Genetic Variation and QTL Analysis of Crude Fibre and Quality Traits in the Doubled Haploid Winter Oilseed Rape (Brassica napus L.) Population R19 x Lord
Funding: The authors gratefully acknowledge the funding provided by the Bundesministerium für Ernährung und Landwirtschaft (FNR 22036418).
ABSTRACT
The oil-extracted meal of oilseed rape is used for feeding livestock and is a potential source of vegetable protein for human consumption. Its protein quality is limited by glucosinolates, phenolic compounds, phytates and crude fibres. The reduction of cellulose, hemicellulose and indigestible lignin in black-seeded oilseed rape may enhance the protein content, colour, sensory quality and digestibility. The objective of this study was to analyse the inheritance of seed fibre components in relation to oil, protein and other quality traits in a DH population derived from the cross of low lignin lines R19 and cv. Lord. The population of 143 lines was tested in field experiments in six environments and was genotyped with Illumina 15K Brassica SNP chip. QTL and candidate genes were identified using the high-quality long-read reference genome of Express 617. Major QTL for fibre components were identified on chromosomes C01 and C03 with opposite additive and epistatic effects. Fibre QTL colocated with each other and QTL for seed colour, oil and protein. Epistatic effects were identified, which resulted in a further decrease of fibre content.
1 Introduction
Oilseed rape (Brassica napus L., AACC, 2n = 38) is an allotetraploid species of winter and spring genotypes that has a high economic importance. It is widely cultivated throughout the world as a source of oil and protein for food and feed purposes. It is the third largest source of vegetable oil (33.06 MMT) after oil palm (79.46 MMT) and soybean (61.93 MMT) and is the second largest source of protein meal (47.55 MMT) after soybean (258.63 MMT) (USDA 2023). With a rising world population and limited environmental resources, food security is a primary challenge for humankind (FAO 2009). The demand for sustainable and renewable food sources is increasing. Plant proteins and by-products from food processing, mostly used for animal feed, are novel protein sources for human consumption that can meet the required macronutrient intake (Sá, Moreno, and Carciofi 2019, 2020; Kowalski et al. 2020; Ismail et al. 2020). In expanding the available sources of plant-based proteins, oilseed rape protein has been suggested as a vegetable protein source (Jia et al. 2021; Wanasundara 2011; So and Duncan 2021; Kotecka-Majchrzak et al. 2020).
The oil-extracted meal consists mainly of the storage proteins cruciferin and napin, as well as of some structural proteins such as oil body proteins and lipid transfer proteins (Wanasundara et al. 2016). The protein has a high biological value and is known for its well-balanced amino acid profile with high quantities of indispensable amino acids, particularly sulphur-containing amino acids such as methionine and cysteine (Campbell, Rempel, and Wanasundara 2016; Bos et al. 2007). For cruciferin and napin, much genetic variation and a link between protein composition and glucosinolate (GSL) content was found, with higher cruciferin to napin ratios for genotypes having low GSL contents (Malabat et al. 2003; Schatzki et al. 2014; Stolte, Vettel, and Möllers 2022). Recently a non-destructive NIRS-based method has been developed to determine their relative concentrations (Stolte, Vettel, and Möllers 2022). However, the nutritional value of the meal and the protein is counterbalanced by the presence of GSL, phenolic compounds, phytates and fibre (Tan et al. 2011; Wittkop, Snowdon, and Friedt 2009; Eifler et al. 2021; Hald et al. 2019). Their biosynthesis competes with the synthesis of oil and protein and can reduce their value (Gacek et al. 2021; Gacek, Bartkowiak-Broda, and Batley 2018). To increase also the value of the oilseed rape meal, higher protein contents need to be obtained by reducing antinutritive compounds in the seed.
In seeds of oilseed rape, the fibre components comprise of cellulose (CC), hemicellulose (HC) and lignin (LC). To analyse the fibre components, Van Soest, Robertson, and Lewis (1991) developed a sequential method to determine neutral detergent fibre (NDF), acid detergent fibre (ADF) and acid detergent lignin (ADL) contents in the oil-extracted dry meal. Subtraction of ADF from NDF, and ADL from ADF yields HC and CC, respectively (Wittkop, Snowdon, and Friedt 2009; Van Soest, Robertson, and Lewis 1991). High fibre content may result in reduced seed oil and protein content, as the synthesis of CC and HC redirects photosynthetic assimilates from oil and protein into fibre biosynthesis (Liu et al. 2013; Wang et al. 2015). Hence, a genetic reduction of fibre content is necessary to enhance seed protein while maintaining high oil content. Especially the black seed coat of oilseed rape has a high content of the indigestible lignin. A lower lignin content (LC) mainly of the seed coat is associated with a more yellowish and brighter seed colour, which could result also in a better protein quality (Carré et al. 2016; Wittkop, Snowdon, and Friedt 2012). However, yellow-seeded genotypes are known only from the Brassica rapa A genome-type Yellow Sarson. Although a large number of QTL for HC, CC, LC and yellowish seed colour on different A and C genome chromosomes of oilseed rape have been identified (Behnke, Suprianto, and Möllers 2018; Wang et al. 2015; Körber et al. 2016; Miao et al. 2019; Liu et al. 2012; Widiarsih 2017; Yusuf and Möllers 2023; Stein et al. 2013; Chao et al. 2022), no clearly yellow-seeded oilseed rape genotypes have yet been reported and approved. Progress in developing low fibre oilseed rape is obviously complicated by interactions among individual fibre components and with QTL for oil and protein content. To reduce fibre content in oilseed rape without impairing the oil and protein content of the seeds, further investigations are necessary. The old oilseed rape cultivar Lord has been reported to have relatively low LC among other oilseed rape line cultivars (Suprianto 2014; Körber et al. 2016). Furthermore, in a preliminary screening, resynthesized B. napus genotype R19 was found to have relatively low LC. We have developed a doubled haploid (DH) population of the cross R19 x Lord. This material was tested in replicated field experiments, and seeds harvested from open-pollinated plants were analysed for the inheritance of different quality parameters as well as of individual fibre components, oil and protein content. Obtained mean phenotypic data were used to perform a QTL analysis based on a 15K SNP marker set. Trait candidate genes were identified by comparing SNP marker positions with the high-quality long-read B. napus reference genome sequence Express 617 (Lee et al. 2020).
2 Material and Methods
2.1 Plant Material
The plant material consisted of a DH population R19 x Lord (n = 143), which was developed by microspore culture at the University of Göttingen (Department of Crop Sciences, Division of Crop Plant Genetics, Göttingen, Germany). One parent, R19, was a resynthesized B. napus line, which was selected for its low lignin content and yellowish-brown seed colour (Figure S1). According to Girke (2002), R19 was developed by a interspecific cross between B. oleracea convar. gemmifera and yellow-seeded B. rapa ssp. oleifera. The cultivar Lord is an old German cultivar developed by KWS Saat (KWS Saat SE & Co. KGaA, Einbeck, Germany), which, according to Suprianto (2014), shows a low fibre content in combination with a relatively high oil content.
