Precise genome editing of Dense and Erect Panicle 1 promotes rice sheath blight resistance and yield production in japonica rice
Summary
The primary goals of crop breeding are to enhance yield and improve disease resistance. However, the “trade-off” mechanism, in which signalling pathways for resistance and yield are antagonistically regulated, poses challenges for achieving both simultaneously. Previously, we demonstrated that knock-out mutants of the Dense and Erect Panicle 1 (DEP1) gene can significantly enhance rice resistance to sheath blight (ShB), and we mapped DEP1's association with panicle length. In this study, we discovered that dep1 mutants significantly reduced rice yield. Nonetheless, truncated DEP1 was able to achieve both ShB resistance and yield increase in japonica rice. To further explore the function of truncated DEP1 in promoting yield and ShB resistance, we generated CRISPR/Cas9-mediated genome editing mutants, including a full-length deletion mutant of DEP1, named dep1, and a truncated version, dep1-cys. Upon inoculation with Rhizoctonia solani, the dep1-cys mutant demonstrated stronger ShB resistance than the dep1 mutant. Additionally, dep1-cys increased yield per plant, whereas dep1 reduced it. Compared to the full DEP1 protein, the truncated DEP1 (dep1-cys) demonstrated a decreased interaction affinity with IDD14 and increased affinity with IDD10, which are known to positively and negatively regulate ShB resistance through the activation of PIN1a and ETR2, respectively. The dep1-cys mutant exhibited higher PIN1a and lower ETR2 expression than wild-type plants, suggesting that dep1-cys modulated IDD14 and IDD10 interactions to regulate PIN1a and ETR2, thereby enhancing ShB resistance. Overall, these data indicate that precise genome editing of DEP1 could simultaneously improve both ShB resistance and yield, effectively mitigating trade-off regulation in rice.
Introduction
Promoting high and stable rice yields is critical to ensuring national food security. Rice diseases significantly affect yield stability, with annual losses due to disease estimated at 30% (Deng et al., 2020; Zhao et al., 2022). Rice sheath blight (ShB) caused by Rhizoctonia solani Kühn can affect rice throughout its growth cycle (Savary et al., 2006), and in severe cases, can reduce yield by more than 50%, severely affecting yield stability (Zheng et al., 2013). Although a few rice varieties with high resistance to ShB have been identified (Zuo et al., 2010), progress in molecular marker techniques has enabled the identification of numerous resistance-related quantitative trait loci (QTL), which facilitate the enhancement of rice resistance to ShB (Chen et al., 2019; Wang et al., 2023; Zuo et al., 2013). However, owing to the antagonistic relationship between growth and disease resistance, high-resistance varieties often fall short in yield (Gao et al., 2024; Huot et al., 2014).
Recent studies have suggested that certain genes may balance yield and resistance, achieving a win-win outcome. Previous studies have indicated that idd10 (indeterminate domain 10) mutants increase rice resistance to ShB by modulating ethylene signalling without reducing yield (Li et al., 2024b). The natural allele UMP1R2115 promotes resistance to various diseases in rice, including ShB, without compromising agronomic traits or yield (Hu et al., 2023). Moderate overexpression of IDD14 (indeterminate domain 14) significantly boosts ShB resistance without affecting yield (Sun et al., 2019). The ammonium transporter (AMT1;1) supports nitrogen uptake and assimilation as well as ethylene signalling, thus enhancing ShB resistance while maintaining yield stability (Li et al., 2024a; Wu et al., 2022). BGL2 serves as a β-glucanase family member, promoting ShB resistance and increasing rice yield by enhancing tillering (Liu et al., 2024). As a GDSL-type esterase/lipase gene, GELP77 contributes to broad-spectrum resistance and significantly boosts yield through jasmonic acid (JA) accumulation (Zhang et al., 2024b). Grassy tiller 1 (GT1) regulates sugar distribution between host and pathogen, as well as rice tillering, achieving a balance between disease resistance and yield (Yang et al., 2023, 2024). Gene-edited RBL1∆12 improves rice disease resistance while maintaining growth and yield with no adverse effects (Sha et al., 2023). Pigm encoding two proteins with opposing roles in disease resistance and yield (PigmR and PigmS) achieves sustained resistance without reducing productivity (Deng et al., 2017). GRF6 (GROWTH-REGULATING FACTOR 6) enhances yield in large panicles and improves bacterial blight resistance by modulating indole acetic acid (IAA) and JA signalling (Yuan et al., 2024). The Ideal Plant Architecture 1 (IPA1) boosts the immunity and yield through phosphorylation modifications (Wang et al., 2018). The natural ROD1 (RESISTANCE OF RICE TO DISEASES1) allele balances resistance and yield by adjusting protein expression based on pathogen presence (Gao et al., 2021). These findings demonstrate the role of specific genes in balancing yield and resistance, providing critical insights for genetic improvement. Identifying genes that enhance resistance without compromising growth can guide sustainable agriculture, laying the groundwork for targeted breeding. Understanding the resistance mechanisms of these genes can improve crop productivity and support food security in the face of challenges.
Research on rice disease resistance and yield is often conducted separately, leaving the impact of certain resistance genes on yield unclear and requiring further exploration of yield-related genes' influence on disease. Natural variation of the DEP1 allele has been shown to enhance rice yield by regulating nitrogen use efficiency (NUE) (Huang et al., 2009; Sun et al., 2014). Known as DN1, qPE9-1, and qNGR9, DEP1 is located at a single gene locus (Taguchi-Shiobara et al., 2011; Yan et al., 2007; Zhou et al., 2009). Studies have further linked the C-terminal length of the DEP1 protein to the balance between rice yield and quality (Huang et al., 2022). Our previous findings demonstrated that DEP1 negatively regulates rice resistance to ShB (Liu et al., 2021a). Nevertheless, the role of the C-terminal-truncated DEP1 in rice resistance to ShB remains unclear.
