Molecular mechanisms and ecological function of far-red light signalling
Abstract
Land plants possess the ability to sense and respond to far-red light (700–760 nm), which serves as an important environmental cue. Due to the nature of far-red light, it is not absorbed by chlorophyll and thus is enriched in canopy shade and will also penetrate deeper into soil than other visible wavelengths. Far-red light responses include regulation of seed germination, suppression of hypocotyl growth, induction of flowering and accumulation of anthocyanins, which depend on one member of the phytochrome photoreceptor family, phytochrome A (phyA). Here, we review the current understanding of the underlying molecular mechanisms of how plants sense far-red light through phyA and the physiological responses to this light quality. Light-activated phytochromes act on two primary pathways within the nucleus; suppression of the E3 ubiquitin ligase complex CUL4/DDB1COP1/SPA and inactivation of the PHYTOCHROME INTERACTING FACTOR (PIF) family of bHLH transcription factors. These pathways integrate with other signal transduction pathways, including phytohormones, for tissue and developmental stage specific responses. Unlike other phytochromes that mediate red-light responses, phyA is transported from the cytoplasm to the nucleus in far-red light by the shuttle proteins FAR-RED ELONGATED HYPOCOTYL 1 (FHY1) and FHY1-LIKE (FHL). However, additional mechanisms must exist that shift the action of phyA to far-red light; current hypotheses are discussed.
Introduction
Light is one of the most important factors affecting growth and development of plants. Importantly, plants utilize energy from light absorption to fuel photosynthesis, but they also gain information on the ambient environment by monitoring the spectral composition, intensity (fluence rate), direction and spatio-temporal patterns of light. As sessile organisms, plants depend on such information to adapt growth and development to the conditions prevailing at the place of germination and to respond to changes in their environment in an adequate manner. Plants possess several different photoreceptors to perceive light of specific wavelengths. ULTRAVIOLET B (UV-B) RESISTANCE 8 (UVR8) is sensitive to UV-B light; phototropins (phots), cryptochromes (crys) and members of the ZEITLUPE (ZTL) family are blue light (B) receptors, and phytochromes (phys) primarily respond to red (R) and far-red light (FR) (Li et al. 2011; Tilbrook et al. 2013; Jenkins 2014; Christie et al. 2015; Liu et al. 2016). A gene duplication event during early evolution of seed plants gave rise to the two major phytochrome clades present in today's monocots and dicots. Phytochrome A (phyA) and phyB are the most prominent members of these clades, but further gene duplication events in the two phytochrome clades resulted in additional phytochromes in many species, for example, phyC, phyD and phyE in Arabidopsis or phyC in rice (Mathews 2010; Li et al. 2015).
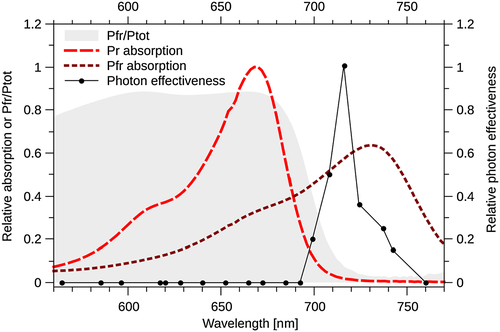
Phytochromes are holoproteins that contain a covalently bound tetrapyrrole chromophore (Li et al. 2011). Phytochromes are synthesized in their ground inactive Pr state, which absorbs maximally in R (Fig. 1). Upon absorption of light, phytochromes can reversibly convert between the inactive Pr and the biologically active Pfr state, which absorbs maximally in FR (Fig. 1). For land plants, where phytochromes bind the chromophore phytochromobilin (PФB), Pr and Pfr have absorption peaks of approximately 665 and 730 nm, respectively (Mancinelli 1994; Eichenberg et al. 2000). The Pr and Pfr absorption spectra overlap (Fig. 1); therefore, light simultaneously triggers both Pr → Pfr and Pfr → Pr photoconversion. The relative amount of active phytochrome (Pfr/Ptot) at equilibrium at a specific wavelength λ is therefore determined by the Pr → Pfr (k1, λ) and Pfr → Pr (k2, λ) photoconversion rates at the respective wavelength and given by Pfr/Ptot = k1, λ / (k1, λ + k2, λ). Additionally, Pfr can convert to Pr in a light-independent thermal relaxation process, referred to as dark reversion (Mancinelli 1994). Given that dark reversion is temperature dependent, it has been suggested that phytochromes could play a role in temperature control of physiological responses, which is supported by recent reports (Schäfer & Schmidt 1974; Hennig & Schäfer 2001; Franklin et al. 2014; Jung et al. 2016; Legris et al. 2016). Based on the Pr and Pfr absorption spectra, it can be calculated that the Pfr/Ptot level in R reaches 0.87 at equilibrium, while it drops to 0.02 or less in FR (Fig. 1) (Mancinelli 1994). The absorption spectra for phyA, phyB and other phytochromes in seed plants for which spectra have been measured are very similar; yet, the action spectrum, that is, the efficiency of photons of different wavelengths to induce a specific biological response, differs dramatically between phyA and phyB (Shinomura et al. 1996; Eichenberg et al. 2000; Klose et al. 2015). PhyB functions as the primary receptor for R, which establishes high Pfr/Ptot levels, whereas phyA has the highest biological activity under light conditions resulting in low Pfr/Ptot ratios, such as FR (Fig. 1) or low intensity light of any wavelength (Smith et al. 1997). The two phyA response types activated by these light conditions are termed high irradiance response (HIR) and very low fluence response (VLFR), respectively. It is well established that the differences between phyA and phyB are important in ecological terms, allowing plants to respond and adapt to highly diverse (light) environments. In this review, we focus on phyA and FR signalling. We discuss current hypotheses, how phyA signalling works at the molecular levels and why the phyA action spectrum differs so dramatically from that of other phytochromes. We also discuss physiological functions of phyA and its role in the ecological context. Far-red light regulated responses have also been described in the moss Physcomitrella patens, the liverwort Marchantia polymorpha and diatom algae, which all do not contain phyA (Possart & Hiltbrunner 2013; Fortunato et al. 2016; Inoue et al. 2016). Although FR sensing in Physcomitrella and Marchantia might rely on partially similar mechanisms as in seed plants, we focus in this review specifically on phyA-mediated FR responses in seed plants.
Far-Red Light Perception
Phytochromes translocate from the cytosol into the nucleus in response to light conditions that trigger physiological responses (Kircher et al. 1999; Yamaguchi et al. 1999; Gil et al. 2000; Hisada et al. 2000; Kim et al. 2000). Phytochrome A induces physiological responses in strong continuous FR (HIRs) and in weak light of any wavelength in the visible spectrum (VLFRs). Phytochrome A is not detectable in the nucleus of dark-grown seedlings but accumulates in the nucleus within minutes following exposure of seedlings to light where it promotes changes in gene expression (Kircher et al. 1999, 2002; Kim et al. 2000; Hiltbrunner et al. 2005; Tepperman et al. 2006). Irradiation with high-intensity FR for several hours further promotes phyA nuclear accumulation. In the following, phyA nuclear transport and downstream signalling will be discussed.
The shuttle proteins FAR-RED ELONGATED HYPOCOTYL 1 and FHY1-LIKE mediate phytochrome A nuclear transport
FAR-RED ELONGATED HYPOCOTYL 1 (FHY1) and FHY1-LIKE (FHL) are plant-specific proteins that contain a nuclear localization signal sequence (NLS) and a nuclear export signal sequence (NES) near the N-terminus and a phyA-binding site at the very C-terminus (Zeidler et al. 2004; Zhou et al. 2005; Hiltbrunner et al. 2006). The NLS/NES and the phyA-binding site are linked by a spacer of roughly 100 to 150 amino acids, which shares only low similarity between FHY1 and FHL; in contrast, the NLS, the NES and the phyA binding site are highly conserved (Zhou et al. 2005; Genoud et al. 2008). FHY1 and FHL are functional homologs, and plants lacking FHY1 and FHL are almost completely insensitive to FR and strongly resemble mutants lacking functional phyA (Zhou et al. 2005; Hiltbrunner et al. 2006; Shen et al. 2009). The NLS and the phyA binding site of FHY1 are essential for proper function, and yeast two hybrid and in vitro Co-IP assays suggest that FHY1 and FHL preferentially bind to the active Pfr form of phyA (Hiltbrunner et al. 2005, 2006; Jaedicke et al. 2012). Moreover, fluorescence recovery after photobleaching (FRAP) and fluorescence loss in photobleaching (FLIP) assays support the idea that FHY1 cycles between the cytosol and the nucleus, suggesting that FHY1 and possibly also FHL bind to light-activated phyA in the cytosol and transport it into the nucleus using a protein shuttle mechanism (Fig. 2, [3]) (Hiltbrunner et al. 2006; Rösler et al. 2007; Genoud et al. 2008; Rausenberger et al. 2011). In the nucleus, FHY1 and FHL are thought to dissociate from phyA and re-cycle back into the cytosol. FHY1 and FHL are specifically required for phyA but not phyB nuclear transport (Hiltbrunner et al. 2006). However, some phyA-mediated responses appear not to depend on FHY1 and FHL. For instance, efficient phototropin-dependent hypocotyl growth orientation in blue light (B) requires phyA but is only slightly affected in the fhy1 fhl double mutant as discussed later in this review (Fig. 2, [1]) (Rösler et al. 2007; Kami et al. 2012).