2.2 Field Trials
Field trials were performed without replicates in the two different growing seasons 2019/2020 and 2020/2021 at the Experimental Field Station Reinshof of the Georg-August-University Göttingen, at KWS Saat SE & Co. KGaA in Einbeck and at Limagrain GmbH in Peine, all located in north-western Germany. Each location per season was considered as an environment. In total, six environments were analysed for the DH population. Sowing was performed in late August or beginning of September with 100 seeds in single-row observation plots. Weed, disease and pest control as well as fertilizer treatment was performed following recommendations for winter oilseed rape production in Germany. At maturity, seeds were harvested and bulked from 10 main racemes of open-pollinated plants from each plot and seed quality traits were analysed by near-infrared reflectance spectroscopy (NIRS).
2.3 Seed Quality Analysis and Phenotypic Characterization
NIRS was applied to determine seed quality with the FOSS monochromator model 6500 equipment (NIRSystem Inc., Silverspring, MD, USA). The spectra from 400 to 2498 nm at 2-nm intervals of 3 g of seeds in small ring cups were recorded. WinISI software (Version 4.12.0.15440, FOSS NIR Systems Inc., USA) was used to record the spectra of the harvested seeds. Calibration ‘raps2020.eqa’ provided by VDLUFA Qualitätssicherung NIRS GmbH (http://www.vdlufa-nirs.de) was used to determine contents of oil (%) and protein (%), GSL (μmol/g seed) and erucic acid (22:1; %). Seed oil (%), protein (%) and GSL (μmol/g seed) contents were determined at 91% seed dry matter basis. Protein content in the defatted meal (PidM) was calculated by using NIRS predicted seed oil and seed protein content (both at 91% dry matter) as % protein in the defatted meal (PidM) = [% protein/(100 − % oil)] × 100. The sum of seed oil and protein content was calculated (Oil + Protein). NDF, ADF, and ADL (≙ lignin; LC) contents were determined in the defatted meal using the calibration equation valid600div7.eqa of Dimov et al. (2012). Hemicellulose (HC) was determined by subtraction of ADF from NDF and cellulose (CC) by subtraction of ADL from ADF, respectively. The original calibration was extended by adding 252 selected samples with a wide variation in seed fibre content as well as including hemicellulose and cellulose as predicted traits. In validation, the standard error of prediction corrected for the bias (SEP(C)) of the extended calibration was 1.78%, 1.62%, 0.93%, 1.14% and 1.48% for NDF, ADF, ADL, HC and CC content, in the defatted seed meal, respectively. All fibre traits were reported as percentage of fibre in the defatted meal. Napin (μg), cruciferin (μg) and the cruciferin/napin ratio were determined with the ‘nap_cru_crunap.eqa’ calibration developed by Stolte, Vettel, and Möllers (2022). Thousand kernel weight (TKW) was determined by counting 500 seeds per plot and multiplying their weight by two. Seed colour was visually scored according to a scale from (1) for yellow, (3) for yellow brown, (5) for brown, (7) for dark brown and (9) for black and also using intermediate scores.
2.4 Germination Test
The preharvest germination (PHG) and germination capacity of the R19 x Lord DH population was determined. The percentage of PHG was determined by counting the number of seeds showing preharvest germination in a sample of 100 randomly selected seeds per DH line in the whole population. The germination capacity was determined with 20 selected DH lines with the highest and lowest LC from the average over six environments from the 2020 and 2021 harvest years. The seed germination test was performed according to Widiarsih (2017) in Petri dishes (92 × 16 mm, Sarstedt, reference code 82.1473) using customized filter paper (90 mm in diameter, Macherey-Nagel, GmbH & Co. KG, reference code 400,866,009.1) with 50 indented holes each to hold seeds. Hundred randomly chosen intact seeds per environment were germinated on moist filter paper in the dark for 10 days. Twelve millilitres of deionized water were carefully added. Petri dishes were lid-covered and placed in large plastic trays covered with cellophane to reduce evaporation. The trays were placed in darkness in a germination chamber at a temperature 16.5°C–17.5°C. After 10 days, seeds were inspected and radicle protrusion (%), germination (%) and contamination with bacteria or fungi (%) were determined. Germination was scored when the radicle was elongated (> 1 cm) and both cotyledons were outside of the seed coat. Radicle protrusion was scored when seed radicle elongated visually and protruded out of seed coat, but the cotyledons were not yet swollen and were still embedded within the seed coat. Contaminated seeds were identified by bacterial/fungal growth around seeds on filter paper. Based on slime or mycelium formation around the seeds, contaminations were considered to be caused by bacteria or fungi, respectively.
2.5 Statistical Analysis
Analysis of variance and descriptive statistics were performed using PLABSTAT software version 3A (Utz 2011). The statistical model used for the ANOVA was Yijk = μ + gi + ej + geij, where Yijk is the observations of a plot and μ is the general mean with gi for the effect of the ith genotype, ej for the effect of the jth environment and geij as interaction between ith genotype and jth environment. In the analysis, the environments were considered as fixed and the genotypes as random.
where σ2g is the variance component for the genotype and σ2ge is the variance component for the genotype × environment interaction, and E is the number of environments, respectively. PLABSTAT was also used to calculate Spearman's rank correlation coefficients between trait's mean values of the genotypes across the environments. The results of the germination test were analysed using R (R Core Team 2023) with the packages ggplot2 (Wickham 2016) and using a t-test with ggsignif (Ahlman-Eltze and Patil 2021).
2.6 DNA Extraction and SNP Markers
The DNA from all the DH and the parental lines was extracted at TraitGenetics GmbH (https://sgs-institut-fresenius.de/en/health-nutrition/traitgenetics) using a proprietary protocol. Genotyping of the DH population and parental lines was performed with a Brassica 15 K Illumina Infinium SNP array, which comprised 13,715 SNP markers at TraitGenetics GmbH (Clarke et al. 2016). The assay data were analysed using Illumina's GenomeStudio Software applying a TraitGenetics GmbH proprietary cluster file.
2.7 Linkage Map Construction
The linkage map was constructed using the ASMap package in R (Taylor and Butler 2017) based on the minimum spanning tree (MST) algorithm (Wu et al. 2008). In total, 43.12% of the markers were polymorphic in the R19 x Lord population. Heterozygous genotype calls were manually set to missing values, and markers with more than 5% missing calls were deleted. Genotypes that had too many double crossovers and markers that were not polymorphic between the two parents were all removed before map construction. Cosegregating markers and those with strongly distorted segregation were also initially excluded from map construction. After the map was constructed, cosegregating and distorted markers that deviated from the 1:1 ratio were included to have a full map. The distance of Kosambi (1943) was used for the final map construction. The threshold distance between markers of 20 cm was used to cluster them into initial linkage groups. MST algorithm (Wu et al. 2008) implemented in R was used for marker ordering within a linkage group. Marker order and chromosome assignment were compared with the map of Lee et al. (2020). The final SNP map used for QTL mapping consisted of 19 chromosomes with 4064 markers and a size of 2117.5 cm. Without colocating markers, chromosome A01 had a low marker density (7.59 Marker/cm). The whole-genome mean marker distance was 2.88 cm (Table S1). The final map is shown in Table S2.