Our research revealed that truncated DEP1 confers superior agronomic traits and disease resistance compared to DEP1 loss-of-function mutants, achieving both ShB resistance and enhanced rice yield. Compared to DEP1, the dep1-cys variant weakened its interaction with IDD14, thereby reducing the inhibitory effect of DEP1 on IDD14-dependent PIN1a activation. Simultaneously, dep1-cys strengthened its interaction with IDD10, enhancing the inhibition of ETR2 expression, thereby reducing rice susceptibility. These findings highlight the dual advantage of dep1-cys in terms of yield and ShB resistance, revealing a novel mechanism by which the natural dep1 variant circumvents trade-offs to simultaneously improve yield and resistance.
Results
dep1 mutants are resistant to ShB but cause yield reduction in rice
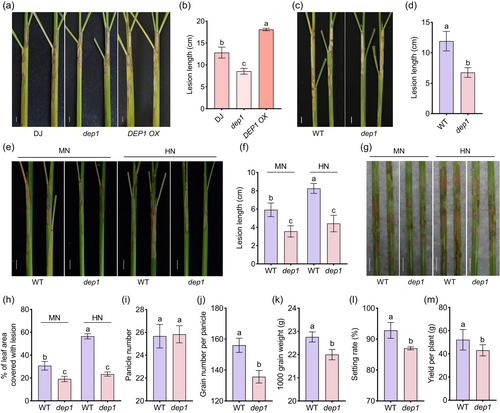
Our previous studies indicated that mutations in DEP1 can enhance ShB resistance and planting density in rice (Figure 1a,b) (Liu et al., 2021a). To further investigate the role of DEP1 in regulating yield and disease resistance, we used CRISPR/Cas9 to create DEP1 knock-out mutants, named dep1, in the japonica rice variety Sasanishiki. Sequence analysis identified insertional mutations near the DEP1 editing target site, with a single nucleotide insertion (c.124_125 ins T) in the first exon causing premature translation termination. This mutation resulted in a truncated protein missing 384 residues (Figure S1). We then evaluated ShB resistance in dep1 mutant plants compared to that in wild-type (WT) plants. The results indicated that dep1 plants exhibited shorter lesion lengths than WT, suggesting that DEP1 negatively regulates rice resistance to ShB (Figure 1c,d). Since DEP1 regulates NUE (Sun et al., 2014), N-dependent ShB resistance of dep1 was analysed. WT and dep1 were grown under medium nitrogen (MN) and high nitrogen (HN) conditions, and R. solani AG1-IA was inoculated. The results showed that ShB was more severe in HN compared to MN in WT plants; however, the lesion length in dep1 was similar in HN and MN conditions, and dep1 is less susceptible to ShB compared to WT (Figure 1e–h). The yield assessments of dep1 mutants and WT revealed no significant differences in panicle number (Figure 1i). However, the number of grains per panicle was notably lower in dep1 compared to WT (Figure 1j). Moreover, the 1000-grain weight and setting rate were reduced in dep1 relative to WT (Figure 1k,l). Consequently, the overall yield per dep1 plant was significantly lower than that of WT (Figure 1m).
dep1-cys improves resistance to ShB and increase yield
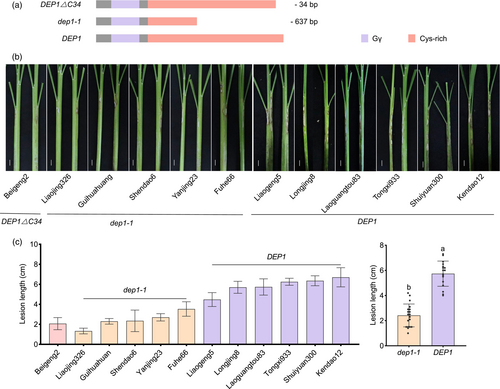
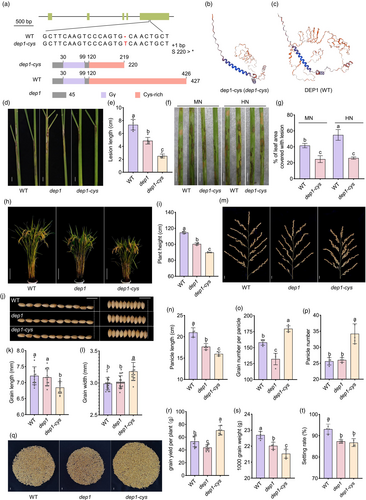
Natural variations in the DEP1 locus have been reported to increase rice yield and balance yield with quality by modifying the C-terminal length of the DEP1 protein (Huang et al., 2009, 2022). However, the influence of DEP1 gene variation on susceptibility to ShB remains unexplored. To address this, we identified rice cultivars harbouring DEP1∆C34 (Beigeng2), dep1-1 (Liaojing326, Guihuahuang, Shendao6, Yanjing23, and Fuhe66), and those with DEP1 (Liaogeng5, Longjing8, Laoguangtou83, Tongxi933, Shuiyuan300, and Kendao12) (Figure 2a). Inoculation with R. solani AG1-IA demonstrated that rice varieties with dep1-1 exhibited enhanced resistance to ShB than those with DEP1 (Figure 2b,c). We proposed that the truncated form of DEP1 in rice could positively regulate resistance to ShB. Sequence analysis of CRISPR/Cas9 mutants revealed the insertion of T between nucleotides 647 and 648 in the DEP1 coding sequence (c.647_648 ins T). This insertion caused a frameshift, advancing the stop codon and resulting in a dep1-cys mutant consisting of 219 amino acids, similar to the naturally occurring truncated DEP1 variant (Figure 3a). In dep1-cys and WT plants, DEP1-associated proteins were dep1-cys and DEP1, respectively (Figure 3b,c). To assess the role of DEP1 in disease resistance, we examined mutants against ShB, bacterial blight, and rice blasts. The inoculation of WT, dep1, and dep1-cys plants with R. solani AG1-IA demonstrated that both dep1 and dep1-cys mutants exhibited enhanced ShB resistance compared with WT, with dep1-cys exhibiting the strongest resistance (Figure 3d,e). WT, dep1, and dep1-cys plants were inoculated with Xanthomonas oryzae pv. oryzae (Xoo) strain PXO99A. The results indicated that the lesion lengths in dep1 and dep1-cys were significantly shorter than those in WT plants, with no significant difference between dep1 and dep1-cys (Figure S2a,b). WT, dep1, and dep1-cys mutant plants were inoculated with the rice blast strain Magnaporthe oryzae Guy11. The results indicated no significant difference in rice blast resistance between dep1 and dep1-cys compared to WT (Figure S2c,d). Since dep1 showed N-independent resistance to ShB, WT and