotenoids (Llorente et al., 2016). The red/far-red light perception via PHYTOCHROMES (PHYs) plays a central role in controlling fruit development and ripening (Bianchetti et al., 2017, 2018; Gupta et al., 2014). Functional PHYs are homodimers with each polypeptide (apoprotein) associated with the linear tetrapyrrole chromophore phytochromobilin (PϕB). In the absence of light, PHYs are inactive in the cytoplasm, and upon red light exposure, an isomeric alteration of the PϕB leads to a rearrangement of the apoprotein structure that exposes the nuclear signalling domain, leading to PHY translocation into the nucleus (Bae & Choi, 2008). In the nucleus, PHYs promote the phosphorylation and further degradation of PHYTOCHROME-INTERACTING FACTORS (PIFs), negative regulators of light signalling. PHYs and other photoreceptors also promote the accumulation of positive regulators, such as ELONGATED HYPOCOTYL 5 (HY5), by down-regulating the negative effectors in light signal transduction pathway CONSTITUTIVE PHOTOMORPHOGENESIS 1 (COP1), DETIOLATED1 (DET1), DAMAGE DNA BINDING1 (DDB1), and CULLIN4 (CUL4; Lau & Deng, 2012; Leivar & Quail, 2011). DET1 silenced fruits displayed higher levels of tocopherol providing evidences of the effect of light signalling over VTE metabolism in tomato (Enfissi et al., 2010); however, no consistent up-regulation of the biosynthetic enzyme-encoding genes was observed. Thus, the increment in tocopherol can be explained as the consequence of the higher number of chloroplasts and Chl accumulation in green fruits, rather than a direct effect of light signalling disturbance.
To better understand the interplay between light and VTE accumulation during tomato fruit ripening, we investigated whether this environmental cue regulates tocopherol biosynthesis and the molecular mechanism underneath. Our results demonstrated that PHY-dependent light perception positively regulates tocopherol production during tomato fruit ripening. Moreover, light-dependent tocopherol accumulation during fruit ripening is mediated by the physical interaction of SlPIF3 transcription factor with the promoter region of SlGGDR, resulting in its transcriptional up-regulation and boosting the de novo biosynthesis of PDP precursor.
2 MATERIALS AND METHODS
2.1 Plant material, growth conditions, and sampling
S. lycopersicum (cv. MicroTom and Moneymaker), Nicotiana benthamiana, and Nicotiana tabacum plants were grown under standard greenhouse conditions (14-hr light at 27 ± 1°C and 10-hr dark at 22 ± 1°C). Seeds of aurea and hp2 mutants, in cv. MicroTom background, were donated by Dr Lázaro Eustáquio Pereira Peres (University of Sao Paulo, Brazil). Seeds of single and multiple phya, phyb1, and phyb2 mutants, in Moneymaker background, were provided by Dr Rameshwar Sharma (University of Hyderabad, India). In aurea, a mutation on PHYTOCHROMOBILIN SYNTHASE-encoding gene prevents the correct synthesis of the PHY chromophore PϕB, leading to a global deficiency in functional PHYs (Kendrick, Kerckhoffs, Tuinen, & Koornneef, 1997; Muramoto et al., 2005; Parks et al., 1987). The hp2 mutant is deficient for DET1 transcription factor, a negative regulator of light signal transduction (Soressi, 1975). phya, phyb1, phyb2, phyab1, phyb1b2, and phyab1b2 are loss-of-function mutants initially characterized by Kerckhoffs et al. (1996); Kerckhoffs, Schreuder, Van Tuinen, Koornneef, and Kendrick (1997); Kerckhoffs et al. (1999); Lazarova, Kerckhoffs, et al. (1998); Lazarova, Kubota, et al. (1998); and Weller, Schreuder, Smith, Koornneef, and Kendrick (2000).
For fruit ripening experiments, fruits at mature green (MG) stage were harvested about 30 days after anthesis (dpa) and were transferred to continuous white light (400 to 800 nm, approximately 50 μmol m−2 s−1) or maintained under absolute darkness until reaching distinct ripening stages in a temperature-controlled growth chamber maintained at 25 ± 2°C and air relative humidity at 80 ± 5%. Top and bottom illumination was applied to homogenize the light environment surrounding the fruits. Pericarp samples (without placenta and locule walls) were harvested at MG (displaying jelly placenta 2 days after harvesting), breaker (BR, 34 dpa displaying the first external yellow color signals), 1 day after BR (BR1), 3 days after BR (BR3), 6 days after BR (BR6), and 12 days after BR (BR12) stages.