2.8 QTL Mapping and Candidate Gene Identification
QTL were analysed using the R/qtl package in R (Broman et al. 2003; Broman and Sen 2009). The QTL mapping was performed using Haley–Knott Regression (Haley and Knott 1992) in R/qtl using a step-wise model selection approach (Manichaikul et al. 2009) with a 1-cm walking speed. The threshold of the significance of the LOD score (p = 0.05) was initially determined using the 50,000-permutation test to get a genome-wide LOD significance threshold. In the second step, a 2-D genome scan was performed, to test for additive effect at a locus, while considering the second locus and epistatic effect between pairs of loci. The LOD significance threshold is also calculated using the 1000-permutation test (p = 0.05). A QTL and a QTL interaction were considered significant when the LOD score was greater than the threshold. Finally, a multiple QTL model was fitted, including all the QTL detected and the interactions. The percentage of phenotypic variance explained by individual, combined QTL and the interactions for a trait was calculated in the fitted model. Identified QTL were named using q + trait with which they were associated, and the corresponding oilseed rape chromosome number (A01–A10 and C01–C09) and serial numbers were added when more than one QTL for the same trait were identified on the same chromosome. QTL explaining more than 10% of the phenotypic variance were termed major QTL. To identify the potential candidate genes of QTL, the positions of the SNP markers on the genetic map were aligned with their physical position by blasting the sequence of each SNP against the B. napus reference genome of Express 617 (Lee et al. 2020). Annotation of the B. napus gene sequences was performed by Pucker, Holtgräwe, and Weisshaar (2017) based on the Araport11 complete reannotation of the Arabidopsis thaliana reference genome (Cheng et al. 2017). To search for the presence of potential candidate genes, the physical region between SNP markers flanking a major QTL was inspected for genes known to be relevant in the respective biosynthetic pathway considering current publications.
3 Results
3.1 Phenotypic Analysis
With the exception of erucic acid (22:1) content, all frequency distributions showed normal or almost normal distributions. The frequency distribution of 22:1 showed a bimodal distribution (Figure S2). The mean values of each trait and DH line of the R19 x Lord DH population are shown in Table S3. The ANOVA showed significant genotypic and environmental effects for the crude fibre fractions and seed quality traits in the DH population R19 x Lord (Table 1). High to very high heritabilities were determined for all quality traits, from protein and PHG (h2 = 0.79) to 22:1 (h2 = 0.98). The DH population showed a large variation for all quality parameters. The mean value of 5.5% for LC in the defatted meal is low with a range between 3.2% and 10.8% (Table 1). The other fibre traits NDF (26.5%–35.3%), ADF (19.4%–27.6%), HC (5.0%–9.0%) and CC (15.6%–23.0%) showed comparatively large variations. The parental lines R19 and Lord showed similar fibre contents; nevertheless, R19 had the lowest contents in all five fibre traits. The colour of the seeds showed a wide range from yellow brown (3.2) to black (9.0) (Figure S1). The TKW ranged between 3.4 and 5.8 g. The mean PHG was 1.6%. The range of preharvest germinated seeds was between 0.0% and 7.9%. Transgressive segregation was observed for all traits in the R19 x Lord DH population.
| Trait | σ2g | σ2e | σ2ge | h2 | Descriptive statistics | Parents | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DF | 142 | 5 | 648 | Min | Max | Mean | LSD 5% | SD | CV | Skewness | Kurtosis | R19 | Lord | |||
| Oil | 6.42 | ** | 2.09 | *** | 2.85 | 93 | 35.8 | 48.9 | 42.6 | 1.9 | 3.329 | 7.8 | −0.1 | −0.26 | 41.3 | 44.2 |
| Protein | 1.08 | *** | 2.49 | *** | 1.72 | 79 | 17.0 | 23.4 | 19.8 | 1.5 | 2.209 | 11.15 | 0.01 | −0.58*** | 21.9 | 17.7 |
| PidM | 2.06 | *** | 3.75 | *** | 2.38 | 84 | 30.3 | 38.7 | 34.5 | 1.8 | 2.741 | 7.95 | −0.11 | −0.46*** | 37.4 | 31.6 |
| Oil + Protein | 4.15 | *** | 0.17 | *** | 0.83 | 97 | 56.4 | 67.2 | 62.4 | 1 | 2.264 | 3.62 | −0.20** | −0.11 | 63.3 | 61.9 |
| Glucosinolates (GSL) | 24.14 | *** | 2.17 | *** | 14.65 | 91 | 7.6 | 40.1 | 15.9 | 4.3 | 6.208 | 39.45 | 1.85*** | 6.61*** | 22.2 | 13.1 |
| 22:1 (Erucic acid) | 252.51 | *** | 1.38 | *** | 23.1 | 99 | 0 | 33.7 | 11.3 | 5.5 | 16.759 | 150.54 | 0.09 | −1.74*** | 24.7 | 0 |
| NDF | 1.88 | *** | 2.04 | *** | 1.99 | 85 | 26.5 | 35.3 | 30.8 | 1.6 | 2.331 | 7.57 | −0.50*** | 0.79*** | 29.6 | 31.5 |
| ADF | 1.86 | *** | 1.59 | *** | 1.24 | 90 | 19.4 | 27.6 | 22.9 | 1.3 | 2.075 | 9.06 | 0.36*** | 1.86*** | 22.2 | 23.5 |
| LC (ADL) | 1.98 | *** | 0.26 | *** | 0.52 | 96 | 3.2 | 10.7 | 5.5 | 0.8 | 1.65 | 29.91 | 0.99*** | 0.47*** | 5.4 | 5.7 |
| HC (hemicellulose) | 0.28 | *** | 0.23 | *** | 0.27 | 86 | 5.0 | 9.0 | 7.8 | 0.6 | 0.859 | 11.03 | −0.75*** | 1.62*** | 7.8 | 8.1 |
| CC (cellulose) | 0.75 | *** | 0.54 | *** | 0.83 | 84 | 15.6 | 23.0 | 17.2 | 1 | 1.38 | 8.04 | 0.94*** | 19.31*** | 16.5 | 17.1 |
| TKW | 0.2 | *** | 0.26 | *** | 0.16 | 88 | 3.4 | 5.8 | 4.6 | 0.5 | 0.762 | 16.39 | 0.08 | −0.13 | 5.5 | 4.3 |
| Preharvest germination (PHG) | 0.78 | *** | 0.24 | *** | 1.24 | 79 | 0.0 | 7.9 | 1.6 | 1.3 | 1.563 | 100.66 | 3.66*** | 21.30*** | 0.2 | 0.8 |
| Seed colour | 1.77 | *** | 0.04 | *** | 0.78 | 93 | 3.2 | 9.0 | 6.6 | 1 | 1.592 | 24.01 | −0.41*** | −0.2 | 7.3 | 6.3 |
| Napin | 8.7 | *** | 2.55 | *** | 7.58 | 87 | 9.3 | 23.9 | 15.7 | 3.1 | 4.223 | 26.97 | 0.21** | −0.27 | 18.1 | 11.4 |
| Cruciferin | 29.38 | *** | 52.92 | *** | 28.87 | 86 | 23.6 | 57.2 | 40 | 6.1 | 10.088 | 25.25 | 0.15* | 0.56*** | 36.2 | 35.9 |
| Cruciferin/napin ratio | 0.05 | *** | 0.11 | *** | 0.06 | 84 | 1.2 | 2.5 | 1.8 | 0.3 | 0.451 | 25.39 | 0.34*** | −0.07 | 1.5 | 1.5 |
- Note: Components of variance and heritabilities for contents of seed oil (in % with 9% residual moisture), protein (in % with 9% residual moisture), protein in defatted meal (PidM), glucosinolates (GSL in μmol/g with 9% residual moisture), erucic acid (22:1; %), five crude fibre traits (% in defatted meal), thousand kernel weight (TKW in g), preharvest germination (%), seed colour, napin (μg), cruciferin (μg), the cruciferin/napin ratio in the DH population R19 x Lord (n = 143).