dep1-cys grown under MN and HN was inoculated with R. solani AG1-IA. The results showed that resistance of dep1-cys to ShB is insensitive to N conditions (Figure 3f,g). We then assessed the agronomic traits of dep1 and dep1-cys mutants. Plant type was a crucial agronomic trait influencing yield, revealing that both mutants exhibited reduced plant height compared to WT, with the dwarfing effect more pronounced in dep1-cys (Figure 3h,i). Grain morphology analysis revealed no significant differences in grain length and width between dep1 and WT. However, the dep1-cys grains were shorter than those of both dep1 and WT but were wider (Figure 3j–l). Additionally, the panicle length in dep1 and dep1-cys was significantly shorter than that in WT, with dep1-cys exhibiting an even shorter panicle length compared to dep1 (Figure 3m,n). Although the grain density per panicle of dep1 remained unchanged, it resulted in a significant reduction in the number of grains per panicle, leading to decreased yield (Figure 3m,o). In contrast, dep1-cys exhibited a significantly increased grain density and a higher number of grains per panicle, enhancing the yield compared to WT and dep1 (Figure 3m,o). Furthermore, dep1-cys exhibited a significant increase in panicle number and yield per plant, whereas dep1 did not exhibit significant changes in panicle number but reduce in yield per plant (Figure 3p–r). Both mutants also demonstrated lower 1000-grain weight and setting rates than the WT, with dep1-cys exhibiting a more reduction (Figure 3s,t). These findings indicated that the dep1-cys mutant had advantages in specific yield traits, such as grain number per panicle and panicle count, ultimately promoting yield and ShB resistance.
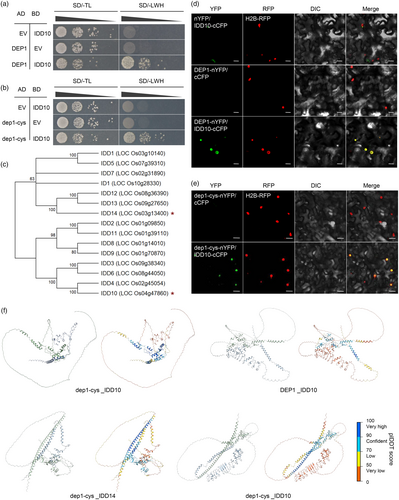
dep1-cys enhanced resistance to ShB by weakening interaction affinity to IDD14
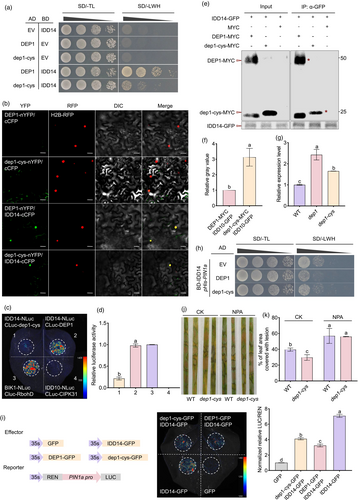
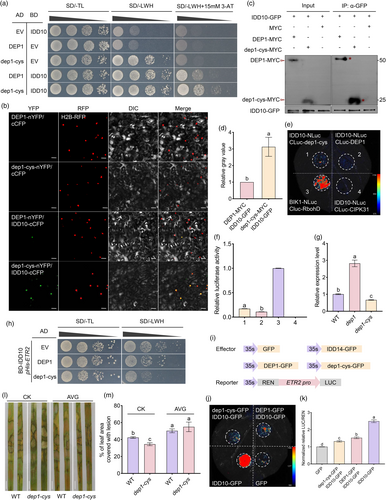
The notable difference in rice resistance to ShB between dep1 and dep1-cys prompted an investigation into the molecular mechanism by which dep1-cys enhances resistance compared to DEP1. Previous studies have shown that IDD14 positively regulates ShB resistance (Figure S3a,b). DEP1 interacts with IDD14 and inhibits its DNA-binding ability, thereby inhibiting the expression of PIN1a (Liu et al., 2021a). To further explore the significance of the interaction between dep1-cys and IDD14 in ShB resistance, the yeast two-hybrid (Y2H) and bimolecular fluorescence complementation (BiFC) assays were conducted. The results demonstrated that dep1-cys interacted with IDD14 in the nucleus (Figure S4a,b). To investigate potential differences in the interaction affinity between DEP1 and dep1-cys with IDD14, a Y2H assay was conducted. The Y2H results indicated that diploid yeast cells harbouring AD-DEP1 and BD-IDD14 exhibited significantly better growth on medium deficient in tryptophan, histidine, and leucine (SD/-LWH) compared to those carrying AD-dep1-cys and BD-IDD14 (Figure 4a). Additionally, BiFC, luciferase complementation assay (LCA), and co-immunoprecipitation (CoIP) assay were performed for further validation. BiFC techniques confirmed that functional YFP was reconstituted more readily when DEP1-nYFP and IDD14-cCFP were co-expressed than when dep1-cys-nYFP and IDD14-cCFP were co-expressed in tobacco (Figure 4b). LCA demonstrated that the interaction affinity between IDD14-NLuc and CLuc-DEP1 was stronger than that between IDD14-NLuc and CLuc-dep1-cys (Figure 4c,d). The CoIP assay results indicated that the protein level of DEP1-MYC was significantly higher than that of dep1-cys-MYC when the protein levels of IDD14-GFP are consistent (Figure 4e,f). In summary, these results indicated that the interaction between IDD14 and DEP1 was stronger than that between IDD14 and dep1-cys. Given that IDD14 activates the expression of PIN1a, our hypothesis suggested that dep1-cys could affect the expression of PIN1a by weakening its interaction with IDD14, thereby inhibiting ShB in dep1-cys. To test this hypothesis, the expression levels of PIN1a were assessed in WT, dep1, and dep1-cys. The results demonstrated significant upregulation of PIN1a expression in both dep1-cys and dep1 compared to the WT, with dep1-cys exhibiting lower expression than dep1 and higher expression than the WT (Figure 4g). Furthermore, DEP1 and dep1-cys were incorporated into the Y1H assay to investigate their influence on the activation of PIN1a by IDD14. The results indicated that the introduction of AD-DEP1 into the yeast significantly inhibited PIN1a activation by BD-IDD14 more than AD-dep1-cys (Figure 4h). Additionally, dual-luciferase (LUC) reporter assays conducted in tobacco leaves revealed that both DEP1 and dep1-cys inhibited the IDD14 activation of PIN1a. However, the inhibitory effect of dep1-cys was significantly weaker than that of DEP1 (Figures 4i and S5). To further validating that whether the activation of PIN1a is the cause of the enhanced resistance in dep1-cys, the experiment involving the treatment with NPA, an inhibitor of polar auxin transport, was conducted. After treatment with NPA, both the WT and dep1-cys were inoculated with R. solani AG1-IA. The results showed that NPA treatment inhibited rice resistance to ShB in WT and dep1-cys (Figure 4j,k). These results suggested that the interaction between dep1-cys and IDD14 was relatively weak, leading to better activation of PIN1a and positive regulation of resistance against ShB in the dep1-cys mutant.