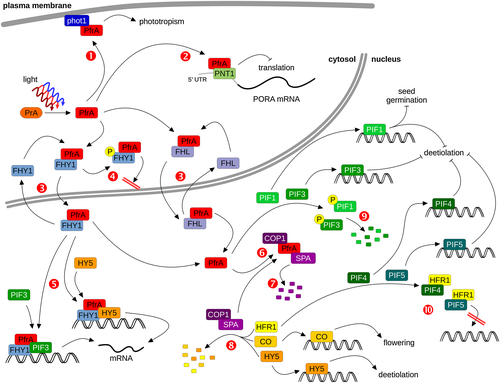
Interestingly, phyA nuclear transport is also abolished in seedlings lacking FAR-RED ELONGATED HYPOCOTYL 3 (FHY3) and FAR-RED IMPAIRED RESPONSE 1 (FAR1), two transposase-related transcription factors (Lin et al. 2007). FHY3 and FAR1 directly bind to the promoter and induce the expression of FHY1 and FHL. In FR, FHY3 and FAR1 transcript levels are down-regulated by phyA, suggesting that there is a negative feedback loop by which strong nuclear accumulation of phyA in FR represses further nuclear transport through inhibition of FHY1 and FHL expression (Desnos et al. 2001; Lin et al. 2007). Moreover, phyA also regulates FHY1 at the protein level. FHY1 is most abundant in etiolated seedlings and is rapidly degraded in light, a process which is partially dependent on phyA (Shen et al. 2005b). Interestingly, functional CONSTITUTIVELY PHOTOMORPHOGENIC 1 (COP1) is required for proper accumulation of FHY1, suggesting that repression of COP1 activity by light-activated phyA might contribute to reduced FHY1 levels in light-grown seedlings (Shen et al. 2005b). PhyA could also regulate the activity of FHY1 by phosphorylation; phosphorylation of FHY1 has been observed in R but not FR in a phyA-dependent manner, but the kinase involved has not yet been identified (Shen et al. 2009). Phosphorylated FHY1 is physiologically less active than non-phosphorylated FHY1, likely as a result of its inability to transport phyA into the nucleus (Fig. 2, [4]) (Chen et al. 2012). Both the FHY1/FHL-dependent nuclear transport of phyA and R induced phosphorylation of FHY1 may be important events that shift the biological activity of phyA to FR light.
Phytochrome A downstream signalling requires inactivation of COP1/SPA and PIFs
There are two major pathways present in the nucleus that link the activity of phytochromes to regulation of gene expression. Both pathways repress light signalling in darkness, but light-activated phyA acts in a tissue-autonomous manner to suppress these pathways and thereby induces light responses (Xu et al. 2015b; Kirchenbauer et al. 2016). COP1 and members of the SUPPRESSOR OF PHYA-105 (SPA) family are the key components of one pathway (Fig. 2, [6] to [8]), while the PHYTOCHROME INTERACTING FACTORs (PIFs), a subgroup of the bHLH transcription factor family, are central to the other pathway (Fig. 2, [9] and [10]). Consequently, seedlings with reduced COP1, SPA or PIF activity exhibit light responses in the dark (Deng et al. 1991; Laubinger et al. 2004; Leivar et al. 2008; Shin et al. 2009).
Light-activated phytochrome A inactivates COP1/SPA
There are four SPA proteins in Arabidopsis (SPA1–SPA4), which form oligomeric complexes with COP1, consisting of two COP1 and either two identical or different SPAs (Zhu et al. 2008). These COP1/SPA complexes form part of the CULLIN 4-DAMAGED DNA BINDING 1 ubiquitin E3 ligase complex (CUL4–DDB1COP1/SPA) and mediate substrate recognition (Lau & Deng 2012). Several positive regulators of light responses, including ELONGATED HYPOCOTYL 5 (HY5), LONG AFTER FAR-RED 1 (LAF1), LONG HYPOCOTYL IN FAR-RED 1 (HFR1), LIGHT-REGULATED ZINC FINGER PROTEIN 1/SALT TOLERANCE HOMOLOG 3/B-BOX DOMAIN PROTEIN 22 (LZF1/STH3/BBX22), PHY RAPIDLY REGULATED 1/2 (PAR1/PAR2) and CONSTANS (CO) are targeted for 26S proteasome-mediated degradation by CUL4–DDB1COP1/SPA (Fig. 2, [8]) (Osterlund et al. 2000; Seo et al. 2003; Duek et al. 2004; Jang et al. 2005, 2008; Yang et al. 2005b; Laubinger et al. 2006; Chang et al. 2011; Zhou et al. 2014). Light-activated phyA down-regulates the ubiquitin E3 ligase activity of CUL4–DDB1COP1/SPA and thereby triggers light responses through stabilization of these positive regulators (Lau & Deng 2012). Prolonged exposure to light promotes export of COP1 from the nucleus into the cytosol, which would lead to down-regulation of CUL4–DDB1COP1/SPA (von Arnim & Deng 1994; von Arnim et al. 1997; Osterlund & Deng 1998; Pacín et al. 2014). Recently, two alternative mechanisms have been described that lead to rapid light-induced inactivation of COP1/SPA. These mechanisms are not mutually exclusive and rely on light-regulation of SPA1/SPA2 turnover and binding to COP1 (Fig. 2, [6] and [7]) (Chen et al. 2015; Lu et al. 2015; Sheerin et al. 2015).
Upon light-induced Pr → Pfr conversion, phyA directly interacts with both SPA1 and SPA2, and inhibits their binding to COP1 (Fig. 2, [6]) (Sheerin et al. 2015). However, phyA also interacts with COP1, and therefore it has been proposed that light-induced binding of phyA to SPA1/SPA2 results in rearrangement of the protein–protein interactions within the COP1/SPA complex, rather than in its dissociation (Sheerin et al. 2015). COP1 mutant versions with reduced binding to SPA1 are poorly active, suggesting that direct interaction of COP1 and SPAs is important for the ubiquitin E3 ligase activity of CUL4–DDB1COP1/SPA (McNellis et al. 1994; Saijo et al. 2003; Zhu et al. 2008). Therefore, binding of light-activated phyA to SPA1/SPA2 is thought to inactivate CUL4–DDB1COP1/SPA and thereby stabilize positive regulators of phyA signalling that are targeted for degradation in dark-grown seedlings. Physiological assays confirm a role for SPA1 and SPA2 in phyA signalling, suggesting that the interaction with phyA is functionally relevant (Hoecker et al. 1998, 1999; Laubinger et al. 2004, 2006; Chen et al. 2015). It is interesting that also phyB, cryptochrome 1 (cry1) and cry2 interact with members of the SPA protein family and that phyB and cry1 also reduce binding of the respective SPAs to COP1 to regulate the activity of the CUL4–DDB1COP1/SPA complex (Lian et al. 2011; Liu et al. 2011; Zuo et al. 2011; Lu et al. 2015).
SPA2 is rapidly degraded, and to a lesser extent SPA1, in Arabidopsis seedlings exposed to light (Fig. 2, [7]) (Balcerowicz et al. 2011; Chen et al. 2015). This turnover of SPA2 in FR is dependent on phyA, but also occurs in weak R or B where phyA also has physiological functions, and in strong R which is dependent on both phyA and phyB (Chen et al. 2015). However, it is remarkable that cry1 and cry2, the primary receptors for B, are not involved in B regulation of SPA2 protein turnover (Chen et al. 2015; Liu et al. 2016). Thus, regulation of the CUL4–DDB1COP1/SPA ubiquitin E3 ligase activity through SPA2 destabilization is specific to phytochromes, while interference with binding of specific SPAs to COP1 is a mechanism shared by phyA, phyB and cry1 (Lian et al. 2011; Liu et al. 2011; Chen et al. 2015; Lu et al. 2015; Sheerin et al. 2015). Interestingly, the light-induced degradation of SPA2 is also dependent on COP1, but not other SPA proteins, indicating that CUL4–DDB1COP1 is active in light, but with altered substrate specificity compared to CUL4–DDB1COP1/SPA (Chen et al. 2015).