- Abbreviations: CV, coefficient of variation; LSD 5%, least significant difference at p-value = 0.05; SD, standard deviation.
- * Significant at the 0.10 probability level,
- ** Significant at the 0.05 probability level, and
- *** Significant at the 0.01 probability level; LSD 5% = least significant difference at p < 5%.
Oil and protein were significantly negatively correlated in the DH population (Table 2). 22:1 was positively correlated with oil. It was also positively correlated with protein but more strongly positively correlated with PidM. The crude fibre fractions NDF, ADF, LC, HC and CC were also positively correlated with oil and negatively with protein. With the exception of the negative correlation between CC and LC, all fibre traits correlated positively with each other. Interestingly, seed colour correlated positively with LC and negatively with CC, but it did not correlate with HC. GSL correlated negatively with oil and positively with protein. All fibre traits correlated negatively with GSL. Further, GSL correlated positively with napin but did not correlate with cruciferin and the cruciferin/napin ratio. All three protein quality traits correlated negatively with oil and positively with protein.
| Oil | 0.36** | |||||||||||||||
| Protein | 0.12** | −0.73** | ||||||||||||||
| PidM | 0.42** | −0.32** | 0.87** | |||||||||||||
| Oil + Protein | 0.66** | 0.73** | −0.11** | 0.37** | ||||||||||||
| GSL | −0.18** | −0.44** | 0.44** | 0.29** | −0.24** | |||||||||||
| NDF | 0.07 | 0.60** | −0.78** | −0.66** | 0.13** | −0.32** | ||||||||||
| ADF | 0.16** | 0.48** | −0.66** | −0.58** | 0.05 | −0.29** | 0.90** | |||||||||
| LC | 0.19** | 0.46** | −0.47** | −0.33** | 0.19** | −0.17** | 0.64** | 0.77** | ||||||||
| HC | −0.23** | 0.33** | −0.50** | −0.47** | −0.01 | −0.12** | 0.53** | 0.29** | 0.11** | |||||||
| CC | 0.04 | 0.09** | −0.31** | −0.36** | −0.15** | −0.24** | 0.45** | 0.39** | −0.19** | 0.28** | ||||||
| TKW | 0.10** | −0.01 | 0.24** | 0.33** | 0.23** | 0.11** | −0.29** | −0.38** | −0.15** | −0.19** | −0.30** | |||||
| PHG | 0.09* | −0.25** | 0.24** | 0.17** | −0.13** | 0.08* | −0.33** | −0.25** | −0.18** | −0.26** | −0.13** | 0.13** | ||||
| Colour | 0.06 | 0.23** | −0.13** | −0.02 | 0.20** | 0.07 | 0.29** | 0.42** | 0.66** | −0.03 | −0.33** | 0.09** | −0.18** | |||
| Napin (NAP) | 0.11** | −0.42** | 0.51** | 0.42** | −0.11** | 0.34** | −0.39** | −0.37** | −0.44** | −0.13** | 0.10** | −0.01 | 0.20** | −0.34** | ||
| Cruciferin (CRU) | 0.07* | −0.30** | 0.52** | 0.52** | 0.08** | 0.04 | −0.47** | −0.60** | −0.58** | −0.32** | −0.02 | 0.43** | 0.14** | −0.37** | 0.18** | |
| CRU/NAP ratio | 0.14** | −0.32** | 0.42** | 0.35** | −0.05 | 0.02 | −0.26** | −0.29** | −0.28** | −0.38** | 0.05 | 0.40** | 0.15** | −0.16** | −0.09* | 0.78** |
| Trait | 22:1 | Oil | Protein | PidM | Oil + Protein | GSL | NDF | ADF | LC | HC | CC | TKW | PHG | Colour | Napin | Cruciferin |
- Note: Correlations for contents of seed oil (in % with 9% residual moisture), protein (in % with 9% residual moisture), protein in defatted meal (PidM), glucosinolates (GSL in μmol/g with 9% residual moisture), erucic acid (%), five crude fibre traits (% in defatted meal), thousand kernel weight (TKW in g), preharvest germination (PHG; %), seed colour, napin (μg), cruciferin (μg) and the cruciferin/napin ratio in the DH population R19 x Lord (n = 143).
- * Significant at the 0.05 probability level,
- ** Significant at the 0.01 probability level.
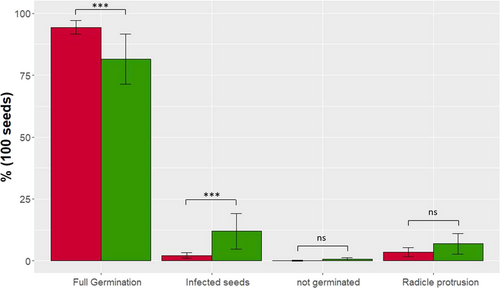
PHG correlated negatively with oil and positively with protein. PHG also correlated negatively with all five fibre traits; among the three individual components, it was more strongly negatively correlated with HC. Comparing the low and the high LC group with each 20 genotypes showed a significantly better germination of the high LC group (~95%) than the low LC group (~80%) (Figure 1). Particularly striking was that there were significantly more seeds contaminated with bacteria or fungi in the low LC group (~10% vs. 2%). The percentages of nongerminated seeds and seeds showing only radicle protrusion were similar in both groups.
3.2 Linkage Map
The final map covered 19 chromosomes with a distance of 2117.55 cm and with 4064 SNP markers for the R19 x Lord DH population (Tables S1 and S2). For QTL mapping, a framework map of 934 markers was used (Table S4). In this genetic map, 69.3% of the SNP markers showed significant deviation from the 1:1 segregation ratio. The Lord alleles were favoured by 54.9%.
3.3 QTL Analysis
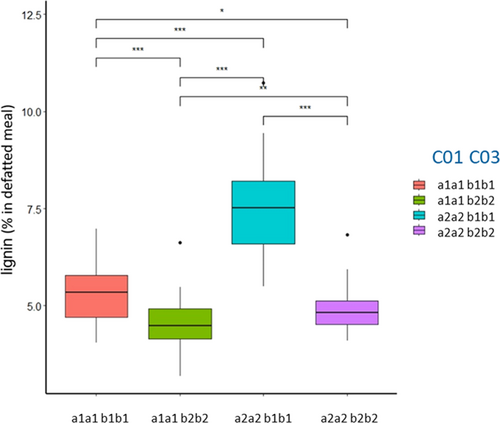
A large number of QTL were mapped for the different seed quality traits (Table 3). In total, six QTL were mapped for oil content. The largest QTL qOil-A08 explained 41.9% of the phenotypic variance and had a negative additive effect, indicating that the R19 allele at this position caused an increase in oil content. The QTL qOil-A08 colocated with q22:1-A08 for erucic acid content, with the qOil+Prot-A08 and qPidM-A08, all with the same direction of the additive effect. The biosynthesis of erucic acid also appeared to affect LC and ADF because of an overlap of the QTL confidence interval with qADL-A08 and qADF-A08 with the same direction of the effect. There was, however, no significant effect of erucic acid on CC. The remaining five QTL for oil content had negative and positive additive effects, which explains the transgressive segregation in the population. The qOil-A06 with a positive additive effect mapped at a similar position as qProt-A06 and qOil+Prot-A06 with an opposite effect, suggesting that the increase in oil content caused a decrease in protein content. This is in line with the negative correlation between oil and protein content. Colocating with these three QTL is as well the major QTL qPHG-A06 with a negative additive effect. This shows that the R19 allele at this position increases PHG, which is in line with its effect on qProt-A06, qOil+Prot-A06 and its opposite effect on qOil-A06. With qPHG-A05 and qPHG-C05, two additional major QTL with opposite effects were identified. The positive effect of qOil-C01 with the Lord allele increasing oil content mapped at a very similar position as qCC-C01 with the opposite effect, indicating that the reduction in CC led to an increase in oil content or vice versa. The reduction of CC at qCC-C01 caused by the Lord allele was balanced by an increase in LC at qADL-C01. This, however, did not lead to a significant change in ADF content at C01. Interestingly, the concurrent increase in LC and decrease in CC led to an increase in seed colour at qCol-C01. Furthermore, the change in fibre composition and increase in oil at qOil-C01 led to a decrease in cruciferin and napin content at qCru-C01 and qNap-C01, which, however, had no significant effect on protein content and on the cru/nap ratio. The largest reduction in LC was caused by a Lord allele at qADL-C03. This QTL did not have a significant effect on oil and protein content but led to an increase in HC and CC at qHC-C03 and qCC-C03, respectively. The reduction of LC at qADL-C03 also led to the change in seed colour towards more yellowish seeds at qCol-C03. Furthermore, a significant shift to more cruciferin content was found at qCru-C03, which, however, had no effect on napin content and on the cru/nap ratio. Although significant differences in HC, CC and LC were detected in the population, not a single significant QTL for the NDF was detected. There were also a number of epistatic interactions between different QTL. Most obvious was the epistatic interaction between the two major QTL qADL-C01:C03 (Figure 2).