2.2 Gene expression analysis
RNA extraction, cDNA synthesis, and quantitative polymerase chain reaction (qPCR) procedures were performed as described by Quadrana et al. (2013). The primers used for qPCR are listed in Table S1. All reactions were performed with two technical replicates and at least three biological replicates. Experiments were performed in a 7500 Real-Time PCR system (Applied Biosystem) using Power SYBR Green Master Mix (Thermo Fischer Scientific). Absolute fluorescence data were analysed with LinRegPCR software (Ruijter et al., 2009) to obtain Ct values and to calculate primer efficiency. Relative mRNA abundance was calculated and normalized with the ΔΔCt method using two reference genes as in Quadrana et al. (2013).
2.3 Tocopherol and Chl quantification
Tocopherols were extracted from approximately 25-mg dry weight as described by Lira et al. (2016). Chl extraction was carried out as described in Porra, Thompson, and Kriedemann (1989). A 1-ml aliquot of dimethylformamide was added to 200 mg of fresh weight fruit samples. After sonication for 5 min at 42 kHz and further centrifugation at 13,000 g for 5 min, the supernatant was collected. The procedure was repeated twice until total removal of tissue green colour and the supernatants were combined. Spectrophotometer measurements were performed at 664 and 647 nm. Chl a content was estimated as (12*Abs 664)-(3,11*Abs 647), whereas Chl b was calculated as (20,78*Abs 647)-(4,88*Abs 664).
2.4 Promoter analysis
Approximately 3 Kb fragments of the promoter sequences of the SlGGDR, SlVTE2, and SlVTE5 genes were retrieved from Sol Genomics Network (Fernandez-Pozo et al., 2015). To gain evidences about the eventual role of SlPIFs in the regulation of these genes, the presence of PIF-binding motifs was analysed using PlantPAN 2.0 platform (Chow et al., 2015).
2.5 Transactivation assay
Full-length cDNAs encoding SlPIF1a (Solyc09g063010), SlPIF1b (Solyc06g008030), SlPIF3 (Solyc06g008030), and SlRIN (Solyc05g012020) were amplified with the primers listed in Table S1. The fragments were cloned into pENTR/DTOPO vector using Gateway technology (Invitrogen). The entry plasmids were recombined into pK7WG2D (Karimi, Inzé, & Depicker, 2002) using LR Clonase (Invitrogen) to produce 35S::SlPIFs/SlRIN effector constructs. Fragments of 2,838 bp for SlPSY1 (Solyc03g031860), 3,123 bp for SlVTE2 (Solyc08g068570), and 2,648 bp for SlGGDR (Solyc01g067890) upstream the ATG starting codon were amplified from genomic DNA, using the primers listed in Table S1. The amplified fragments were digested with XhoI and BamHI restriction enzymes and cloned into the multicloning site of pGreenII 0800 LUC (Hellens et al., 2005) to produce the target constructs. All the constructs were sequenced and introduced into Agrobacterium tumefaciens (GV3101). For transient expression in N. tabacum leaves, A. tumefaciens cells carrying the different constructs were grown at 28°C for 48 hr in YEP medium (Sambrook, Fritsch, & Maniatis, 1989) with appropriate antibiotics. The cells were harvested, washed twice, and resuspended in infiltration buffer (50-mM MES pH 5.6, 2-mM sodium phosphate buffer pH 7, 0.5% glucose, and 200-μM acetosyringone).
Leaves of 4-week-old plants were coinfiltrated with a mix of equal volumes of effector and target cultures, both at a final OD600 of 0.05. After 3 days, Firefly Luciferase and Renillia Luciferase activity were assayed using the Dual-Luciferase Reporter Assay System (Promega) as described by Hellens et al. (2005), with slight modifications. Two leaf discs of 2 cm were harvested and grounded in 500 μl of Passive Lysis Buffer. Ten microlitre of this crude extract were assayed in 40 μl of Luciferase Assay Reagent and the chemiluminescence was measured. Then, 40 μl of Stop and Glow™ Reagent was added and a second chemiluminescence measurement was made. Absolute relative light units (RLU) were measured by Synergy H1 (Biotek) luminometer, with a 5-s delay and 15-s measurement. Data were collected as Luciferase/Renilla activity ratio and subsequently normalized relative to the control condition (leaves infiltrated with A. tumefaciens harbouring pK7WG2D empty vector).