Mechanisms for phytochrome A-dependent inactivation of PIF proteins
In parallel to COP1 and SPAs, the phytochrome interacting bHLH transcription factors, PIFs, suppress light responses in the dark. Only a subset of the PIF family, PIF1 and PIF3, interact with phyA, and this is through an interaction motif distinct to the conserved active phyB binding motif (APB) (Khanna et al. 2004; Al-Sady et al. 2006; Shen et al. 2008; Leivar & Quail 2011). Phytochrome A promotes both the phosphorylation and degradation of PIF1 and PIF3 (Fig. 2, [9]), similar to the mechanism by which phyB regulates PIF1, PIF3, PIF4 and PIF5 (Bauer et al. 2004; Al-Sady et al. 2006; Shen et al. 2007, 2008; Lorrain et al. 2008). A recent report provides strong evidence that phyA itself has kinase activity and directly phosphorylates PIF1 and PIF3 (Shin et al. 2016). PIF4 and PIF5 do not physically interact with phyA and are stable in FR, which is in contrast to PIF1 and PIF3; thus, it is surprising that mutants lacking functional PIF4 and PIF5 are hypersensitive to FR and show altered FR-regulated gene expression (Al-Sady et al. 2006; Lorrain et al. 2008, 2009; Shen et al. 2008). However, in FR, HFR1 expression is up-regulated, and the HFR1 protein is stabilized by phyA-dependent repression of CUL4–DDB1COP1/SPA activity (Fairchild et al. 2000; Soh et al. 2000; Duek & Fankhauser 2003; Yang et al. 2005a, 2005b). HFR1 inhibits PIF4 and PIF5 by forming non-DNA binding HFR1/PIF heterodimers (Fig. 2, [10]) (Hornitschek et al. 2009). HFR1 also heterodimerizes with other PIFs and possibly affects their binding to target promoters (Bou-Torrent et al. 2015); for PIF1, such regulation through HFR1 has been demonstrated (Shi et al. 2013). Similar to HFR1, PAR1 also interacts with PIF4 and inhibits binding of PIF4 to target promoters (Hao et al. 2012).
COP1/SPA- and PIF-independent phytochrome A signalling mechanisms
Based on extensive phyA chromatin immunoprecipitation and RNA seq analyses, it has been suggested that phyA can directly regulate gene expression by associating, likely through bound transcription factors, with promoters of target genes (Chen et al. 2014b). Interestingly, FHY1 facilitates the association of phyA with HFR1 and LAF1, and phyA bound to FHY1 is recruited to the chalcone synthase promoter through PIF3 or HY5 (Fig. 2, [5]), giving rise to the idea that FHY1 not only mediates phyA nuclear transport but also plays a role in phyA downstream signalling (Yang et al. 2009; Chen et al. 2012). Yet, it is important to note that only 15% of all genes regulated by phyA are direct phyA target genes and, therefore, the PIF and COP1/SPA-dependent signalling pathways are possibly the predominant pathways downstream of phyA (Chen et al. 2014b). FHY1 can even associate with promoters that are not bound by phyA (Chen et al. 2014a). Therefore, FHY1 has been suggested to control the expression of these genes in FR in a phyA-independent manner; yet, which photoreceptor would be responsible for FHY1-dependent FR responses in the absence of phyA is unknown. It should also be noted that expression of phyA fused to an SV40 NLS in fhy1 background rescues the fhy1 mutant phenotype (Genoud et al. 2008). This would imply that FHY1 is not required for phyA downstream signalling though it is possible that FHL can substitute for FHY1 in fhy1. Further research is needed to clarify potential roles of FHY1 and FHL beyond phyA nuclear transport.
How to Shift the Phytochrome A Action Peak Towards Far-Red?
The spectral properties of phytochromes from different land plants are very similar with Pr and Pfr absorption peaks at 665 and 730 nm, respectively (Fig. 1) (Mancinelli 1994; Eichenberg et al. 2000). Given that Pfr is the biologically active form of phytochromes and that the Pfr/Ptot level is high in R but low in FR, one would expect that phytochromes are activated by R and inactivated by FR. Phytochrome B meets these expectations, and R/FR reversible activation/inactivation of physiological responses is even used as a diagnostic tool to demonstrate regulation by phyB. In contrast, phyA-mediated responses in the HIR mode are most efficiently induced by monochromatic FR with a wavelength between 716 and 720 nm (Fig. 1) or by multichromatic light that establishes the same Pfr/Ptot level as 716/720 nm light (i.e. Pfr/Ptot ≈ 0.03–0.05) (Hartmann 1966, 1967; Mancinelli 1994; Shinomura et al. 2000; Dieterle et al. 2005). Why a Pfr/Ptot ratio of ≈ 0.03–0.05 is much more efficient to trigger HIRs than light establishing higher Pfr/Ptot levels – or in other words, why phyA is an excellent sensor for high-irradiance FR but not R – is still not fully understood, but it appears likely that multiple mechanisms contribute to shifting the phyA action from R to FR (Rausenberger et al. 2011).
Multiple mechanism might shape the phytochrome A action spectrum
The current view is that there are several mechanisms that specifically suppress the physiological activity of phyA in R but not in FR, resulting in a R → FR shift of the action peak (Rausenberger et al. 2011). Although the spectral properties of phyA and phyB are very similar, there are a number of differences that might be important to explain this phenomenon. Nuclear accumulation of phyA is most efficient in continuous high-intensity FR and therefore is itself an HIR, suggesting that the mechanism by which phyA is transported into the nucleus contributes to the shift of the phyA action peak towards FR (Kim et al. 2000; Hiltbrunner et al. 2005). PhyA Y242H, a phyA mutant that is constitutively in a Pfr-like state, interacts with FHY1 and FHL in a light-independent manner (Su & Lagarias 2007; Rausenberger et al. 2011). Interestingly, phyA Y242H is poorly transported into the nucleus and inhibits nuclear transport of endogenous phyA, which appears counterintuitive. However, light-activated phyA reduces nucleocytoplasmic shuttling of FHY1, possibly by trapping it in phyA/FHY1 complexes (Rausenberger et al. 2011). Therefore, it has been suggested that constitutive binding of phyA Y242H to FHY1 and FHL decreases the pool of free FHY1/FHL available for phyA (Y242H) nuclear transport, and that Pfr → Pr conversion promotes dissociation of nuclear phyA-FHY1/FHL complexes and recycling of FHY1/FHL into the cytosol (Rausenberger et al. 2011). Binding of phyA to FHY1/FHL in the cytosol requires conversion of Pr to Pfr, which is most efficient in R, while dissociation of FHY1/FHL from phyA in the nucleus is enhanced by FR-induced Pfr → Pr conversion. Thus, the optimal wavelength for phyA nuclear transport is between 660 and 730 nm, which is consistent with action spectra for phyA HIRs having a peak at 716 to 720 nm (Fig. 1) (Hartmann 1967; Shinomura et al. 2000; Dieterle et al. 2001, 2005). A mathematical modelling approach confirmed that FHY1/FHL-mediated phyA nuclear transport can generate a shift of the phyA action peak from R towards FR and that the dissociation rate of phyA Pfr-FHY1/FHL complexes must be low so that dissociation of FHY1/FHL from phyA depends on phyA Pfr → Pr conversion. Moreover, phyA-dependent phosphorylation and inactivation of FHY1 in R but not FR could also be important for the R → FR shift of the phyA action peak (Shen et al. 2009; Chen et al. 2012). The mathematical model also predicted that additional mechanisms working in parallel to FHY1/FHL-dependent phyA nuclear transport are required to obtain an action spectrum corresponding to that of phyA measured in plants; yet, the molecular nature of these additional mechanisms is still unknown (Hartmann 1967; Shinomura et al. 2000; Dieterle et al. 2005; Rausenberger et al. 2011).
The mathematical modelling approach suggested that light-induced phyA degradation is critical to obtain the shift of the phyA action peak from R to FR (Rausenberger et al. 2011). Rapid light-dependent degradation is a key feature of phyA that distinguishes phyA from phytochromes having an action peak in R (Sharrock & Clack 2002). In this regard, the recently described phyA K206R mutant is of special interest (Rattanapisit et al. 2016). Light-induced degradation of phyA K206R is delayed compared to the wild type, and it will be interesting to measure detailed action spectra for phyA K206R expressing seedlings to verify the prediction that phyA degradation is critical to shape the phyA action spectrum.
Sequestered areas of phytochrome (SAPs) are cytosolic complexes that specifically contain phyA but not other phytochromes; they form under light conditions (e.g. R) that establish high Pfr/Ptot levels (Mackenzie et al. 1975; Saunders et al. 1983; Speth et al. 1986; Kircher et al. 1999). The mathematical model in Rausenberger et al. did not include SAPs, but given that they are phyA specific they might contribute to the R → FR shift of the phyA action peak (Rausenberger et al. 2011). This notion is supported by the phyA E229K mutant, which, compared to the wild type, shows reduced SAP formation and has an action peak extending to R (Dieterle et al. 2005).
Only few mutants with altered phyA action spectrum have been described. Most noticeable among them is the empfindlicher in dunkelrotem Licht 1 (eid1) mutant, which is strongly hypersensitive to light and has a phyA action peak in R instead of FR (Büche et al. 2000; Dieterle et al. 2001). EID1 is a nuclear localized F-box protein that possibly targets phyA signalling components for degradation. However, the targets of EID1 and the mechanism by which it shifts the phyA action spectrum towards FR are still unknown.
Can plants distinguish between phytochrome A PfrPfr homo- and PfrPr heterodimers?