| Trait/QTL | LG | Peak (cm) | CI (cm)a | Additiveb | LOD | R2c | TR2d | p | Left marker | Right marker |
|---|---|---|---|---|---|---|---|---|---|---|
| Oil | ||||||||||
| qOil-A06 | A06 | 143.0 | 135.0–150.2 | 1.01 | 9.25 | 11.38 | 70.41 | 3.24E-10 | Bn-A06-p2437284 | Bn-A06-p1768254 |
| qOil-A08 | A08 | 55.0 | 51.5–57.0 | −1.82 | 25.10 | 41.93 | <2e-16 | Bn-A08-p13060591 | Bn-A08-p6066021 | |
| qOil-A09 | A09 | 21.0 | 10.0–44.0 | 0.65 | 5.84 | 6.74 | 3.66E-06 | Bn-A09-p37019231 | Bn-A09-p28463402 | |
| qOil-C01 | C01 | 20.6 | 17.7–38.0 | 0.96 | 9.56 | 11.82 | 1.66E-10 | Bn-scaff_17827_1-p1027570 | Bn-scaff_19244_1-p380858 | |
| qOil-C05 | C05 | 131.1 | 0.0–150.0 | −0.43 | 4.07 | 4.55 | 0.000554 | Bn-scaff_15856_1-p81608 | Bn-scaff_16414_1-p1016430 | |
| qOil-C09 | C09 | 35.0 | 24.0–53.0 | −0.65 | 4.60 | 5.20 | 9.23E-06 | Bn-scaff_15743_1-p26515 | Bn-scaff_20903_1-p380659 | |
| qOil-A08:C05 | A08:C05 | 0.25 | 0.79 | 0.83 | 6.68E-02 | |||||
| qOil-A09:C05 | A09:C05 | 0.30 | 1.01 | 1.07 | 0.038118 | |||||
| Protein | ||||||||||
| qProt-A06 | A06 | 124.0 | 116.1–164.0 | −0.40 | 3.50 | 10.60 | 24.99 | 2.26E-04 | Bn-A06-p4001378 | Bn-A06-p163388 |
| qProt-C06 | C06 | 7.0 | 1.0–17.0 | 0.49 | 5.52 | 16.91 | 2.47E-06 | Bn-scaff_24104_1-p111316 | Bn-scaff_17799_1-p2065343 | |
| PidM | ||||||||||
| qPidM-A08 | A08 | 55.0 | 52.2–63.3 | −0.84 | 12.03 | 31.35 | 41.0 | 2.09E-13 | Bn-A08-p12599446 | Bn-A08-p372048 |
| qPidM-C06 | C06 | 6.0 | 0.0–36.0 | 0.45 | 3.76 | 8.41 | 4.00E-05 | Bn-scaff_24104_1-p111316 | Bn-scaff_16874_1-p292060 | |
| Oil + Protein | ||||||||||
| qOil+Prot-A06 | A06 | 145.8 | 136.0–164.0 | 0.49 | 4.61 | 5.34 | 69.89 | 6.52E-06 | Bn-A06-p2437284 | Bn-A06-p163388 |
| qOil+Prot-A08 | A08 | 55.0 | 53.0–57.0 | −1.62 | 30.41 | 58.31 | <2e-16 | Bn-A08-p12556455 | Bn-A08-p6066021 | |
| qOil+Prot-A09 | A09 | 34.7 | 5.0–67.0 | 0.38 | 2.84 | 3.19 | 0.000399 | Bn-A09-p37019231 | Bn-A09-p23552530 | |
| qOil+Prot-C01 | C01 | 22.8 | 19.0–44.0 | 0.69 | 8.45 | 10.50 | 1.03E-09 | Bn-scaff_17827_1-p586047 | Bn-A01-p17081703 | |
| Glucosinolates (GSL) | ||||||||||
| qGSL-A03–1 | A03 | 37.5 | 30.0–51.0 | 1.86 | 5.91 | 16.79 | 27.33 | 2.63E-07 | Bn-A03-p22976648 | Bn-A03-p20000930 |
| qGSL-A03–2 | A03 | 111.0 | 100.8–216.0 | 1.57 | 3.74 | 10.22 | 4.19E-05 | Bn-A03-p10011955 | Bn-A03-p135145 | |
| ADF | ||||||||||
| qADF-A08 | A08 | 39.7 | 33.1–61.0 | −0.36 | 3.06 | 6.66 | 41.86 | 0.000211 | Bn-A08-p15994149 | Bn-A08-p1166566 |
| qADF-C03 | C03 | 176.1 | 174.6–182.0 | −0.78 | 11.64 | 29.66 | 5.11E-13 | Bn-scaff_18322_1-p826786 | Bn-scaff_22728_1-p349077 | |
| LC (lignin) | ||||||||||
| qADL-A08 | A08 | 52.0 | 38.3–60.0 | −0.35 | 5.60 | 5.44 | 75.20 | 6.74E-07 | Bn-A08-p15615914 | Bn-A08-p2461697 |
| qADL-C01 | C01 | 17.0 | 10.0–19.0 | 0.75 | 20.90 | 27.20 | <2e-16 | Bn-scaff_16055_1-p1543652 | Bn-scaff_17827_1-p586047 | |
| qADL-C03 | C03 | 177.0 | 175.3–178.0 | −0.80 | 30.59 | 48.51 | <2e-16 | Bn-scaff_18322_1-p920570 | Bn-scaff_22728_1-p349077 | |
| qADL-C01:C03 | C01:C03 | −0.55 | 11.51 | 12.48 | 1.05E-12 | |||||
| HC (hemicellulose) | ||||||||||
| qHC-A03 | A03 | 75.8 | 65.5–105.0 | 0.21 | 5.06 | 13.46 | 31.42 | 1.25E-05 | Bn-A03-p16017186 | Bn-scaff_19248_1-p357191 |
| qHC-C03 | C03 | 176.1 | 171.0–179.0 | 0.25 | 7.97 | 22.38 | 1.88E-08 | Bn-scaff_18322_1-p42485 | Bn-scaff_22728_1-p349077 | |