2.6 Subcellular localization and chromatin immunoprecipitation assay
Full-length cDNA encoding SlPIF1a, SlPIF1b, SlPIF3, and SlHY5 without the stop codon were amplified with the primers listed in Table S1. The fragments were cloned into pENTR/DTOPO using Gateway technology (Invitrogen). The entry plasmids were recombined into pK7FWG2 (Karimi et al., 2002) using LR Clonase (Invitrogen) to produce 35S::SlPIF/SlHY5-GFP fusion proteins. The constructs obtained were introduced into A. tumefaciens (GV3101) for further subcellular localization assay and for chromatin immunoprecipitation (ChIP). For subcellular localization, 35S::SlPIF/SlHY5-GFP fusion proteins were agroinfiltrated in N. benthamiana leaves and 3 days after the infiltration the fluorescence was detected using a Leica TCS SP5 confocal laser-scanning microscope. Excitation filter of 450–490 nm was used for detection of GFP fluorescence.
ChIP assay followed by qPCR was performed as described in Ricardi, González, and Iusem (2010) with some modifications. Briefly, MG fruits were agroinfiltrated with 35S::SlPIF3-GFP construct, kept for 3 days under light or dark conditions, and fixed with formaldehyde to promote the cross linking between DNA and proteins. Following nuclei enrichment with a Percoll (GE Healthcare) gradient, the chromatin was fragmented by sonication (10 s on/20 s off, amplitude 70, during 10 min using QSonica700 device) and then incubated with Dynabeads Protein-A (Invitrogen) with either anti-GFP or anti-HA antibodies (Invitrogen). Next, the immunoprecipitated DNA was purified by phenol:chloroform:isoamyl alcohol extraction and used as template for qPCR analysis. Specific primer pairs flanking the predicted transcription factor binding motifs for each promoter region and the coding region of SlACTIN4 (Fujisawa, Nakano, & Ito, 2011) as normalizer (Table S1) were used.
2.7 Fruit transient overexpression
For transient SlPIF3 overexpression assay, MG fruits were agroinfiltrated (Orzaez, Mirabel, Wieland, & Granell, 2006) with the 35S::SlPIF3 construct used for transactivation experiments, and after 3 days in the dark, the gene expression of SlGGDR and SlPIF3 was addressed by qPCR as previously described using the primers listed in Table S1.
2.8 Data analyses
Differences in parameters were analysed in Infostat software version 17/06/2015 (Di Rienzo et al., 2011). When the data set showed homoscedasticity, t test (P < 0.05) was performed to compare genotypes or treatments. In the absence of homoscedasticity, a nonparametric comparison was performed by applying Mann–Whitney test (P < 0.05). All values represent the mean of at least three biological replicates.
3 RESULTS
3.1 Tocopherol accumulation is transcriptionally regulated by light during fruit ripening
To investigate whether light regulates VTE biosynthesis during fruit ripening, tocopherol levels were quantified in fruits from wild-type (WT) plants and two light-related mutants, the light-hyposensitive PϕB deficient aurea and the light-hypersensitive DET1 deficient high-pigment 2 (hp2). Fruits at the MG stage were detached from the plants and let to ripen under constant white light or darkness (Figure 2a). As previously reported (Almeida et al., 2015), the tocopherol levels increased during ripening in WT fruits. However, the accumulation was higher when the fruits were maintained under constant white light, indicating that this stimulus promotes tocopherol biosynthesis. Interestingly, this light-associated increase in tocopherol contents was not observed in the aurea mutant (Figure 2a), strongly suggesting a PHY-mediated effect. Consistently, fruits from the light hypersensitive mutant hp2 displayed a pronounced increment of total tocopherols when ripened in the light, reaching higher absolute levels than those observed from fruits ripened in the darkness (Table S2).