Phytochromes form obligate dimers that can exist in three different states: PrPr, PfrPr and PfrPfr. For simplicity, this fact is omitted in many models, but recent work by Klose et al. has shown that the different properties of PfrPfr and PfrPr are important to reduce the activity of phyB at wavelengths above 680 nm (Klose et al. 2015). PfrPfr homodimers are abundant below 680 nm, while PfrPr heterodimers are the dominant Pfr containing phytochrome species above 690 nm (Mancinelli 1994). Thus, plants could efficiently shift the action peak of phyA towards FR if they were able to distinguish phyA PfrPfr from phyA PfrPr and selectively suppress the physiological activity of phyA PfrPfr. It is still unknown if plants can do this and, if yes, how they would do it. Future research will have to address these questions. Furthermore, it is crucial to develop (mathematical) models that do not neglect the fact that phyA is a dimer and that include the possibility that phyA PfrPfr and phyA PfrPr may behave differently regarding, nuclear transport, degradation, formation of nuclear bodies and recruitment into SAPs.
Phytochrome A-mediated Responses and Ecological Relevance of Phytochrome A
For many decades, phytochromes were only known as R/FR absorbing pigments in plants that had physiological effects on plant growth and development. Different phytochrome-mediated responses have been grouped into VLFRs, low fluence responses (LFRs) and HIRs, depending on the light conditions under which they can be observed. VLFRs are saturated at very low Pfr/Ptot ratios (typically below 10−3) that are established by low intensity R (≈10−4–10−1 μmol m−2) but also by light of other wavelengths, including FR (≈10−1–10 μmol m−2) and even green light (Botto et al. 1996; Shinomura et al. 1996; Casal et al. 1997, 1998b; Mazzella et al. 1997; Yanovsky et al. 1997). Given that VLFRs can be induced by FR, they are not R/FR reversible, but they obey the reciprocity law (Bunsen–Roscoe law), that is, the total fluence required to induce a response is independent of the duration over which the exposure occurs (Shinomura et al. 1996; Mazzella et al. 1997). Compared to VLFRs, LFRs require higher Pfr/Ptot ratios (≈0.1–0.87) that can be obtained in R (≈10 μmol m−2 or higher) but not in FR; thus LFRs are R/FR reversible (Botto et al. 1996; Shinomura et al. 1996; Casal et al. 1997, 1998b; Mazzella et al. 1997). LFRs like VLFRs, follow the reciprocity law (Shinomura et al. 1996; Mazzella et al. 1997). HIRs require a low Pfr/Ptot ratio (≈0.03–0.05) typically established by FR (Hartmann 1967; Shinomura et al. 2000). HIRs are not R/FR reversible and depend on the fluence rate (≈1 μmol m−2 s−1 or greater), that is, the reciprocity law does not apply except for very frequent light pulses (Mazzella et al. 1997; Casal et al. 1998b; Cerdán et al. 1999; Büche et al. 2000; Shinomura et al. 2000; Huq & Quail 2002; Laubinger et al. 2004). Analyses of phytochrome mutants of Arabidopsis and other species revealed that phyA is responsible for VLFRs and HIRs, while phyB mediates LFRs; other phys, such as phyE in Arabidopsis, also contribute to some LFRs (Whitelam et al. 1993; Weller et al. 1995, 1997; Botto et al. 1996; Shinomura et al. 1996, 2000; Mazzella et al. 1997; Smith et al. 1997; Takano et al. 2001; Hennig et al. 2002). It should be noted that differences between Arabidopsis and other species are possible; in rice, for instance, phyA mediates some LFRs and phyC contributes to FR responses (Takano et al. 2005).
Far-red light can affect development and growth of plants either by activating phyA or by inactivation of phyB. The response to canopy shade is repressed by active phyB and possibly the most prominent example of a response that is ‘induced’ in light with a low R:FR ratio through inactivation of phyB. However, phyB and phyB regulated responses are the subject of other review articles in this issue of Plant, Cell and Environment. In this section, we summarize responses that are mediated by phyA, discuss the underlying molecular mechanisms and highlight, why these responses are, or could be important for plants growing in their natural environment.
Phytochrome A regulates seed germination
Similar to seedlings and adult plants, seeds sense different environmental signals and germinate when these signals indicate favourable conditions for seedling establishment (Casal & Sánchez 1998). The light environment perceived by phytochromes plays an important role in regulation of seed germination (Strasser et al. 2010). Seeds of many species show biphasic responses to the Pfr/Ptot levels established under specific light conditions. The first phase is a VLFR and typically saturated at Pfr/Ptot levels below 10−3, while the second phase is a LFR that requires Pfr/Ptot levels higher than 0.01 (Botto et al. 1996; Casal et al. 1997, 1998b; Casal & Sánchez 1998).
Different pretreatments can increase the light sensitivity of seeds. For instance, seeds of Datura ferox kept in dry storage only germinated under LFR but not VLFR conditions (i.e. Pfr/Ptot > 0.02 was required); in contrast, the light sensitivity of D. ferox seeds buried in the soil for some months was four orders of magnitude higher and a Pfr/Ptot ratio of ≈ 10−4–10−6 was sufficient for induction of germination (Scopel et al. 1991). Burial in soil apparently established the competence to respond to light as a VLFR, and similar sensitization of seeds has also been described for many other species (Batlla & Benech-Arnold 2014). Seeds buried in the soil are protected from predation and desiccation, but emerging seedlings will not have immediate access to light to start photosynthesis. It has been hypothesized that light penetrating through soil is an important signal, indicating that seeds are close enough to the surface so that the emerging seedlings will reach the surface before the energy stored in the seeds is depleted (Ballaré et al. 1992; Batlla & Benech-Arnold 2014). In contrast, germination is inhibited if seeds are buried too deep. Seeds of some species can persist in the soil seed bank for several years before they germinate under more favourable conditions; this delay of germination not only increases the probability of successful establishment and completion of the life cycle but also allows plants to disperse their seeds through time (Long et al. 2015). Short exposure to light, as occurring during tillage operations for example, can induce germination of seeds in the seed bank, and it has been demonstrated that conducting soil cultivation during night can substantially reduce the emergence of weeds (Hartmann & Nezadal 1990; Ascard 1994; Scopel et al. 1994; Jensen 1995). Rough estimations predict that light exposure during soil cultivation is not sufficient to trigger LFRs and that few milliseconds of full sunlight promote germination; thus, phyA working in the VLFR mode is responsible for this response (Scopel et al. 1991; Botto et al. 1996). Interestingly, in addition to phyA, phyE is also required for FR-induced seed germination in Arabidopsis, while other responses to FR are independent of phyE (Hennig et al. 2002). It is worth mentioning that longer wavelengths, especially FR, penetrate deeper into soil than shorter wavelengths and that the type of soil, the particle size and moisture not only affect the total light that is transmitted but also the R:FR ratio (Bliss & Smith 1985). Thus, even below-ground, light can provide important information on the environment.
In many species, prolonged exposure to continuous FR inhibits seed germination (Hendricks et al. 1968; de Miguel et al. 2000; Shichijo et al. 2001; Górski et al. 2013; Batlla & Benech-Arnold 2014). In the ecological context, this may be important to prevent germination when under strong canopy shade, where emerging seedlings would have only very limited access to light for photosynthesis and the chance of successful completion of the life cycle would be low (Smith & Whitelam 1997). Experiments with D. ferox and tomato suggest that phyA working in the HIR mode perceives the high FR content characteristic of canopy shade (de Miguel et al. 2000; Shichijo et al. 2001). However, it is important to note that there are species in which seed germination is not inhibited under shade conditions (Górski et al. 2013). In Arabidopsis, for instance, monochromatic FR and light with a low R:FR ratio promote seed germination even under continuous irradiation with high fluence rates, suggesting that phyA enhances seed germination through a VLFR but does not inhibit it under HIR conditions (Johnson et al. 1994; Botto et al. 1996; Shinomura et al. 1996; Hennig et al. 2001, 2002; Yanovsky et al. 2002). Whether this response, which could allow for germination in strong canopy shade, provides a benefit to plants is currently unclear, but may be important for shade-tolerant species that have adapted photosynthesis to operate under these light conditions (Gommers et al. 2013).