dep1-cys achieves ShB resistance through enhanced interaction affinity to IDD10
dep1-cys exhibited less susceptibility to ShB than dep1, whereas the expression level of PIN1a was higher in dep1 than in dep1-cys, suggesting that dep1-cys may also regulate other signalling pathways. Consequently, the interactions of DEP1 and dep1-cys with other members of the IDD family were investigated to determine their impact on ShB resistance. Y2H experiments were conducted to screen proteins capable of interacting with both DEP1 and dep1-cys within the IDD family, and IDD10 was identified as interactor of DEP1 (Figure 5a,b). IDD10 and IDD14 belong to different subfamilies based on phylogenetic analysis (Figure 5c) and did not interact with each other (Sun et al., 2020). BiFC experiments further confirmed the interaction between IDD10 and both DEP1 and dep1-cys in the nucleus (Figure 5d,e). Further, the complex structures of dep1-cys and dep1 with IDD14 or IDD10 were predicted using AlphaFold3. The predictions suggested that these proteins might form complexes, though the high proportion of disordered regions and moderate confidence scores indicate the dynamic nature of these potential interactions (Figure 5f). Hence, the interaction strength between DEP1 and IDD10, as well as between dep1-cys and IDD10, was assessed using the Y2H assay, revealing that the interaction between IDD10 and DEP1 was significantly weaker than that between IDD10 and dep1-cys (Figure 6a). The interaction between IDD10 and DEP1 was less intense than that between IDD10 and dep1-cys, as confirmed using BiFC, CoIP, and LCA (Figure 6b–f). Previous studies have demonstrated that IDD10 inhibited rice resistance to ShB (Figure S6a,b). IDD10 activated the expression of ETHYLENE RESPONSE2 (ETR2), a negative regulator of ethylene signalling (Li et al., 2024b). RT-qPCR results indicated that the ETR2 expression levels were lower in dep1-cys and higher in dep1 compare to WT (Figure 6g). Furthermore, the expression levels of PIN1a in IDD10 OX and idd10, as well as the expression levels of ETR2 in IDD14 OX and idd14 mutants, were investigated. The results indicated that PIN1a expression was lower in idd10 and IDD10 OX than in WT, whereas ETR2 expression was also lower in idd14 and IDD14 OX than in WT (Figure S7), suggesting that there is no specific regulatory mechanism of IDD10 on PIN1a or IDD14 on ETR2. Also, the expression patterns for IDD10, IDD14, DEP1, PIN1a and ETR2 was performed (Figure S8). Among these, DEP1 exhibited higher expression in the anther, stigma, and panicle. The expression patterns of IDD10 and ETR2 are similar, with both being highly expressed in the root, sheath, and seed. Specifically, the high expression of PIN1a and IDD14 in nodes and collars may be associated with the overexpression of IDD14 activating PIN1a, thereby affecting disease resistance (Sun et al., 2019). Furthermore, after inoculation of WT rice with R. solani AG1-IA, the upregulation of DEP1 coincides with a slight downregulation of both PIN1a and ETR2 expression, which is consistent with the findings of this study that DEP1 interacts with IDD14 or IDD10 to reduce the expression levels of PIN1a and ETR2 (Figure S8). Given that ETR2 exhibits differential expression in dep1-cys and dep1 competitive Y1H experiments was conducted. The result revealed that both DEP1 and dep1-cys effectively suppressed IDD10 activation on pETR2, while the inhibitory effect of dep1-cys was significantly stronger than that of DEP1 (Figure 6h). Transient assays indicated that IDD10 activated ETR2 promoter-driven LUC activity. However, addition of DEP1 and dep1-cys significantly inhibited LUC activity, with dep1-cys demonstrating a stronger inhibitory effect (Figures 6i–k and S9). To evaluate ethylene function in dep1-cys regulating rice ShB resistance, AVG, an inhibitor of ethylene biosynthesis was treated, and the WT and dep1-cys were inoculated with R. solani AG1-IA. The results showed that the AVG treatment inhibited rice ShB resistance in WT and dep1-cys (Figure 6l,m). These findings suggested that dep1-cys exhibited a higher interaction affinity with IDD10 to activate ethylene signalling by which positively regulating rice resistance to ShB.