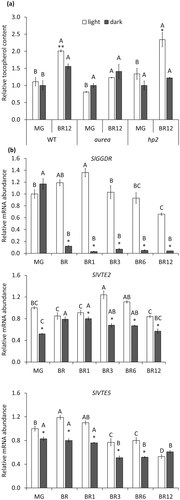
The transcript levels of the genes involved in VTE biosynthesis were next profiled in WT fruits along ripening under constant light or dark conditions (Figures 2b and S1). We observed that SlGGDR, SlVTE1, SlVTE2, SlVTE4, and SlVTE5 mRNA levels were lower when the fruits were incubated in the dark. Particularly interesting were the reductions in SlGGDR, SlVTE5, and SlVTE2 mRNA levels (Figure 2b). The two formers are responsible for PDP production either de novo (SlGGDR) or from Chl degradation (SlVTE5), whereas SlVTE2 encodes the enzyme responsible for the condensation of PDP and homogentisic acid in the first committed step of tocopherol biosynthesis (Figure 1).
Together, these results revealed that tocopherol accumulation in tomato fruits is induced by light, which might be explained, at least in part, by the transcriptional regulation of tocopherol biosynthetic genes.
3.2 PHY-mediated light signal transduction enhances tocopherol accumulation
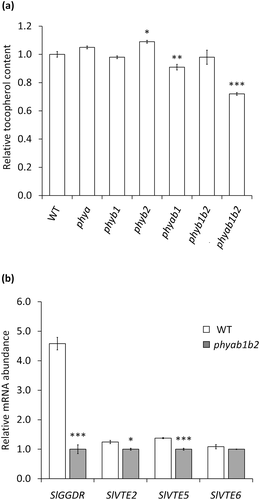
To test whether the observed positive effect of light on the regulation of fruit tocopherol content is mediated by PHYs, we analysed the tocopherol accumulation in fruits from WT and six loss-of-function PHY mutants (phya, phyb1, phyb2, phyab1, phyb1b2, and phyab1b2). Interestingly, only the double phyab1 and triple phyab1b2 mutants displayed a reduction in tocopherol content in ripe fruits (Figure 3a). These results reinforce our previous conclusion that light induces tocopherol accumulation during ripening in a PHY-mediated manner. They further reveal redundancy of individual phytochrome functions in the regulation of VTE biosynthesis. Quantification of tocopherol biosynthetic gene expression in WT and phyab1b2 mutant fruits showed reduced levels of SlGGDR, SlVTE2, and SlVTE5 (but not SlVTE6) transcripts in the PHY-defective triple mutant (Figure 3b), hence confirming that the PHY-dependent regulation of fruit tocopherol content is mediated by the transcriptional regulation of key tocopherol biosynthetic genes.
3.3 SlPIF3 represses SlGGDR transcription by physically interacting with its promoter
As PIFs are major transcription factors acting downstream of PHY-mediated light signalling, we investigated the eventual role of SlPIFs in the PHY-dependent regulation of tocopherol accumulation in tomato fruits. SlPIF loci were previously identified and transcriptionally characterized, being SlPIF1a, SlPIF1b, and SlPIF3 the most abundantly expressed in fruits (Rosado et al., 2016). Thus, we addressed whether the light treatment (Figure 2a) or the PHY deficiency (Figure 3a) affected the expression of these SlPIFs. Interestingly, these genes were found to be up-regulated in WT fruits maintained in darkness (Figure S2a), as well as in the triple mutant phyab1b2 fruits (Figure S2b), reinforcing their candidature as mediators of the PHY-dependent regulation of tocopherol accumulation. As an additional step in their functional characterization, we aimed to confirm their expected nuclear localization. In order to do so, the coding region of these genes was fused to the Green Fluorescence Protein (GFP) and transiently expressed in N. benthamiana leaves. As reported for A. thaliana PIFs (Leivar & Monte, 2014), SlPIF1a, SlPIF1b, and SlPIF3 proteins localized as nuclear speckles (Figure 4a). Such nuclear speckles, also referred to as photobodies, have been associated to the interaction of PHYs with photolabile PIFs for their phosphorylation and further degradation of these transcription factors (Al-Sady, Ni, Kircher, Schäfer, & Quail, 2006). Indeed, a GFP-tagged version of SlHY5, a transcription factor that does not directly interact with PHYs, showed a homogeneous distribution in the nucleus (Figure S3). Together, these results strongly support that, as demonstrated for SlPIF1a (Llorente et al., 2016), SlPIF1b and SlPIF3 are true PIFs, that is, PHY-interacting factors.