| qHC-C03:A03 | C03:A03 | −0.10 | 1.22 | 3.04 | 0.0196 | |||||
| CC (cellulose) | ||||||||||
| qCC-C01 | C01 | 16.9 | 12.0–21.3 | −0.50 | 15.73 | 38.19 | 48.80 | <2e-16 | Bn-scaff_17369_1-p1017003 | Bn-scaff_25149_1-p45585 |
| qCC-C03 | C03 | 188.6 | 171.0–198.0 | 0.20 | 3.09 | 5.93 | 0.000196 | Bn-scaff_18322_1-p42485 | Bn-scaff_18936_1-p269153 | |
| Seed colour | ||||||||||
| qCol-C01 | C01 | 22 | 18–34 | 0.61 | 11.01 | 17.41 | 63.51 | 2.14E-11 | Bn-scaff_17827_1-p1027570 | Bn-scaff_19244_1-p380858 |
| qCol-C03 | C03 | 176.1 | 175–178 | −0.86 | 20.22 | 38.19 | < 2e-16 | Bn-scaff_18322_1-p920570 | Bn-scaff_18322_1-p2583107 | |
| Cruciferin | ||||||||||
| qCru-A07 | A07 | 81.0 | 77.3–97.0 | −1.67 | 7.15 | 10.89 | 61.90 | 1.71E-07 | Bn-A07-p14029820 | Bn-A07-p18955989 |
| qCru-C01 | C01 | 16.9 | 0.0–67.0 | −0.79 | 1.36 | 1.87 | 0.01511 | Bn-scaff_15712_3-p804356 | Bn-A01-p17081703 | |
| qCru-C03 | C03 | 176.0 | 174.6–179.7 | 2.98 | 15.60 | 27.83 | 1.78E-15 | Bn-scaff_18322_1-p826786 | Bn-scaff_22728_1-p349077 | |
| qCru-C09 | C09 | 31.0 | 0.0–91.0 | −1.11 | 2.46 | 3.44 | 0.00109 | Bn-A10-p15227741 | Bn-scaff_17297_1-p214513 | |
| qCru-A03 | A03 | 32.0 | 28.0–38.3 | −1.45 | 4.19 | 6.05 | 1.99E-05 | Bn-A03-p23711362 | Bn-A03-p21945987 | |
| qCru-A07:C03 | A07:C03 | −0.70 | 0.99 | 1.34 | 0.03864 | |||||
| Napin | ||||||||||
| qNap-A09 | A09 | 17.7 | 0.0–29.0 | −0.85 | 3.16 | 7.49 | 36.24 | 0.000178 | Bn-A09-p34482075 | Bn-A09-p30391674 |
| qNap-C01 | C01 | 23.0 | 10.3–34.0 | −1.27 | 6.58 | 16.60 | 6.27E-08 | Bn-scaff_16055_1-p1543652 | Bn-scaff_15838_5-p441651 | |
| qNap-C02 | C02 | 31.0 | 0.0–51.0 | −0.90 | 2.45 | 5.73 | 0.000971 | Bn-scaff_15714_1-p2481342 | Bn-scaff_17298_1-p720480 | |
| Cruciferin/Napin ratio | ||||||||||
| qCruNap-A07 | A07 | 85.0 | 49.4–86.9 | −0.09 | 3.46 | 11.46 | 11.46 | 7.63E-05 | Bn-A02-p61866 | Bn-A07-p18955989 |
| Erucic acid 22:1 | ||||||||||
| qC22:1-A08 | A08 | 54 | 53.0–55.9 | −15.75 | 72.58 | 92.36 | 92.36 | 0 | Bn-A08-p12559372 | Bn-A08-p11458466 |
| TKW | ||||||||||
| qTKW-A08 | A08 | 11 | 6.0–17.7 | −0.22 | 7.63 | 20.22 | 34.84 | 4.03E-08 | Bn-A08-p19676498 | Bn-A08-p18069035 |
| qTKW-C09 | C09 | 1 | 0.0–7.0 | −0.16 | 4.43 | 11.09 | 0.0000503 | Bn-A10-p15227741 | Bn-scaff_27705_1-p179294 | |
| Preharvest germination | ||||||||||
| qPHG-A05 | A05 | 7.0 | 0.0–18.0 | 0.29 | 6.40 | 15.34 | 39.74 | 7.00E-07 | Bn-A05-p140798 | Bn-A05-p5057096 |
| qPHG-A06 | A06 | 151 | 145.8–164.0 | −0.41 | 9.42 | 23.87 | 8.74E-10 | Bn-A06-p2345966 | Bn-A06-p163388 | |
| qPHG-C05 | C05 | 0.73535 | 0.0–10.0 | −0.36 | 5.10 | 11.81 | 2.35E-06 | Bn-scaff_15856_1-p81608 | Bn-scaff_17441_1-p569870 | |
| qPHG-A06:A05 | −0.30 | 3.97 | 9.10 | 2.85E-05 | ||||||
- Note: QTL were mapped for four fibre components (% in defatted meal) ADF, ADL, hemicellulose and cellulose, seed oil (in % with 9% residual moisture), protein (in % with 9% residual moisture), the sum of oil and protein (in % with 9% residual moisture), glucosinolates (GSL in μmol/g with 9% residual moisture), napin (μg), cruciferin (μg) and the cruciferin/napin ratio in the R19 x Lord DH population (n = 143).
- a QTL confidence interval at p ≤ 0.01.
- b positive sign indicates alleles from Lord increase trait values and negative R19.
- c R2 percentage of the phenotypic variance explained by a QTL.
- d TR2 percentage of the phenotypic variance explained by all the QTL for that trait.