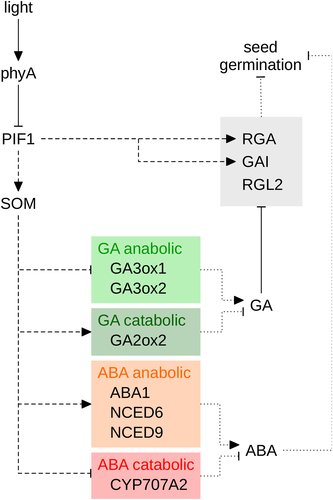
The phytohormones gibberellin (GA) and abscisic acid (ABA) play a pivotal role in regulation of seed germination. ABA is required for establishing and maintaining seed dormancy, while GA promotes breaking of seed dormancy and germination (Finch-Savage & Leubner-Metzger 2006). Light increases the levels of bioactive GA in germinating seeds by up-regulation of GA anabolic (GA3ox1 and GA3ox2) and down-regulation of catabolic genes (GA2ox2) (Fig. 3) (Yamaguchi et al. 1998; Oh et al. 2006; Seo et al. 2006, 2009). The DELLA proteins GA INSENSITIVE (GAI), REPRESSOR OF GA1 (RGA), RGA-LIKE 1 (RGL1) and, in particular, RGL2 inhibit seed germination; high GA levels induce the degradation of these DELLAs and thereby promote germination (Tyler et al. 2004; Cao et al. 2005; Seo et al. 2009). Interestingly, Arabidopsis mutants lacking functional PIF1 (= PIL5) germinate independent of light; this phenotype is specific for pif1, while mutants lacking other PIFs still require light for germination (Oh et al. 2004, 2006, 2007; Shin et al. 2009). Similar to pif1, mutants carrying defects in the CCCH-type zinc finger protein SOMNUS (SOM) cannot repress germination in the dark (Kim et al. 2008). In seeds, light-activated phyA and phyB target PIF1 for degradation by the proteasome, similar to light-regulated PIF1 turnover in seedlings (Shen et al. 2005a, 2008; Oh et al. 2006). PIF1 directly binds to the promoters of GAI and RGA and up-regulates their transcript levels, thereby inhibiting germination (Fig. 3) (Oh et al. 2007). In addition, PIF1 also inhibits seed germination by directly activating the transcription of SOM, which enhances the expression of GA catabolic (GA2ox2) and ABA anabolic genes (ABA1, NCED6 and NCED9), while expression of GA anabolic (GA3ox1 and GA3ox2) and ABA catabolic genes (CYP707A2) is repressed (Oh et al. 2007; Kim et al. 2008). Interestingly, the cop1 mutant which presents other light-induced responses in darkness, still requires light for germination, suggesting suppression of CUL4–DDB1COP1/SPA is not involved in germination induction (Deng et al. 1991; McNellis et al. 1994). To summarize, light destabilizes PIF1 and thereby controls the expression of key factors involved in germination, including SOM and DELLAs (Fig. 3).
Depending on when a FR pulse is applied, it has opposite effects on germination of Arabidopsis seeds. Early upon imbibition, a FR pulse cancels the effect of a preceding R pulse by reverting phyB to the inactive Pr form, suggesting that phyA cannot induce germination at this early time point (Shinomura et al. 1996). In contrast, when applied after 2 d of imbibition, a FR pulse promotes germination through phyA acting in the VLFR mode (Shinomura et al. 1996). This effect has been attributed to the observation that phyA is not present in Arabidopsis seeds imbibed for 3 h, while it accumulates after imbibition for 2 d (Shinomura et al. 1996). However, more recent work shows that phyA accumulates in seeds imbibed for 12 h but that a FR pulse given at this time still does not promote seed germination; thus, an alternative explanation has been proposed (Lee et al. 2012). Mature Arabidopsis seeds consist of the embryo, which is surrounded by an inner layer (endosperm) and an outer layer (testa) (Weitbrecht et al. 2011). During regulation of seed germination, phyB acts in the endosperm and phyA in the embryo. A FR pulse early upon imbibition inactivates phyB in the endosperm, resulting in stabilization of PIF1, up-regulation of ABA anabolic genes and thus high ABA concentrations (Lee et al. 2012). The same FR pulse activates phyA signalling in the embryo and would promote germination, but ABA originating from the endosperm and released towards the embryo overrides the positive effect of phyA and therefore prevents germination. However, the inhibitory effect of ABA produced in the endosperm decreases over time and a FR pulse given after 2 d of imbibition can induce germination through phyA acting in the embryo (Lee et al. 2012). Why the inhibitory effect of ABA decreases over time is not yet clear (Rodríguez-Gacio et al. 2009).
Phytochrome A regulates seedling photomorphogenesis
When germination of seeds is induced during soil cultivation, the emerging seedlings are initially growing in the dark beneath the soil surface. Strong elongation growth of the hypocotyl, closed cotyledons and an apical hook that protects the apical meristem from mechanical damage when the seedlings push towards the soil surface are key features of skotomorphogenesis, the developmental programme in dark-grown seedlings (Arsovski et al. 2012). Phytochromes and cryptochromes promote deetiolation, that is, the transition from skotomorphogenesis to photomorphogenesis, as soon as the seedlings reach the soil surface and become exposed to light (Li et al. 2011; Liu et al. 2016). Phytochrome A is required for deetiolation in dense canopy shade, where the R:FR ratio is very low, while in more open places, phyB and cry1 are the primary photoreceptors promoting the transition from skoto- to photomorphogenic development (Yanovsky et al. 1995; Sellaro et al. 2010). Hypocotyl growth is strongly reduced during photomorphogenesis, cotyledons open and expand, and the apical hook unfolds; in addition, chlorophyll is synthesized, and the photosynthetic apparatus is assembled, allowing seedlings to switch from heterotrophic to photoautotrophic growth (Arsovski et al. 2012). As discussed earlier, COP1, SPAs and PIFs are required to suppress photomorphogenic development in the absence of light (Fig. 2, [8] to [10]) (Deng et al. 1991; Laubinger et al. 2004; Leivar et al. 2008; Shin et al. 2009). Down-regulation of COP1/SPA and PIF activity in light results in massive changes at the transcriptome level, thereby promoting deetiolation. In the following, we will discuss different aspects of the deetiolation process regulated by phyA working in the VLFR and/or HIR mode and give a schematic overview on the underlying molecular mechanisms.
Phytochrome A inhibits hypocotyl growth in far-red light and strong canopy shade
Tissue-specific expression has shown that epidermis-localized phyA contributes to inhibition of hypocotyl growth in FR, but that phyA in other cell/tissue types is also required for normal regulation of hypocotyl growth (Kirchenbauer et al. 2016). Inhibition of hypocotyl growth in monochromatic FR shows two phases: a VLFR and an HIR. Hourly pulses of FR result in a slight reduction of hypocotyl elongation compared to dark-grown control seedlings and are considered a VLFR (Yanovsky et al. 1997, 2002; Casal et al. 1998a, 2000; Büche et al. 2000; Hennig et al. 2002; Luccioni et al. 2002). More frequent FR pulses only increase the response if the dark phase between FR pulses is shorter than 30 min or if continuous FR is applied, and even short dark phases considerably reduce the response compared to continuous FR, indicating a second HIR phase to the response (Yanovsky et al. 1997, 2002; Casal et al. 1998a, 2000; Büche et al. 2000; Hennig et al. 2002; Luccioni et al. 2002; Trupkin et al. 2007).
HY5, HFR1, LAF1, PIF1 and the redundantly acting PIF4 and PIF5 are key factors working downstream of phyA in regulation of hypocotyl growth (Fig. 4) (Koornneef et al. 1980; Fairchild et al. 2000; Fankhauser & Chory 2000; Soh et al. 2000; Ballesteros et al. 2001; Oh et al. 2004; Shin et al. 2007; Chang et al. 2008; Lorrain et al. 2009; Klose et al. 2012). HY5 transcript levels are strongly up-regulated in FR, and the HY5 protein is stabilized through light-dependent down-regulation of the ubiquitin E3-ligase activity of CUL4–DDB1COP1/SPA (Osterlund et al. 2000; Tepperman et al. 2006; Vandenbussche et al. 2007; Chang et al. 2008; Lorrain et al. 2009; Peschke & Kretsch 2011). Several genes coding for AUXIN/INDOL-3-ACETIC ACID proteins (AUX/IAAs) and AUXIN RESPONSE FACTORs (ARFs) are among the targets of HY5, suggesting that modulation of auxin signalling and responsiveness is part of the mechanism by which HY5 regulates hypocotyl growth (Fig. 4) (Cluis et al. 2004; Sibout et al. 2006; Lee et al. 2007; Lau & Deng 2010). A divergent ortholog of Arabidopsis HY5 also enhances the expression of the GA catabolic gene GA2ox2 in pea, promoting the inactivation of bioactive GA (Fig. 4); moreover, exposure to FR reduces the levels of bioactive GA in wild type but not in pea phyA mutant seedlings (Reid et al. 2002; Weller et al. 2009). In Arabidopsis, GA levels are tightly controlled by light, in particular through regulation of GA catabolic genes (GA2ox1, 2, 3 and 7) (Achard et al. 2007; Alabadí et al. 2008). Reduced levels of bioactive GA in GA biosynthesis mutants or in seedlings treated with an inhibitor of GA biosynthesis result in partial deetiolation and inhibition of hypocotyl elongation in dark-grown seedlings (Alabadí et al. 2004). The positive effect of GA on growth is mediated to a large extent through PIFs (Fig. 4) (Alabadí et al. 2008). High levels of GA in dark-grown seedlings destabilize DELLAs and thereby enhance the activity of PIF3, PIF4 and, potentially, PIF1 and PIF5 (de Lucas et al. 2008; Feng et al. 2008; Gallego-Bartolomé et al. 2010, 2011b). Gibberellin also promotes COP1-dependent degradation of HY5, although the molecular mechanism is still unknown (Alabadí et al. 2008). However, recent work suggests that PIFs may form the link between GA and COP1-dependent turnover of HY5 (Fig. 4). PIF1 forms a complex with COP1 and SPA1 and enhances targeting for degradation of HY5 by COP1/SPA; HY5 is therefore stabilized in pif1 (Xu et al. 2014). HY5 is progressively more abundant in pif double, triple and quadruple mutants, suggesting that also other PIFs have a positive effect on COP1/SPA-dependent HY5 turnover (Xu et al. 2014). Thus, hypothetically, binding of DELLAs to PIFs might reduce the positive effect of PIF1 (and potentially other PIFs) on COP1/SPA activity, which could explain how GA enhances COP1-dependent targeting for degradation of HY5.