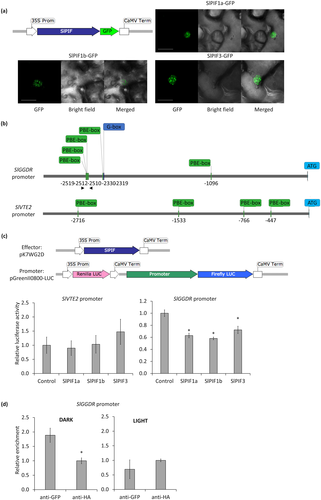
Being demonstrated that PHY-mediated light perception affected SlPIF expression in fruits and that SlPIFs localized in speckles, which is indicative of their light-induced PHY-mediated degradation, we investigated whether SlPIFs are responsible for the light-regulated expression of tocopherol biosynthetic genes. A de novo search for putative PIF-binding motifs in 3,000 bp fragments upstream the ATG starting codon of SlGGDR, SlVTE2, and SlVTE5 retrieved from the Heinz reference tomato genome (Sol Genomics Network, Fernandez-Pozo et al., 2015) revealed the presence of G-boxes and PBE-boxes (Chen et al., 2013; Song et al., 2014; Toledo-Ortiz et al., 2014; Zhang et al., 2013) in the promoter regions of SlGGDR and SlVTE2 (data not shown). To test the functionality of these putative PIF-binding motifs, transient transactivation assays were performed in N. tabacum (Figure 4). Promoter regions of 2,648 bp for SlGGDR and 3,123 bp for SlVTE2 were cloned from MicroTom genotype and sequenced (Figure S4). Polymorphisms were identified compared with the reference Heinz sequence, which do not alter the previously identified motifs on SlGGDR but reduce to 4 the number of PBE-boxes identified on SlVTE2 (Figure 4b). As positive control, the previously reported inductive effect of the transcription factor RIPENING INHIBITOR (SlRIN) on the SlPSY1 promoter (Martel, Vrebalov, Tafelmeyer, & Giovannoni, 2011) was also tested (Figure S5). The activity of the SlGGDR promoter was found to be down-regulated in the presence of SlPIF1a, SlPIF1b, and SlPIF3, whereas none of these SlPIFs affected the activity of the SlVTE2 promoter (Figure 4c).
The direct interaction between SlPIF3, the most highly expressed SlPIF gene in tomato fruits (Rosado et al., 2016), and the SlGGDR promoter was further addressed by chromatin immunoprecipitation followed by quantitative PCR (ChIP-qPCR). MG fruits were infiltrated with A tumefaciens harbouring a 35S::SlPIF3-GFP construct and maintained in constant dark or light conditions for 3 days. After chromatin purification, an enrichment in SlGGDR promoter sequences harbouring PBE boxes (Figure 4b) was observed in anti-GFP immunoprecipitated samples, that is, those containing SlPIF3-GFP, in comparison with anti-HA immunoprecipitated control in those fruits maintained in the darkness. This enrichment was not observed when the fruits were kept in the light, hence indicating that SlPIF3 physically interacts with the SlGGDR promoter preferably in the darkness (Figure 4d).
3.4 Transient SlPIF3 overexpression reduces the expression of SlGGDR
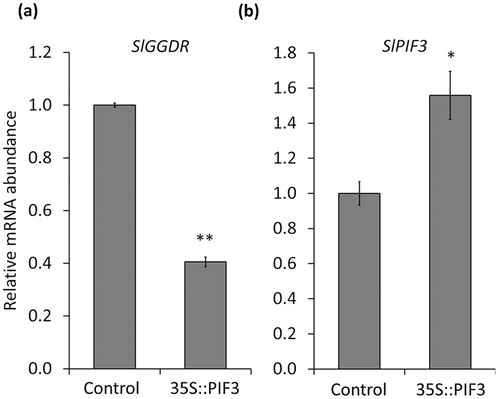
The fact that SlPIF3 is the most abundantly expressed SlPIF gene in MG tomato fruits (Rosado et al., 2016), together with the results obtained by transactivation and ChIP-qPCR assays, strongly pointed this gene as the most evident candidate for down-regulating SlGGDR expression in darkness. To test this hypothesis, we transiently overexpressed SlPIF3 in MG tomato fruits by agroinfiltration and analysed the impact of increasing SlPIF3 levels on the expression level of SlGGDR. A decrease in SlGGDR mRNA levels was verified (Figure 5a) that inversely correlated with the level of SlPIF3 transcripts (Figure 5b). Altogether, the data indicate that SlPIF3 mediates the PHY-dependent regulation of VTE biosynthesis via the transcriptional inhibition of SlGGDR expression in tomato fruit.