Discussion
Rice ShB is one of the three major diseases affecting rice and is characterized by its extensive incidence, wide-ranging influence, and rapid spread. The DEP1 gene encodes a G-protein γ-subunit that plays a crucial role in rice growth and development (Chakravorty et al., 2011; Mao et al., 2010). It is essential for regulating various biological processes in rice, including auxin distribution, responses to nitrogen and ABA, and the balance between carbon and nitrogen metabolism (Liu et al., 2021a; Sun et al., 2014; Zhang et al., 2015; Zhao et al., 2019). Research has shown that the DEP1 gene is closely linked to the growth conditions of rice, significantly affecting both yield and quality (Huang et al., 2022; Xu et al., 2016). Since DEP1 was identified as a key pleiotropic gene regulating rice yield, research into its effects on yield and quality has intensified, yielding remarkable achievements. Our previous findings indicated that DEP1 negatively modulates rice resistance to ShB (Liu et al., 2021a). Furthermore, studies have demonstrated that DEP1, also known as qPE9-1, can govern ear length and kernel length while regulating yield (Huang et al., 2009; Zhou et al., 2009). Our study further substantiated that the deletion of the DEP1 gene enhanced rice resistance to ShB and the disease resistance remained stable despite the increase in nitrogen application. However, it also resulted in a decrease in the number of grains per panicle and 1000-grain weight of dep1 plants, leading to a reduction in overall yield.
With the advancement of research on DEP1, the dominant allele at the DEP1 locus was identified. The naturally mutated DEP1 allele, dep1-1, undergoes a base substitution between the 586th and 587th bases, resulting in premature generation of a stop codon and translation of a truncated protein consisting of only 196 amino acids. Studies have shown that the dep1-1 allele influences the panicle morphology of rice and increases production primarily by reducing panicle length and enhancing grain density in the japonica background (Huang et al., 2009; Wang et al., 2009). However, transgenic lines containing the dep1-1 showed reduced yield in the indica background (Zhou et al., 2009). The differential impacts of the dep1-1 gene on rice yield in japonica and indica rice varieties are primarily attributed to variations in the number of primary and secondary branches of the rice panicle. In the japonica background, rice containing dep1-1 is characterized by dense and erect panicles, which are consistent with many high-yielding japonica cultivars (Li et al., 2010; Wen, 2001). Moreover, studies have shown that DEP1 and dep1 can interact with GNA to inhibit the expression of OsCKX2, thereby increasing the content of cytokinin and grain number per panicle (Zhang et al., 2024a). GNA expression levels were higher in NIL-dep1-1 compared to NIL-DEP1, while OsCKX2 was lower, indicating that dep1-1 was superior in terms of number of grains per panicle in the japonica context (Huang et al., 2009; Zhang et al., 2024a). However, no significant difference was observed in panicles number, although the panicles maintained erect in the indica background (Zhou et al., 2009). In the context of japonica and indica rice backgrounds, the mechanism by which dep1-1 causes variation in the number of grains per panicle remains unclear. The dep1-1 gene originates from japonica rice and confers superior yield traits specifically in japonica rice variety (Huang et al., 2009). How to transfer dep1-1 into indica rice background and maintain high yield needs further exploration. DEP1 is capable of interacting with Gα and Gβ subunits to inhibit nitrogen reactions. Under conditions of limited nitrogen application, rice carrying dep1-1 enhances nitrogen uptake and assimilation, thereby increasing yield (Sun et al., 2014). Studies have indicated that WYJ8 harbouring dep1-1 can enhance drought resistance by promoting the expression of ABA and stress-inducing genes, whereas the rice variety WYJ8 (qPE9-1), containing DEP1, negatively regulates drought resistance (Zhang et al., 2015). Additionally, DEP1 can interact with GS3, another G-protein gamma subunit in rice, influencing both yield and quality. The combination of WYJ7-dep1-2 with GGL, vWFC1, and part of vVFC2 domains enhances yield per plant while improving the length-to-width ratio of grains, achieving a dual increase in yield and quality (Huang et al., 2022). Overall, previous studies have demonstrated that DEP1-C-terminated mutants can influence photosynthetic efficiency, nutrient uptake, and distribution in rice, thereby enhancing yield and quality. Simultaneously, DEP1 has been implicated in stress resistance processes, including drought tolerance and disease resistance. In this study, we generated the DEP1 gene knock-out mutant plants (dep1) and truncated DEP1 mutant plants (dep1-cys), validating the individual impacts of dep1-cys on disease resistance and yield. These findings indicate that dep1-cys can enhance yield while providing resistance to both ShB and bacterial blight. Moreover, the disease resistance of dep1-cys did not decrease with the increase of nitrogen application. Notably, dep1-cys specifically enhanced rice resistance to ShB, in contrast to dep1.