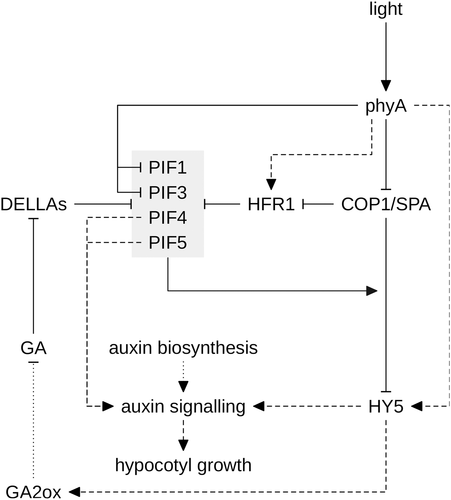
Similar to HY5, the expression of HFR1 is up-regulated by FR and repression of CUL4–DDB1COP1/SPA activity in FR also stabilizes the HFR1 protein (Fig. 4) (Fairchild et al. 2000; Soh et al. 2000; Sheerin et al. 2015). HFR1 promotes photomorphogenesis by reducing PIF4 and PIF5 activity through formation of non-DNA binding HFR1/PIF heterodimers (Hornitschek et al. 2009). In canopy shade, PIF4 and PIF5 promote hypocotyl growth by increasing the transcript levels of genes involved in auxin signalling; therefore, it appears likely that inhibition of PIF4 and PIF5 by HFR1 reduces hypocotyl elongation in FR (Lorrain et al. 2008, 2009; Hornitschek et al. 2009, 2012). It is worth mentioning that not only light and GA but also brassinosteroids and temperature promote hypocotyl growth through PIF4, which therefore plays a critical role in signal integration (Koini et al. 2009; Oh et al. 2012; Chaiwanon et al. 2016; Choi & Oh 2016).
Phytochrome A promotes apical hook unfolding and cotyledon opening
Opening of the apical hook and unfolding of the cotyledons must be tightly coordinated with the emergence of the seedling at the soil surface (Mazzella et al. 2014). Light and different hormones play a pivotal role in this process. Very low light intensities or hourly pulses of FR are sufficient to promote unfolding of the apical hook and partial cotyledon opening, suggesting that these responses are mediated by phyA working in the VLFR mode; however, full opening of the cotyledons in FR requires continuous irradiation, indicating that this is an HIR (Yanovsky et al. 1997; Casal et al. 2000; Hennig et al. 2002; Luccioni et al. 2002; Trupkin et al. 2007).
Unfolding of the apical hook depends on the interplay between light, auxin, GA, ethylene and brassinosteroids and has recently been reviewed (Fig. 5) (Mazzella et al. 2014). The apical hook is the result of asymmetric growth at opposite sides of the apical part of the hypocotyl. A local auxin maximum at the inner side of the hook inhibits growth and thereby contributes to the development of the apical hook. Although normal auxin biosynthesis is essential for hook development, the auxin gradient in the apical hook is not established by asymmetric synthesis but depends on auxin transport (Zhao et al. 2001; Stepanova et al. 2008; Vandenbussche et al. 2010; Zádníková et al. 2010). Ethylene enhances the expression of auxin carriers, including PINs, and thereby further increases auxin levels at the inner side of the apical hook (De Grauwe et al. 2005; Zádníková et al. 2010). PIF1, PIF3 and PIF5 also promote hook development in darkness, and overexpression of PIF5 enhances ethylene biosynthesis through up-regulation of several ACC SYNTHASE (ACS) genes (Fig. 5) (Wang et al. 2002; Khanna et al. 2007; Leivar et al. 2008; Gallego-Bartolomé et al. 2011b). One of the ACS genes, ACS8, is a direct PIF5 target, and it has been shown that GAI and potentially other DELLAs repress ACS8 through inhibition of PIF5 (Gallego-Bartolomé et al. 2011b; Willige et al. 2012). PIF5 also up-regulates the expression of WAVY ROOT GROWTH 2 (WAG2), which encodes a protein kinase that enhances auxin transport (Willige et al. 2012). Thus, light and GA signals are integrated at the level of PIF5, which regulates hook development through auxin and ethylene (Mazzella et al. 2014). Light also affects hook development through AUXIN RESPONSE FACTOR 2 (ARF2) and HOOKLESS 1 (HLS1), a negative regulator of ARF2 protein stability (Fig. 5) (Li et al. 2004). ARF2 is a repressor of auxin signalling and therefore inhibits hook development. HLS1 transcript levels are up-regulated by ethylene through ETHYLENE INSENSITIVE 3 (EIN3) and EIN3-LIKE 1 (EIL1), and the HLS1 protein is destabilized by light, resulting in high levels of ARF2 in light (Li et al. 2004; An et al. 2012). Similar to PIFs, also EIN3/EIL1 is inhibited by direct interaction with DELLAs, providing an additional route for GA to regulate hook development (An et al. 2012). Thus, the HLS1/ARF2 module integrates negative (light) and positive signals (GA, ethylene) during hook development (Fig. 5). Given that FR induces hook unfolding, it appears possible that phyA contributes to light regulation of HLS1/ARF2 although experimental investigation is required.
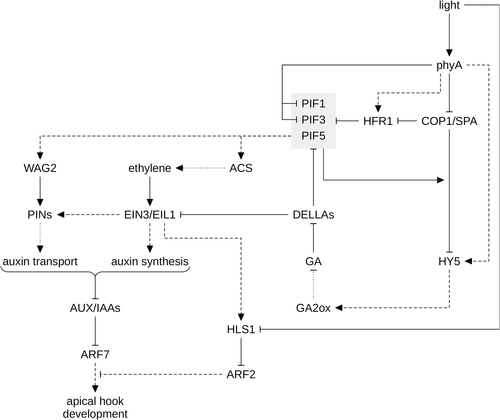
HY5 promotes hook opening, cotyledon unfolding and inhibition of hypocotyl growth in response to light, whereas brassinosteroids act in opposition, promoting etiolated growth (Liscum & Hangarter 1993; Chaiwanon et al. 2016; Choi & Oh 2016). In this regard, it is interesting that brassinosteroid-dependent repression of apical hook unfolding and cotyledon opening is impaired in the hy5 mutant, while the effect of brassinosteroids on hypocotyl elongation appears not to depend on functional HY5 (Li & He 2016). BRASSINAZOLE-RESISTANT 1 (BZR1) is a key transcription factor involved in brassinosteroid downstream signalling; in the absence of brassinosteroids, the kinase BRASSINOSTEROID-INSENSITIVE 2 (BIN2) phosphorylates and thereby inactivates BZR1 (Zhu et al. 2013). HY5 specifically interacts with the non-phosphorylated, active BZR1, which has been proposed to inhibit binding of BZR1 to target promoters; furthermore, HY5 destabilizes the BZR1 protein by an as yet unknown mechanism (Li & He 2016). Thus, stabilization of HY5 in light would repress the positive effects of brassinosteroids on hook development and cotyledon unfolding and thereby promote deetiolation.