4 DISCUSSION
Manipulation of tomato genes involved in light signalling has been shown to impact the nutritional quality of tomato fruits (Azari et al., 2010; Liu et al., 2004; Llorente, Martinez-Garcia, Stange, & Rodriguez-Concepcion, 2017). Down-regulation of the photomorphogenic transcription factor SlHY5 resulted in thylakoid deficient chloroplasts with larger plastoglobules at green stages and reduced carotenoid levels in ripe fruits (Liu et al., 2004). On the contrary, silencing of the negative regulators of light signalling SlDDB1 and SlCUL4 led to a significant increment in the number of plastids that resulted in enhanced carotenoid and flavonoid accumulation during ripening (Wang et al., 2008). In particular, a positive role of PHY-dependent light response cascade in fruit carotenogenesis has been demonstrated (Alba, Cordonnier-Pratt, & Pratt, 2000; Bianchetti et al., 2018; Llorente et al., 2016).
Compared with the well-studied effect of light on carotenoid accumulation in fruits, little is known about the role of this environmental stimulus on the regulation of tocopherol biosynthesis, another important family of antioxidant health-promoting compounds. To address this issue, here, we analysed tocopherol accumulation along ripening in fruits from the PHY chromophore deficient aurea, the light hyperresponsive hp2, single and multiple phya, phyb1, and phyb2 mutants, and corresponding control genotypes.
Tocopherol biosynthesis is highly dependent on Chl degradation-derived phytol (Almeida et al., 2016), and consequently, the rising of tocopherol content from MG to the ripe stage of fruit development correlates with the amount of Chl right before the onset of ripening. In agreement, the more sensitive to light the genotype is, the higher the level of Chl at the MG stage (Figure S6) and the higher the tocopherol accumulation in ripe fruits. Interestingly, our results showed that ripening under constant light conditions boosts (over 20%) tocopherol production in WT genotype from the last green stage of tomato fruit (i.e., MG stage) onwards, once there is no more Chl synthesis. This increment was not observed in the aurea mutant, indicating PHY-mediated modulation of tocopherol biosynthesis. The observed light effect on tocopherol accumulation can be explained by the light-triggered changes in the expression profile of the biosynthetic genes. Light up-regulated not only tocopherol core pathway genes but also SlGGDR and SlVTE5, which produce the limiting precursor PDP. The PHY-mediated induction of tocopherol biosynthesis was reinforced by the analysis of phyab1b2 triple mutant. Although the reduced levels of tocopherol could be, at least in part, due to the reduced amount of Chl (Weller et al., 2000), the expression profiles observed in ripe fruits from phyab1b2 mimicked those from WT fruits ripened in the darkness, thus demonstrating that this effect on gene expression is mediated by PHYs. In agreement with our results, regulation of tocopherol biosynthesis by light has been recently reported in vegetative tissues of A. thaliana. Leaves from plants maintained in the dark displayed lower levels of tocopherol than those from plants exposed to light, which correlated with the down-regulation of AtVTE1, AtVTE2, AtVTE3, and AtVTE4 genes (Tanaka et al., 2015).