Our findings reveal that dep1-cys is a crucial element in the positive modulation of rice yield and disease resistance. In terms of disease resistance, we investigated the mechanism by which dep1-cys enhanced rice resistance to ShB. Both dep1 and dep1-cys exhibited similar functions against bacterial blight, with both mutants exhibiting less susceptibility than WT rice. However, DEP1 and dep1-cys did not regulate resistance to rice blast, indicating a specific regulatory role of dep1-cys in ShB resistance. We determined that dep1-cys influenced the strength of protein interactions and regulated ethylene and auxin signalling pathways. Ethylene is a crucial phytohormone that significantly influences plant growth, development and participates in plant defence responses (Mersmann et al., 2010; Zheng et al., 2024). Ethylene plays a positive regulatory role in enhancing the resistance of ShB and rice blast (Xue et al., 2020; Yang et al., 2017). ETR2, as a negative regulator in ethylene signalling pathway, enhances the susceptibility of rice to ShB (Hua and Meyerowitz, 1998; Li et al., 2024b). Auxin plays an irreplaceable role in plant architecture regulation, root morphogenesis and stress response of rice (Guo et al., 2021; Song et al., 2023; Yamamoto et al., 2007). IDD14 activate expression of auxin polar transporter PIN1a to enhance rice resistance of rice plants to ShB. Also, exogenous auxin treatment promoted rice resistance to ShB (Sun et al., 2019). Our results found that DEP1 and IDD10 exhibited weak interactions in WT rice, with most IDD10 binding to the promoter region of ETR2 to activate its expression. In contrast, DEP1 and IDD14 had strong interactions, resulting in less IDD14 binding to the promoter region of PIN1a, which weakened PIN1a activation. Consequently, ETR2 expression was high, whereas PIN1a expression was low in WT. Conversely, in the dep1-cys mutant, the interaction intensity between dep1-cys and IDD10 was relatively strong, whereas the interaction with IDD14 was weak. As a result, the ETR2 expression was low, and the PIN1a expression was high in the dep1-cys mutant. ETR2 may increase susceptibility to ShB, whereas PIN1a may enhance resistance (Figure 7). In addition, NPA or AVG, the inhibitor of auxin transport or ethylene biosynthesis inhibited ShB resistance of dep1-cys, suggesting that dep1-cys might activate auxin and ethylene signalling to promote rice ShB resistance. In conclusion, dep1-cys demonstrated stronger resistance to ShB than WT by altering its binding affinities to IDD10 and IDD14, thus activating both ethylene and auxin signalling (Figure 7). RT-qPCR results indicated that IDD10 did not regulate PIN1a while IDD14 did not regulate ETR2. The co-expression analysis showed that IDD10, DEP1, and ETR2 or IDD14, DEP1, and PIN1a were co-expressed in some organ or R. solani inoculated tissue, but their expression patterns were not exactly the same, indicating there are the certain correlations between these genes. Among them, the expression patterns of DEP1 may be associated with the function of DEP1 in reproductive development and panicle formation (Huang et al., 2009; Wang et al., 2009; Xu et al., 2016). In vegetative organs, DEP1 showed higher expression in the sheath, node, and collar, which may be related to its role in regulating stem structure, enhancing plant lodging resistance, and possibly in cell wall biosynthesis (Wang et al., 2024). IDD10 and ETR2 are co-expressed in multiple organizations, which is consistent with the finding that IDD10 can activate ETR2 (Li et al., 2024b). Furthermore, the two genes exhibit differential expression in root hair, anther wall, and pollen. IDD10 participates in nitrogen metabolism and cellular responses by activating the transcription of AMT1;2, GDH2 and CIPKs, thereby regulating root responses to ammonium and affecting root growth and function (Xuan et al., 2013a, 2019). This indicates that although IDD10 and ETR2 have similar expression patterns in some organs, they may exert independent functions through different mechanisms in other organs. The high expression of IDD14 in nodes and collars may be related to its multiple functions in plant growth and development. Studies have shown that IDD14 promotes the biosynthesis and transport of auxin, thereby coordinately regulating the morphogenesis of lateral organs and the geotropism response (Cui et al., 2013; Sun et al., 2019). PIN1a is highly expressed in roots, nodes and collars. This may be related to its role in regulating plant structure and defence mechanisms. Furthermore, we utilized AlphaFold3 to further investigate the reasons why IDD10 and IDD14, both members of the IDD family, exhibit opposite interaction affinities when interacting with dep1-cys. The visualization of the pLDDT (predicted Local Distance Difference Test) scores indicates significant differences in the confidence levels of structural predictions across various regions. While the core interaction regions exhibited relatively high confidence, the peripheral regions, particularly the extended loop structures and termini, displayed markedly lower confidence. This variation in confidence suggests that these areas possess inherent flexibility or disorder. Consequently, due to the complexity of the predicted protein interaction interface and the lower confidence in some areas, it is challenging to accurately assess the differences in relative binding affinities between dep1-cys and IDD14 or IDD10 relying solely on structural simulations. The reason why dep1-Cys exhibits opposite interaction affinities with IDD10 and IDD14 has not been fully explained and needs further investigation.