Anthocyanin accumulation is regulated by phytochrome A
Anthocyanins are red and blue flavonoid pigments in plants (Chalker-Scott 1999; Gould 2004; Xu et al. 2015a). The accumulation of anthocyanins is strongly enhanced by FR and B; phyA is required for FR induction of anthocyanin biosynthesis and, together with cry1, also promotes synthesis of anthocyanins in B (Neff & Chory 1998; Fankhauser & Chory 2000; Soh et al. 2000; Duek & Fankhauser 2003; Vandenbussche et al. 2007; Lorrain et al. 2009; Gangappa et al. 2013; Shin et al. 2013). Anthocyanins have been implicated in tolerance to several different stress conditions, including defence against high light stress. Anthocyanins protect plants from oxidative damage by absorption of excess light and scavenging reactive oxygen species (Gould 2004). Continuous irradiation with high-intensity FR is most effective to induce anthocyanin biosynthesis, while light pulses are completely ineffective; thus, phyA-dependent anthocyanin biosynthesis is an HIR (Luccioni et al. 2002; Trupkin et al. 2007). HY5 plays a pivotal role in the transcriptional regulation of enzymes involved in flavonoid biosynthesis, and hy5 mutants accumulate greatly reduced anthocyanin levels (Fig. 6) (Shin et al. 2007, 2013; Chang et al. 2008). HY5 binds to the promoters of early flavonoid biosynthesis genes (CHS, CHI, F3H, F3′H), common to the biosynthetic pathway of all flavonoids, and late biosynthesis genes (DFR, LDOX), which are specific to anthocyanin biosynthesis (Lee et al. 2007; Shin et al. 2007; Das et al. 2012; Li 2014). Furthermore, HY5 also directly regulates the expression of LZF1/STH3/BBX22 and PRODUCTION OF ANTHOCYANIN PIGMENT 1 (PAP1)/MYB75, two positive regulators of anthocyanin biosynthesis genes; PAP1 expression is also up-regulated by LZF1 (Chang et al. 2008; Shin et al. 2013). Light regulation of many late biosynthesis genes requires the MBW (MYB/bHLH/WD40) complex, a ternary complex consisting of a MYB (PAP1 or PAP2) and a bHLH (TRANSPARENT TESTA 8 [TT8], GLABRA 3 [GL3], or ENHANCER OF GL3 [EGL3]) transcription factor and the WD40 protein TRANSPARENT TESTA GLABRA 1 (TTG1) (Fig. 6) (Li 2014). Except for TTG1, all components of the MBW complex are up-regulated by light at the transcript level (Cominelli et al. 2008). In addition, PAP1 and PAP2 interact with COP1 and several members of the SPA family, and are targeted for 26S proteasome-mediated degradation by CUL4–DDB1COP1/SPA (Maier et al. 2013). PIF4 and PIF5 do not contribute to regulation of anthocyanin biosynthesis in FR, but PIF3 binds to the promoters of CHS, CHI, F3H, F3′H, DFR and LDOX and enhances anthocyanin biosynthesis in FR in a HY5-dependent manner (Shin et al. 2007; Lorrain et al. 2009). However, it should also be noted that the pif3 mutant accumulates normal levels of anthocyanin, and the effect of PIF3 on anthocyanin biosynthesis can only be observed in PIF3 overexpressing seedlings (Shin et al. 2007). It is interesting that both HY5 and PIF3 promote anthocyanin biosynthesis in FR, whereas they have an opposite effect on many other light-regulated responses (Shin et al. 2007). HFR1 also enhances anthocyanin biosynthesis in FR, although the molecular mechanism is unclear; given that PIF3 is a positive regulator of anthocyanin biosynthesis, HFR1 appears not to work through repression of PIF activity (Fankhauser & Chory 2000; Shin et al. 2007). Several factors involved in up-regulation of anthocyanin biosynthesis, including HY5, HFR1, PAP1, PAP2 and LZF1, are stabilized in cop1 and higher order spa mutants, and these mutants even accumulate anthocyanin in darkness. Therefore, FR enhancement of anthocyanin accumulation could be mediated by phyA suppression of CUL4–DDB1COP1/SPA and thus stabilization of its targets (Deng et al. 1991; McNellis et al. 1994; Osterlund et al. 2000; Laubinger et al. 2004; Yang et al. 2005a, 2005b; Yang & Wang 2006; Chang et al. 2011; Maier et al. 2013; Maier & Hoecker 2015; Ordoñez-Herrera et al. 2015).
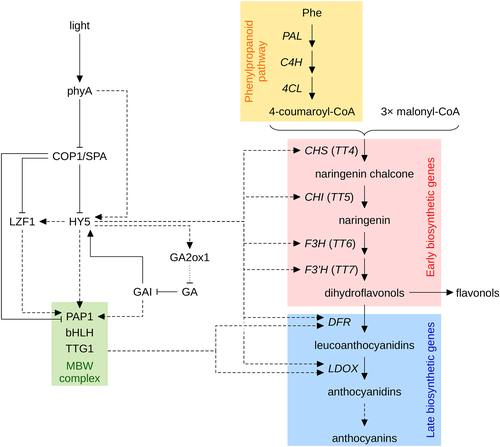
Dark reactions of photosynthesis are slower at low temperature, while light capture is temperature independent (Long et al. 1994; Gould 2004). Therefore, seedlings growing at low temperature are particularly prone to photooxidative damage caused by absorption of excess light. Expression of HY5 is increased at low temperature, and the HY5 protein is stabilized (Catalá et al. 2011; Zhang et al. 2011b). In addition, HY5 up-regulates GA2ox1 under low temperature conditions in a light-dependent manner, which leads to decreased levels of bioactive GA and stabilization of DELLAs (Fig. 6) (Zhang et al. 2011a). GIBBERELLIN INSENSITIVE (GAI) directly up-regulates PAP1 expression and, in addition, may further stabilize HY5 (Alabadí et al. 2008; Gallego-Bartolomé et al. 2011a). Thus, HY5 also promotes anthocyanin biosynthesis in response to low temperatures to protect seedlings from photooxidative damage.
Down-regulation of PORA by phytochrome A inhibits greening upon growth in far-red light
Light induces the assembly of the photosynthetic apparatus, which requires tight coordination of chlorophyll biosynthesis and expression of proteins that bind chlorophyll and chlorophyll precursors (Apel 2001). This is important because free chlorophyll and many intermediates in the biosynthetic pathway of chlorophyll, such as protochlorophyllide, can trigger the production of reactive oxygen species when exposed to light. Reduction of protochlorophyllide to chlorophyllide, the immediate precursor of chlorophyll, is catalysed by protochlorophyllide oxidoreductase (POR) and requires light absorbed by protochlorophyllide (Runge et al. 1996; Björn 2015). This step cannot be completed in seedlings grown in the dark and is also very inefficient in FR, because protochlorophyllide primarily absorbs in the B and R, but not the FR range of the light spectrum (Björn 2015). Therefore, protochlorophyllide accumulates in dark- and FR-grown seedlings (Runge et al. 1996). One of the POR enzymes, PORA, is expressed at high levels in dark-grown Arabidopsis seedlings; binding of protochlorophyllide by PORA normally prevents the accumulation of free protochlorophyllide and leads to rapid accumulation of chlorophyll when dark-grown seedlings are exposed to white light (e.g. when seedlings growing in soil emerge at the soil surface). In contrast, PORA expression is repressed by phyA in seedlings grown in FR and cytosolic phyA Pfr also inhibits the translation of PORA mRNA through PENTA 1 (PNT1) (Fig. 2, [2]) (Barnes & Nishizawa 1996; Runge et al. 1996; Paik et al. 2012). As a consequence, high levels of free protochlorophyllide accumulate in FR and therefore FR-grown seedlings exposed to white light suffer from severe photooxidative damage and are unable to green (Barnes & Nishizawa 1996; Runge et al. 1996). This phenomenon has been called ‘FR block of greening’ (Barnes & Nishizawa 1996). The phyA mutant does not repress PORA expression and mRNA translation in FR and, therefore, accumulates chlorophyll in white light even after growth in FR (Barnes & Nishizawa 1996; Runge et al. 1996). Moreover, fhy1, fhy3 and pnt1 but not hy5 and hfr1 mutants are partially resistant to the FR block of greening, while lines overexpressing PNT1 are hypersensitive (Barnes & Nishizawa 1996; Soh et al. 2000; Paik et al. 2012). Etiolated pif1 mutant seedlings express reduced amounts of PORA, PORB and PORC, and accumulate higher levels of protochlorophyllide than the wild type. As a consequence, dark-grown pif1 seedlings suffer from photooxidative damage when exposed to white light (Huq et al. 2004; Moon et al. 2008; Zhong et al. 2009). Chlorophyll levels are slightly reduced in wild-type seedlings pretreated with hourly pulses of FR followed by irradiation with white light, and pretreatment with continuous FR results in further reduction of chlorophyll levels; thus, the FR block of greening is controlled by phyA working in the VLFR and HIR mode (Barnes & Nishizawa 1996; Luccioni et al. 2002; Trupkin et al. 2007).
Phytochrome A suppresses gravitropism and promotes phototropic growth
Phototropic growth allows plants to optimize light capture for photosynthesis. The blue light receptors phototropin 1 and 2 (phot1/2) are essential for phototropism in visible light, and UVR8 mediates phototropic growth in light supplemented with UV-B (Liscum & Briggs 1995; Huala et al. 1997; Sakai et al. 2001; Vandenbussche et al. 2014). Phytochromes are not essential for phototropism, but they enhance growth in the direction of light (Strasser et al. 2010). Phytochrome A is required for enhancement of phototropism by pretreatment with FR and B and also promotes phototropic growth in response to pre-irradiation with R (Parks et al. 1996; Janoudi et al. 1997; Stowe-Evans et al. 2001; Kirchenbauer et al. 2016; Sullivan et al. 2016). Tissue-specific expression has shown that this response is mediated by mesophyll-localized phyA (Kirchenbauer et al. 2016). The mechanism by which phyA promotes phototropism is still unclear but may include both cytosolic and nuclear signalling events. This is suggested by two studies that investigated phototropism in the fhy1 fhl double mutant, which is impaired in phyA nuclear transport, and in transgenic lines expressing constitutively nuclear localized phyA (Rösler et al. 2007; Kami et al. 2012). Phot1 localizes to the plasma membrane in the dark but is partially internalized upon activation by B (Sakamoto & Briggs 2002). Interestingly, pretreatment with R inhibits internalization during subsequent B irradiation in a phyA-dependent manner, and therefore it has been suggested that phyA might enhance phototropic growth by blocking the internalization of phot1 (Han et al. 2008; Sakai & Haga 2012). Split-YFP assays indicate that phyA interacts with phot1 at the plasma membrane, which appears to support this hypothesis (Jaedicke et al. 2012). However, transgenic plants expressing a phot1 version that is stably anchored to the plasma membrane do not provide evidence for a role of phot1 internalization in the attenuation of phot1 signalling, and therefore it is still unclear how cytosolic phyA promotes phototropism (Fankhauser & Christie 2015; Preuten et al. 2015). An alternative explanation for phyA-mediated enhancement of phototropism relies on light-induced phyA nuclear transport, which can also occur in R and B (Kircher et al. 1999, 2002; Kami et al. 2012). Thus, pretreatment with FR, R or B increases phyA levels in the nucleus, where phyA up-regulates several genes known to play a role in phototropic growth, including genes involved in auxin transport or signalling (Nagashima et al. 2008; Sakai & Haga 2012; Goyal et al. 2013; Hohm et al. 2013; Liscum et al. 2014). It is worth mentioning that pretreatment with R accelerates phototropic bending in response to unilateral B only in wild-type seedlings but not in seedlings expressing phyA-NLS (Kami et al. 2012). This might be because nuclear transport of phyA is light independent in seedlings expressing phyA fused to an NLS and would support the idea that pre-irradiation enhances phototropism by promoting phyA nuclear transport. Negative phototropism in roots also depends on phot1 and phyA and requires phyA-dependent expression of PHYTOCHROME KINASE SUBSTRATE 1 (PKS1) (Boccalandro et al. 2008).