Being demonstrated that PHY-mediated light perception controls tocopherol accumulation during fruit ripening, PIF proteins, hub players of light response (Leivar & Quail, 2011), appeared as the most likely transcription factors involved in this process. Indeed, the fruit most abundantly expressed SlPIFs showed higher levels of mRNA accumulation in response to darkness or PHY deficiency. Moreover, SlPIF localization as nuclear speckles is directly involved in their light-dependent degradation as demonstrated for AtPIF3 in A. thaliana (Al-Sady et al., 2006). The two PIF-binding motifs described so far, PBE- and G-boxes, are present in the promoter regions of photosynthesis-related genes. In A. thaliana, AtPIF1 directly binds the G-box motifs located in the promoters of the Chl and carotenoid biosynthetic genes PROTOCHLOROPHYLLIDE OXIDOREDUCTASE (AtPORC) and PHYTOENE SYNTHASE (AtPSY), inducing and inhibiting their transcription, respectively (Moon, Zhu, Shen, & Huq, 2008; Toledo-Ortiz, Huq, & Rodríguez-Concepción, 2010). Moreover, AtPIF4 and AtPIF5 interact with the G-box motifs of the senescence transcription factor ORESARA 1 (AtORE1) and Chl degrading enzyme-encoding genes, such as STAY GREEN 1 (AtSGR1) and NON-YELLOW COLORING 1 (AtNYC1), up-regulating them during dark-induced senescence (Sakuraba et al., 2014; Song et al., 2014; Zhang, Liu, Chen, He, & Bi, 2015). Regarding tomato, it was discovered recently that SlPIF1a modulates carotenoid biosynthesis during fruit development, by binding to the PBE-box motifs of SlPSY1 gene leading to its repression (Llorente et al., 2016). It was proposed that the presence of Chl in the chloroplasts of green (e.g., MG) fruits results in a “self-shading” effect that leads to PHY deactivation and subsequent accumulation of PIFs in the inner layers of the pericarp (Llorente et al., 2016). High SlPIF1a levels in MG fruit result in the repression of SlPSY1 expression. As Chl degrades during ripening, PHYs are activated degrading PIFs, which in turn release SlPSY1 gene expression and carotenoid biosynthesis.
Here, our data obtained from transactivation and ChIP assays, demonstrated the light-dependent direct interaction of SlPIF3 with a PBE-box rich region of the SlGGDR promoter leading to the down-regulation of its expression. Based on our results, we propose a model that describes PHY- and SlPIF3-mediated transcriptional control of SlGGDR that regulates the influx of the PDP precursor for the light-dependent production of VTE during fruit ripening (Figure 6). Although Chl degrades during ripening, the recycling of Chl breakdown derived phytol enhances the availability of PDP for tocopherol biosynthesis (Almeida et al., 2016), increasing tocopherol content in the ripe fruits, both in the darkness and under light conditions. However, when fruits ripen in the light, PHY-mediated SlPIF3 degradation and the consequent increment in SlGGDR expression provide an extra input of PDP, from the de novo synthesis, resulting in higher tocopherol levels.
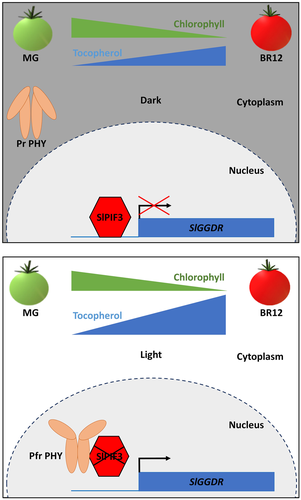
Our study, together with that from Llorente et al. (2016), reveals that the regulation of SlPIF levels in tomato fruits could serve as a mechanism to coordinate the production of carotenoids (provitamin A) and tocopherols (vitamin E). Manipulation of these underlying mechanisms in plants therefore appears as an effective strategy to increase fruit nutraceutical value, highlighting the importance of light signalling modulation as a biotechnological approach to improve functional properties in crops.
FUNDING INFORMATION
G. G. and D. R. were recipients of FAPESP fellowships, and M. R. was funded by a fellowship from CNPq. M. S. M. and B. L. were funded by Spanish Ministry of Economy and Competitiveness (MINECO) FPI (BES-2015-072725) and Juan de la Cierva (FPDI-2013-18882) fellowships, respectively. This work was partially supported by grants from FAPESP (2016/01128-9, Brazil), CNPq (Brazil), CAPES (Finance Code 001, Brazil), and USP (Brazil). M. R. C. was funded by grants from the MINECO (BIO2014-59092-P, BIO2015-71703-REDT, and BIO2017-84041-P) and Generalitat de Catalunya (2014SGR-1434 and 2017SGR-710). The financial support of the MINECO Severo Ochoa Programme for Centres of Excellence in R&D 2016–2019 (SEV-2015-0533) and Generalitat de Catalunya CERCA Programme to CRAG is also acknowledged.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.