3.4 Identification of Candidate Genes
Candidate genes were searched only for individual major QTL explaining more than 10% of the phenotypic variance (Table S5). For a number of traits with overlapping QTL confidence intervals and composite traits, for example, NDF, ADF and seed colour, candidate genes are mentioned only for major contributing individual QTL. And among the possible candidates, only the most likely candidate genes are mentioned below. Additional candidate genes for minor QTL are included in Table S5. For qOil-A06 with the positive effect of the Lord allele, the acetyl-CoA carboxylase 1 (ACC1) gene was identified as candidate. This QTL showed overlapping confidence interval with qProt-A06 with a negative effect, suggesting that the increase in oil content likely caused a decrease in protein content, which is in line with the negative correlation between the two traits. For oil content, the largest QTL qOil-A08 colocated with the QTL q22:1-A08. Between the two flanking SNP markers of QTL q22:1-A08, two 3-ketoacyl-CoA synthase genes (KCS17 and KCS18) were identified that are known to be involved in the fatty acid chain elongation. Among other genes, for qOil-C01, a glycerol-3-phosphate acyltransferase (GPAT9) gene and fatty acid desaturase (FAD6) were identified. For the minor qOil-C05, two glycerol-3-phosphate acyltransferase genes (GPAT2 and GPAT4) were spotted. Between the flanking SNP markers of qProt-A06 and qProt-C06 each, a number of bifunctional inhibitor/lipid-transfer protein/seed storage 2S albumin superfamily protein genes were identified. The only two major QTL for GSL content were located on the Brassica A03 genome chromosome. For qGSL-A03-1, the two MYB transcription factor genes, MYB28 and MYB34, and the methylthioalkylmalate synthase MAM1 genes were detected. For the qGSL-A03-2, a MYB29, a cytochrome P450, and two glutathione S-transferase genes (GSTF9 and GSTF10) were identified. Among the fibre components QTL qADF-C03, qADL-C03, qHC-C03 and qCC-C03 with overlapping confidence intervals, qADL-C03 explained the largest fraction of variance. The flanking markers were mapped at 6.66 and 13.55 Mbp. Inspecting this physical region for putative candidate genes known to be involved in the phenylpropanoid and lignin biosynthesis, MYB transcription factors MYB19, MYB36 and MYB101 and an O-methyltransferases (OMT) gene were found. Cinnamate-4-hydroxylase (C4H), as part of the phenylpropanoid pathway, and cellulose synthase-like A02 (CSLA02) were identified for qHC-C03 and qCC-C03. Moreover, phosphofructokinase 7 (PFK7) is identified as an enzyme of the glycolysis. Among the individual QTL qADL-C01, qCC-C01, qCol-C01, qOil-C01, qOil+ProtC01, qCru-C01 and qNap-C01 with overlapping confidence intervals, qCC-C01 explained the largest fraction of explained variance. Within the confidence interval of qCC-C01, a number of cellulose synthase genes (CSLC5, CESA1, CSLG1, CSLG2) were located. Different MYB transcription factors were identified as well: MYB85, MYBR1 and MYB101. Cruciferin and napin are the main storage proteins of oilseed rape. For the largest cruciferin QTL qCru-C03, a large number of bifunctional inhibitor/lipid-transfer protein/seed storage 2S albumin superfamily protein and lipid transfer protein genes were identified. The QTL qCru-A07 and qCruNap-A07 colocated. For qCru-A07, the R19 allele increased the cruciferin content and equally the cru/nap ratio of qCruNap-A07. In this region, various transmembrane amino acid transporter family proteins, a vacuolar sorting receptor homologue gene (VSR1) and bifunctional inhibitor/lipid-transfer protein/seed storage 2S albumin superfamily proteins were found. Within the confidence interval of the QTL for napin qNap-C01, different genes were found, among others vacuolar sorting receptor (VSR7), seed storage albumin 4 (SESA4) and amino acid permease 1 (AAP1) genes.
4 Discussion
Oilseed rape (B. napus) is a worldwide grown oil and protein crop. The demand for sustainable and renewable plant proteins is increasing. After oil extraction, rapeseed meal contains a high-quality protein that can be used as a valuable animal feed and additionally as a novel protein source for human consumption. It has a high biological value and well-balanced amino acid composition (Jia et al. 2021; Wanasundara 2011; So and Duncan 2021; Kotecka-Majchrzak et al. 2020; Sá et al. 2021; Langyan et al. 2021; Arrutia et al. 2020). However, oilseed rape meal contains relatively high amounts of anti-nutritive fibre compounds, phenolic acids, phytate and GSLs (Wittkop, Snowdon, and Friedt 2009; Arrutia et al. 2020; Eifler et al. 2021). Increasing the oil and protein content of the seeds requires that other seed components like crude fibre are reduced.
Although both parents of the DH population had quite similar contents for the sum of oil and protein, the DH population segregated with a difference of 10.8% between the min and max value (56.4%–67.2%) for the sum of both. The closest positive correlation between the sum of both traits was for oil, followed by erucic acid. A strong negative correlation between oil and protein content was detected. This is in line with many earlier published results (Yusuf and Möllers 2023; Zum Felde et al. 2007; Liu et al. 2012; Chao et al. 2022; Gacek et al. 2021; Yan et al. 2009). The observed positive correlations between all fibre components and oil are in contrast to the published results of Miao et al. (2019) who reported negative correlations between oil and LC, HC and CC in the KN DH population, respectively. Somewhat different results were reported for two half-sib DH populations by Yusuf and Möllers (2023). They found a positive correlation between oil and HC, and CC, and a negative correlation to LC. Obviously, the outcome of individual QTL for fibre components depends on their specific mutation and on their parental genotype. As found by Yusuf and Möllers (2023), all fibre components in the present population correlated negatively with protein and PidM. Furthermore, none of the QTL for individual fibre components with opposite effects mapped at same or similar positions as for the two major QTL for seed protein content on A06 and C06. Hence, in this population, there is no evidence that a reduction in fibre components causes increased seed protein contents. Obviously, additional genetic variation for fibre components that leads to increased protein content needs to be identified. For the major qCC-C01, a large number of candidate genes involved in the cellulose biosynthesis were identified (Table S5; Pedersen et al. 2023).
The largest QTL for oil qOil-A08 explained 42% of the phenotypic variance. Notably, qOil-A08 colocated with q22:1-A08 for erucic acid, which is in line with the earlier observed effect of erucic acid on oil content (Ecke, Uzunova, and Weißleder 1995; Behnke, Suprianto, and Möllers 2018). This QTL also did not have a significant effect on protein content but did have an effect on qPidM-A08. This suggests that erucic acid reduces some other minor constituents, among which fibre components may be. The second largest QTL qOil-C01 with the Lord allele increasing oil content colocated with the major QTL qCC-C01 with an opposite effect on cellulose content, suggesting that a reduction in cellulose content may have led to the increase in oil. In line with this hypothesis is that the decrease in cellulose caused concomitantly an increase at the colocating qADL-C01. However, alternatively, the identified candidate gene GPAT4 is a likely explanation for the observed increase in oil and decrease in cellulose content (Yang et al. 2022). There were, with qOil-A09, qOil-C05 and qOil-C09, three QTL for oil with positive and negative effects that did not colocate with QTL for individual fibre components with opposite effects. Interestingly, qOil-C05 showed an increase in oil caused by an allele of the resynthesized parent R19. The QTL flanking marker Bn-scaff_15856_1-p81608 is mapped at 45.8 Mbp on C05 in Express 617. In an earlier work, QTL for oil, lignin and seed hull content on C05 were located at a very similar position (Yusuf and Möllers 2023; Behnke, Suprianto, and Möllers 2018), which later turned out to be caused by a homoeologous nonreciprocal translocation (HNRT) from A05 to C05 (Schilbert et al. 2023). Further work would be required to investigate if the qOil-C05 in R19 is caused by a similar translocation event. However, in contrast to Schilbert et al. (2023), in the present population, there was no significant QTL for LC at the same position with an opposite effect. Supporting the possible A05 to C05 translocation hypothesis are the qPHG-A05 and qPHG-C05 with opposite additive effects. In an early genome-wide association study of Wang et al. (2015), a major QTL for low lignin and seed hull content on A05 and C05 has been reported that may have been caused by a similar translocation event. It was also detected that the low lignin lines may have a thinner seed coat than the high lignin lines (Schilbert et al. 2023; Behnke, Suprianto, and Möllers 2018; Ding et al. 2021; Wang et al. 2015; Wittkop, Snowdon, and Friedt 2012). The oilseed rape seeds are mainly composed of two parts, embryo and seed coat. Oil and protein are concentrated in the embryo, whereas the seed coat is part of protection against biotic and abiotic stresses, determination of seed dormancy, germination and control of seed size (Radchuk and Borisjuk 2014; Moïse et al. 2005). The seed coat is controlled by secondary cell wall formation, including the biosynthesis of cellulose, hemicellulose and lignin (Jiang and Deyholos 2010). A negative correlation between fibre and PHG as well as TKW was detected, which could be caused by a reduced seed hull content. Further investigations are necessary.