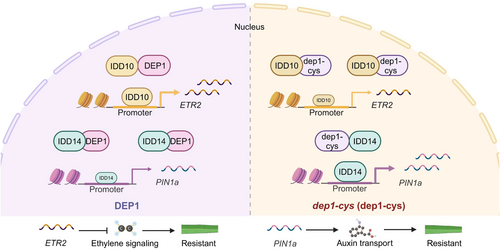
Simultaneously, enhancing rice disease resistance and yield presents a significant challenge in modern agriculture. This study demonstrated that dep1-cys was a crucial factor in the positive regulation of both rice yield and disease resistance, providing valuable genetic resources for breeding rice varieties that achieve a simultaneous increase in yield and disease resistance, with broad applications for improving these traits. Furthermore, the natural variant of dep1 also improved rice panicle traits and resistance to sheath blight among different populations, offering a useful resource for resistant breeding. Also, HN cultivation condition promoted rice susceptibility to ShB; however, DEP1 mutation or truncation blocked HN defect in ShB susceptibility, suggesting NUE and HN-induced ShB susceptibility might be associated with DEP1. Further research aimed at understanding the mechanisms of dep1-cys is expected to yield novel ideas and approaches for genetic improvement of rice.
Materials and methods
Plant materials and growth conditions
This study utilized wild-type rice cultivars (Oryza sativa Dongjin, Hwayoung, and Sasanishiki). Previous studies described IDD10 OX, idd10 (Xuan et al., 2013b), IDD14 OX, idd14 (Liu et al., 2016), DEP1 OX, and dep1 (Liu et al., 2021a). The dep1 and dep1-cys mutants were generated using CRISPR/Cas9 technology (Baige Gene Technology, Jiangsu, China). The rice for yield determination was planted at the Rice Research Institute of Shenyang Agricultural University (123.6° E, 41.8° N), whereas the rice for disease resistance evaluation was grown in pots at Nankai University (117.2° E, 39.1° N). Before transplantation, the temperature was maintained between 24 and 30°C and 70% relative humidity. After transplantation, the rice was grown under natural climatic conditions. Nicotiana benthamiana was cultivated in a plant growth chamber with a 24°C temperature setting and a 10-h light/14-h dark cycle.
Strain culture conditions and disease inoculation methods
Rhizoctonia solani AG1-IA was cultured on potato dextrose agar (PDA) at 28°C. Sterilized veneers (3 mm × 7 mm) were placed on the edge of the PDA medium, and R. solani AG1-IA sclerotia were positioned in the centre and cultured at 28°C. Rice plants with uniform stalk thickness and in a comparable booting stage were selected for inoculation following vaccination methods (Zuo et al., 2013). The activation and inoculation of Xanthomonas oryzae pv.oryzae (Xoo) strain PXO99A was consistent with that reported in a previous study (Ke et al., 2017). Two-month-old rice plants were inoculated with PXO99A using the leaf tip-clipping method (Yang and Bogdanove, 2013), where approximately 1 cm of each leaf tip was clipped with sterilized, bacteria-dipped scissors and re-dipped in bacterial solution for each leaf. Magnaporthe oryzae strain Guy11 was used to inoculate rice under spore activation and production conditions, as described previously (Zhai et al., 2019). Isolated leaves were inoculated by applying a spore suspension to the wounds (Zhang et al., 2014).
Compound treatment
Six-week-old rice plants were selected, and several green leaves were clipped for the inhibitor treatment. A sprayer was used to evenly apply ddH2O, a 0.5 μM solution of naphthylphthalamic acid (NPA), a polar auxin transport inhibitor or a 0.2 μM solution of 2-aminoethoxyvinyl glycine (AVG), an ethylene biosynthesis inhibitor to the leaf surfaces. The treated leaves were then placed on petri dishes, maintaining a moist environment throughout the period. Inoculation of detached leaves was conducted 24 h after treatment. Nitrogen treatments were administered according to the standards of medium nitrogen (MN 150 kg/ha) and high nitrogen (HN 300 kg/ha), with sheath inoculation of R. solani AG1-IA performed 7 days after the nitrogen treatment. (Liu et al., 2021b).
Yeast two-hybrid (Y2H)
The target genes DEP1 and dep1-cys were cloned into the pGAD424 vector at EcoR I (Takara, Dalian, China) and Sma I restriction sites. The IDD10 gene was cloned into the pGBT9 vector at the EcoR I and Hind III sites, whereas IDD14 was inserted into pGBT9 at the EcoR I and Sma I sites. The constructed plasmids were transfected into yeast strain PJ69-4A using the lithium acetate method (Clontech, http://www.clontech.com/). The transformed yeast was grown at 28°C on selective medium deficient in leucine and tryptophan (SD/-Leu/-Trp) to identify the successful transformants. The yeast cultures were diluted to four gradients (OD = 1, OD = 10−1, OD = 10−2, and OD = 10−3) and 10 μL samples were inoculated onto medium lacking leucine, tryptophan, and histidine (SD/-Leu/-Trp/-His) at 28°C for 2–3 days to confirm the protein interaction. To suppress self-activation, 3-amino-1,2,4-triazole (3-AT), a histidine synthesis inhibitor, was used. The primers used are listed in Table S1.