The phyA-mediated enhancement of hypocotyl phototropism by R and B is also partially an indirect effect caused by the inhibition of negative gravitropic growth (Lariguet & Fankhauser 2004; Kiss et al. 2012). Phytochrome A working in the VLFR mode is required to inhibit negative gravitropism in FR and B, and in R it works together with phyB to mediate this response (Poppe et al. 1996; Hennig et al. 2002; Lariguet & Fankhauser 2004; Rösler et al. 2007). PIF1, PIF3, PIF4 and PIF5 promote negative gravitropic growth, while HFR1 reduces negative gravitropism of hypoctoyls, possibly through inhibition of PIFs (Fairchild et al. 2000; Soh et al. 2000; Oh et al. 2004; Lorrain et al. 2009). Phytochrome interacting factors are active in etiolated seedlings and function by inhibiting the conversion of gravity-sensing endodermal amyloplasts to other plastid types (Kim et al. 2011). Activation of phyA by FR, R or B inactivates PIFs, either by promoting their degradation or inhibiting their activity through stabilization of HFR1, leading to the loss of endodermal amyloplasts and thereby loss of gravity sensing (Duek et al. 2004; Al-Sady et al. 2006; Hornitschek et al. 2009; Kim et al. 2011; Sheerin et al. 2015).
Phytochrome A promotes induction of flowering
Light down-regulates the abundance of phyA at the transcript level and also destabilizes the protein, suggesting that phyA is most important during the dark to light transition; in contrast, other phytochromes, and among them primarily phyB, play a dominant role in light-grown seedlings and adult plants (Cantón & Quail 1999; Sharrock & Clack 2002). Nevertheless, phyA contributes to photoperiodic regulation of flowering, which requires CO and FLOWERING LOCUS T (FT) (Shim et al. 2017). In long-day plants, if day-length exceeds a critical threshold, CO accumulates to levels where it promotes induction of flowering by up-regulation of FT expression. The expression of CO is regulated by the circadian clock and increases in the afternoon (Suárez-López et al. 2001; Yanovsky & Kay 2002). However, the CO protein is targeted for proteasome-mediated degradation by CUL4–DDB1COP1/SPA in the dark, while it is stabilized in light by repression of COP1/SPA activity (Laubinger et al. 2006; Jang et al. 2008). Up-regulation of CO mRNA levels coincides with the light phase under long- but not under short-day conditions, allowing CO to accumulate in the afternoon of long days. White light supplemented with FR is much more effective in promoting flowering than white light alone, and it has been shown that this effect depends on phyA, and phyA is also required for induction of flowering when extending the day length with either monochromatic FR or white light supplemented with FR (Takimoto 1957; Friend et al. 1961; Vince et al. 1964; Johnson et al. 1994; Jackson & Thomas 1997; Weller et al. 1997, 2001; Mockler et al. 2003; Laubinger et al. 2006; Trupkin et al. 2007; Kirchenbauer et al. 2016). Light-activated phyA stabilizes CO by suppressing the ubiquitin E3 ligase activity of CUL4–DDB1COP1/SPA and thereby promotes FT expression and induction of flowering (Fig. 2, [8]) (Suárez-López et al. 2001; Yanovsky & Kay 2002; Valverde et al. 2004). Tissue-specific expression of phyA demonstrates that phyA in the vascular tissue is essential and sufficient for proper regulation of flowering, consistent with CO and FT expression being primarily limited to the vascular bundle (Kirchenbauer et al. 2016).
Additional physiological functions of phytochrome A
Several other phyA-influenced responses of plants to their environment that are not discussed in detail by this review include effects on the circadian clock, the shade avoidance response and root growth. Entrainment of the circadian clock to the daily light/dark cycles is critical for proper regulation of flowering and adaptation of plants to their environment. Phytochrome A contributes to entrainment of the circadian clock under low-light conditions and is required in FR (Somers et al. 1998). How light input to the circadian clock works at the molecular level is discussed in detail in another review article in this issue of Plant, Cell and Environment. Responses to canopy shade and competition by neighbouring plants primarily depend on phyB. However, it is interesting that phyA but not phyB is required to limit the loss of chlorophyll in plants that are only partially shaded (Brouwer et al. 2014). It has been proposed that phyA allows shaded leaves to adjust the photosynthetic machinery to very low light intensities to maintain a positive carbon balance (Brouwer et al. 2014). Shoot-derived phyA signals also play a role in regulation of root growth and, in tomato, phyA has been shown to increase the tolerance to high evaporative demand, suggesting that phyA is involved in several responses that one might not expect to be regulated by light (Salisbury et al. 2007; Warnasooriya & Montgomery 2011; Auge et al. 2012; Kirchenbauer et al. 2016). Thus, further responses traditionally not investigated in photobiology might be modulated by phyA.
Molecular differences between very low fluence response and high irradiance response
Although VLFRs and HIRs can be distinguished at the physiological level, it is less clear how they differ at the molecular level. In this regard, it is notable that the phyA-302 (phyA E777K), phyA-303 (phyA R384K) and phyA Δ6-12 (phyA lacking amino acids 6 to 12) mutants are defective in the HIR mode but retain an almost normal VLFR (Yanovsky et al. 2002; Mateos et al. 2006; Trupkin et al. 2007). Furthermore, the vlf1, vlf2, owl, gigantea (gi), pks1 and pks2 mutants have altered VLFRs but normal HIRs, while it is opposite for eid1 and bell-like homeodomain 1 (blh1) (Yanovsky et al. 1997; Büche et al. 2000; Dieterle et al. 2001; Zhou et al. 2002; Lariguet et al. 2003; Oliverio et al. 2007; Kneissl et al. 2009; Staneloni et al. 2009). The Lhcb1*2 promoter of tobacco can be induced by light treatments specific for either HIRs, LFRs or VLFRs (Cerdán et al. 1997, 2000). It is interesting that truncating the Lhcb1*2 promoter at the 5′ end only abolished the light regulation in the HIR but not in the LFR and VLFR mode (Cerdán et al. 2000). The truncated part of Lhcb1*2 promoter contains a nucleotide motif that is essential for binding of the Bell-like homeodomain transcription factor BHL1, which is required for HIRs but not VLFRs (Staneloni et al. 2009). Thus, it appears possible that HIRs and VLFRs employ partially different molecular mechanisms for regulation of gene expression. Given that differences between VLFRs and HIRs have been observed at the level of phyA itself as well as at the level of phyA downstream signalling components and target promoters, it is possible that specific residues in phyA regulate HIR- or VLFR-specific downstream signalling components that target specific regulatory elements in target promoters. Nevertheless, further research is required to support this hypothesis.
Final Remarks
Several factors not discussed in detail in this review also play a role in phyA-mediated responses. However, their involvement at the molecular level is not well understood, and they have therefore either not been discussed, or not in detail.
We also want to highlight that most of the work referred to in this review has been performed using Arabidopsis. Although basic light signalling components, such as phytochromes, COP1, HY5 and PIFs, are conserved between Arabidopsis and other species, differences in the light signalling network will exist. In particular, species that grow in different habitats and, therefore, are adapted to different ecological environments may respond to the same light conditions in a different manner. Thus, care should be taken when extrapolating from one species, such as Arabidopsis, to another.
Conclusions and Perspectives
The conclusion of the first publication reporting on the isolation of an Arabidopsis phyA mutant was that ‘… the function of PHYA might be highly specialized and restricted to certain phases of Arabidopsis development.’ (Nagatani et al. 1993). Research done over the next two and a half decades clearly supports this notion and provided insight into the physiological and ecological role of phyA as well as into molecular mechanisms underlying FR signalling. However, this has also taught us what we still do not know and what questions we have to address in the future to understand phyA in all its aspects. The foremost questions include ‘What is the molecular basis of the R → FR shift of the phyA action peak’, ‘How did far-red light signalling evolve?’ and ‘How does phyA interact with other signalling pathways to regulate the adaptation of growth and development to different environmental conditions?’.
Acknowledgment
This study was supported by the Excellence Initiative of the German Federal and State Governments (EXC 294, project C20) and grants from the German Research Foundation (HI 1369/5-1) and the Human Frontier Science Program (HFSP; RGP0025/2013) to A.H.