With qADL-C03, the largest QTL for LC did not colocate with a QTL for oil or protein content. At the same chromosome and at very similar positions, Widiarsih (2017) identified QTL for LC in two half-sib DH populations. In both populations, there was no effect on protein content, and only in one of the populations did low lignin content lead to increased oil content. In the study of Widiarsih et al. (2021), a gene copy of cinnamate-4-hydroxylase C4H was identified as a potential candidate gene for low lignin content. The same C4H gene was identified in the confidence interval of qADF-C03, qADL-C03 and qHC-C03 in the R19 x Lord population (Table S5). C4H is responsible for the conversion of cinnamic acid to p-coumaric acid, which is the second enzymatic step within the phenylpropanoid pathway and plays an important role in flavonoid- and lignin-related metabolites (Fraser and Chapple 2011; Hong et al. 2017; Liu, Osbourn, and Ma 2015). As Widiarsih (2017) discussed, an impaired activity of C4H can lead to decreased contents of downstream metabolites of this pathway.
Seed colour was most closely positively correlated with LC, and it was not correlated with HC and even significantly negatively correlated with CC. This is underlined by colocation of qLC-C03 and qCol-C03, and of qCC-C01 and qCol-C01, respectively, with opposite additive effects. Because lignin is mainly located in the seed hull (Carré et al. 2016), this indicates that a reduction in lignin and an increase in cellulose would lead to brighter more yellowish seeds. Some QTL for lignin content and seed colour were also found to colocate in other studies (Badani et al. 2006; Liu et al. 2012). As lignin and pigment synthesis share common precursors (Fraser and Chapple 2011), the accumulation of both in the seed coat may be regulated by same or different genes.
The R19 x Lord DH population is the first to which the NIRS calibration of Stolte, Vettel, and Möllers (2022) for the main seed storage proteins cruciferin and napin is applied. The results obtained confirmed earlier reports (Malabat et al. 2003; Schatzki et al. 2014) with respect to the positive correlation between napin, GSL and seed protein. Both cruciferin and napin were positively correlated with protein and negatively with oil in the population (Table 3). A positive correlation for the sum of cruciferin and napin to protein, as found by Stolte, Vettel, and Möllers (2022), can hence be expected. The positive cruciferin/napin ratio indicates that quantitatively more cruciferin than napin is present in the population, which, however, shows a large variation from 0.6 to 3.7 (Table 2). Schatzki et al. (2014) reported colocating QTL for GSL, napin, cruciferin and the cru/nap ratio on chromosome N19 (C09). In the present population, the major QTL for cruciferin was found on C03 with an explained variance of 27.8%. Two bifunctional inhibitor/lipid-transfer protein/seed storage 2S albumin superfamily protein genes were identified as candidates. The QTL qCru-A07 and qCruNap-A07 colocated with the same direction of the additive effect. In this region, bifunctional inhibitor/lipid-transfer protein/seed storage 2S albumin superfamily protein, various transmembrane amino acid transporter family proteins and a vacuolar sorting receptor homologue gene (VSR1) that functions as sorting receptor for storage proteins (Shimada et al. 2003) were located. Within the confidence intervals of major qNap-C01 that showed overlapping confidence interval with qCru-C01, a large number of seed storage protein genes were identified (Table S5).
Because of the negative correlation between GSL and fibre components and the location of both qGSL-A03-1 and qGSL-A03-2 on the oilseed rape Brassica A genome, it appeared reasonable to search for candidate genes involved in the biosynthesis of phenylalanine derived benzyl- and phenethyl-GSL. Conspicuously, Shang et al. (2022) reported the two transcription factor genes MYB34 (BraA03g043830.3C) and MYB28 (BraA03g044380.3C) as well as the methylthioalkylmalate synthase 1 gene (MAM1; BraA03g043740.3C) as candidates for the specific qGSL-A03-1 in B. rapa (Table S5). Furthermore, Shang et al. (2022) reported a sulfotransferase gene (ST2B) as candidate for qGSL-A03-2. Although the NIRS method did only allow quantification of total GSL content, it seems likely that limited phenylalanine led to the negative correlation between the products of the phenylpropanoid and GSL biosynthetic pathway and QTL colocation with opposite effects.
The selected 20 DH lines with the lowest and highest seed lignin content showed clear and significantly lower seed germination and percentage of infected seeds. Lower germination and vigour, and increased preharvest germination of yellow-seeded genotypes were discussed as cause (Boerjan, Ralph, and Baucher 2003; Widiarsih 2017; Zhou et al. 2020). In the R19 x Lord population, the positive correlation between PHG and protein; the negative correlation to oil; and the colocation of qPHG-A06, qProt-A06 and pOil-A06 with different direction of additive effects suggest that breeding of protein-rich, high-yielding yellow-seeded cultivars may be compromised by increased PHG. However, it may be that there are a larger number of genes involved in the inheritance of these traits, which may segregate independently and may allow to select genotypes with improved seed germination and lower PHG. The higher percentage of infected seeds points to ‘seed microbiome’ (Rybakova et al. 2017) and ‘soil fatique’ that has been discussed in dependence of crop rotation, crop species, soil types, soil-borne diseases and so forth (Wolińska et al. 2018). Hence, multiple environmental factors affect PHG, seed longevity and germination (Nagel et al. 2011; Schatzki et al. 2013).
The cultivar Lord was identified in much earlier studies as a genotype with a very low lignin content by Suprianto (2014) and Körber et al. (2016). Similarly, a low LC content of 5.7% was confirmed in the present study. The low lignin line R19 (5.4%) is a resynthesis of B. rapa (AA, 2n = 20) and B. oleracea (CC, 2n = 18). Recombination is one of the major sources of genetic variation and for novel allelic combinations. The cross was made to introduce new alleles for low fibre content into the B. napus gene pool. Several published studies carried out work about genotypes and resynthesized lines with reduced lignin content, which usually is associated with the appearance of yellow or at least brown-yellowish seed phenotypes (Rahman and McVetty 2011; Bagheri et al. 2012; Wang et al. 2015; Rahman 2001). However, no improved cultivar with reduced fibre content has been successfully established in the market, even though it was proven that a reduced lignin content has no negative effect on seed yield (Holzenkamp, Link, and Möllers 2022). The present study shows that the R19 genotype has as well a low lignin content but that epistatic interactions between the two major QTL for LC on C01 and C03 with opposite effects complicate the selection of genotypes with yellow seeds and an even lower lignin content.
Author Contributions
Christian Möllers conceived the study. Karin Holzenkamp performed the experiments and analysed the data. Karin Holzenkamp wrote the manuscript draft. All authors read and approved the manuscript.
Acknowledgements
The authors gratefully acknowledge the funding provided by the Bundesministerium für Ernährung und Landwirtschaft (FNR 22036418). Special thanks to Dietrich Kaufmann and Jutta Schaper for their excellent technical support and to Boas Pucker for providing the annotation of the Brassica gene sequences. The authors very much appreciate the support of KWS SAAT SE & Co. KgaA and Limagrain GmbH for performing the field experiments. Open Access funding enabled and organized by Projekt DEAL.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