Bimolecular fluorescence complementation (BiFC)
The Gateway cloning technique was adopted to fuse DEP1 and dep1-cys with N-terminal YFP sequences in the pXNGW vector, as well as IDD10 and IDD14 with C-terminal CFP sequences in the pXCGW vector. The constructed expression plasmids were transformed into Agrobacterium strain GV3101, and successful clones were screened on a medium containing Spectinomycin and Rifampicin. Following a published protocol, the Agrobacterium solution was proportionally mixed and infiltrated into N. benthamiana leaves for transient expression (Taylor et al., 2012). Leaves near the infiltration site were observed 48 h after infiltration using a laser scanning confocal microscope (Fluoview FV 1000, http://www.olympus-global.com/). Primers used in this study are listed in Table S1.
Co-immunoprecipitation (CoIP) and western blot (WB) assays
IDD10 and IDD14 were cloned into the pGD3GGM vector, whereas DEP1 and dep1-cys were integrated into the PGD6MYC vector to construct IDD10-GFP, IDD14-GFP, DEP1-MYC, and dep1-cys-MYC expression plasmids. The resulting vectors were transformed into the Agrobacterium strain GV3101 and infiltrated into tobacco leaves. Sixty hours after tobacco infiltration, total protein was extracted for co-immunoprecipitation and WB analysis, following a modified protocol based on published literature (Masters, 2004). Key modifications included protein extraction using SDS without bromophenol blue. Protein concentration was quantified using the Bradford Protein Assay Kit (Sangon, Shanghai, China) via spectrophotometry, with a final sample concentration of 90 ng. The primers used are listed in Table S1.
Luciferase complementation assay (LCA)
Infusion cloning technology was employed to construct a fusion protein expression vector linking DEP1, dep1-cys, and CIPK31 into pCAMBIA1300-CLuc, while IDD10 and IDD14 were inserted into pCAMBIA1300-NLuc. Following the experimental protocol, tobacco was infiltrated, and luciferase activity was subsequently monitored (Zhou et al., 2018). BIK1-NLuc and CLuc-RbohD served as positive controls (Zhou et al., 2018), whereas the CIPK31-IDD10 pair was used as the negative control (Li et al., 2024b). The primers used are listed in Table S1.
RNA extraction and RT-qPCR
Three-week-old rice seedlings were ground in liquid nitrogen, and total RNA was extracted using the RNAiso Plus reagent (Takara, Dalian, China). Genomic DNA was removed using a PrimeScript™ RT Reagent Kit with gDNA Eraser (Takara, Dalian, China). Quantitative real-time PCR was conducted using ChamQ Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China) according to the manufacturer's instructions. Ubiquitin was used as an internal control gene, and its expression levels were calculated based on established methods (Xuan et al., 2013b). The primers used are listed in Table S1.
Yeast one-hybrid assay
The fragment containing the binding motifs was cloned from the PIN1a or ETR2 promoter region using PCR and inserted into the pHISi vector (Li et al., 2024b; Sun et al., 2019). The constructed vector was then linearized with the restriction enzyme Xho I and transformed into the yeast strain YM4271. Transformed monoclonal clones were selected using histidine-deficient medium (SD/-His). IDD14 or IDD10 was combined with either the pGAD424 empty vector, pGAD424-DEP1, or pGAD424-dep1-cys and introduced into the monoclonal strains. Yeast strain growth was observed on SD/-Leu/-Trp/-His and SD/-Leu/-Trp media (Gu et al., 2022).
Dual luciferase reporter assay
Using infusion cloning, the promoters of PIN1a and ETR2 were inserted into the pGreenII0800-LUC vector and transformed into Agrobacterium tumefaciens GV3101 (pSoup) competent cells. Transformed bacterial colonies were selected and cultured for 12–14 h in LB liquid medium containing Rifampicin and Kanamycin. The Agrobacterium suspension was then prepared and infiltrated into tobacco leaves. To maintain experimental consistency, the bacterial suspensions for the control and experimental groups were infiltrated into different parts of the same leaf to ensure a similar growth background. Luciferase activity was observed 48–60 h after inoculation. The primers used are listed in Table S1.
Co-expression analysis
Anatomical expression data about IDD10, IDD14, PIN1a, ETR2 and DEP1 was retrieved from ROADv2 database (https://roadv2.khu.ac.kr/). R. solani responsive transcriptomic data refer to published articles (Yuan et al., 2020). The co-expression of genes was shown as a heatmap which was constructed using MeV software. The colour scheme represents the strength of normalized log2 intensity values. The expression data is listed in Tables S2 and S3.
Statistical analysis
ImageJ (imagej.nih.gov/ij/) was used to calculate the incidence rates, while Prism 8.0 (GraphPad, San Diego, CA, USA) was used for statistical analysis and graphical representation. The data were presented as mean ± SE. One-way analysis of variance (ANOVA) was performed to compare the mean values across treatment groups, followed by a Bonferroni multiple comparison test (Brady et al., 2015). Student's t-test was used for comparisons between two groups, with significance set at P < 0.05.
Author contributions
QX and YHX designed and supervised the experiments. HYZ, TGZ and ZL conducted all experiments and validations. QX, HYZ and LZ preserve experimental plants. HYZ and TGZ analysed data. QX, HYZ and YHX wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This work was supported by the National Science Foundation of China (32372482). We appreciate very much for Professor Sun Jian of Shenyang Agricultural University and Yazhouwan National Laboratory for providing us with rice varieties selected for population genetics experiments. We extend our gratitude to Professor Zhou Jianmin from the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences for generously providing plasmids essential for LCA research, including pCAMBIA1300-NLuc, pCAMBIA1300-CLuc, BIK1-NLuc and CLuc- RbohD.
Conflict of interest
The authors declare that they have no known conflicts of interest in this article.
Open Research
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




