Lysine deacetylase TaSRT1 mediates wheat drought tolerance by deacetylating TaDT-A to reduce its protein stability and transcriptional activity
The author(s) responsible for the distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors is: Zhaorong Hu ([email protected]).
Summary
Drought is one of the major environmental stresses limiting crop growth and yield. Epigenetic regulations play crucial roles in plant adaptation to environmental changes, whereas the epigenetic mechanism of drought resistance in crops remains largely elusive. Here, we report that the nicotinamide adenine dinucleotide (NAD+)-dependent deacetylase TaSRT1 negatively regulates drought tolerance in wheat. Compared with the wild type, the tasrt1 mutant had higher relative water contents, along with a smaller stomatal aperture and improved water use efficiency under drought conditions, whereas TaSRT1 overexpression plants exhibited opposite phenotypes. TaSRT1 directly interacted with the drought-resistant pivotal factor TaDT-A to regulate its protein stability and transcriptional activity through lysine deacetylation. Furthermore, the key lysine residue of TaDT-A was identified as a deacetylation/acetylation site that plays an important role in regulating its stability. In addition, genetic analysis indicated TaDT-A functions downstream of TaSRT1 to modulate drought resistance. These findings uncover how the functional interplay between epigenetic regulator and transcription factors regulates drought resistance in plants, and illustrate a mechanism by which lysine deacetylase affects gene transcription via influencing non-histone protein acetylation and regulating their function.
Introduction
Wheat is one of the most important and widely cultivated cereals that guarantee global food security, supporting 60% of the world's population for their daily calorific and protein needs (Tadesse et al., 2017). While wheat is highly susceptible to water scarcity. Studies have shown that a 40% water reduction can cause a 20.6% yield reduction in wheat, a threat further exacerbated due to climatic change and global warming (Daryanto et al., 2016; Hickey et al., 2019; Lesk et al., 2016; Sallam et al., 2019). Therefore, it is of great significance to dig out excellent drought resistance genes and speed up the breeding process of drought-resistant wheat varieties using modern molecular breeding technology (Bapela et al., 2022).
Despite the complexity of drought resistance, tremendous progress has been made in understanding the mechanisms of drought resistance in plants (Fang and Xiong, 2015). Drought response mechanisms are involved in morph-physiological, biochemical, cellular and molecular processes in plants (Ilyas et al., 2021). These processes include improvement in the root system, leaf structure, osmotic adjustment, relative water content and stomata regulation (Abdelraheem et al., 2019; Iqbal et al., 2013; Moustakas et al., 2011; Urban et al., 2017; Xie et al., 2012). In response to drought stress, stomata closure is the first step to reduce transpiration in most plants. There is an opposite relationship between drought resistance and stomatal conductance, stomatal closure decreases transpiration which improves drought resistance, while stomatal opening increases transpiration and subsequently weakens plant drought resistance (Clauw et al., 2015).
Transcription factors (TFs) coordinate complex signalling pathways and networks that trigger multiple biochemical and developmental pathways to improve plant response to drought stress (Gong et al., 2020; Gupta et al., 2020; Hu and Xiong, 2014; Zhu, 2016). Precise control of TFs function is essential for the organization and function of biological systems. Among different regulatory processes, post-translational modifications (PTMs) such as phosphorylation, acetylation and ubiquitination are crucial regulators of protein function, and specific patterns of PTMs characterize plant responses to various environmental stresses (Hashiguchi and Komatsu, 2017; Kim et al., 2015; Narita et al., 2019). As a major epigenetic regulatory mechanism, lysine acetylation of histone has been extensively studied in the context of transcriptional regulation (Buszewicz et al., 2016; Chen et al., 2018, 2019; Gao et al., 2015; Gu et al., 2017; Shen et al., 2015; Song et al., 2022). Recently, increasing studies have indicated that non-histone proteins are frequently acetylated and constitute a major portion of the acetylome in mammalian cells, which is involved in key cellular processes, such as gene transcription, cell cycle, DNA damage repair, cellular signalling, protein folding, RNA processing and stability, autophagy and metabolism (Downey, 2021; Narita et al., 2019). In plants, it has been reported that histone deacetylase HDA6 can interact with and deacetylate BIN2 to repress its kinase activity, thereby enhancing brassinosteroid signalling (Hao et al., 2016). WRKY53 is post-translationally modified by lysine acetylation at multiple sites, some of which are deacetylated by HDA9, resulting in the inhibition of WRKY53 transcription activity in Arabidopsis (Zheng et al., 2020). Recently, it was reported that the histone deacetylase OsHDA716 represses rice cold tolerance by interacting with and deacetylating the transcription factor OsbZIP46 (Sun et al., 2024). However, studies on the functions and mechanisms of non-histone protein acetylation in plants are limited. However, whether and how the non-histone protein acetylation regulatory networks involved in wheat drought resistance are largely unknown.
In this study, we demonstrate that the loss-of-function mutants of a SIR2 lysine deacetylase TaSRT1 exhibit drought-resistant phenotype by influencing stomatal aperture, while TaSRT1-OE lines are sensitive to drought stress in wheat. We identified a TaSRT1-interacting protein TaDT-A, which we recently reported contributes to drought resistance as a vital positive regulator in wheat due to its genetic variation (Liu et al., 2024). We demonstrate that TaDT-A is a direct target of the TaSRT1 in vivo and in vitro, and identify the deacetylated lysine residue. Deacetylation of the residue reduces the stability of TaDT-A by promoting ubiquitination-mediated degradation, thus affecting drought resistance. Our results reveal an important mechanism of plant response to drought stress, illustrating how the interaction between lysine deacetylase and transcription factor regulates drought resistance in wheat, which provides important genetic resources for breeding drought-tolerant varieties.
Results
TaSRT1 is a putative histone deacetylase in wheat
In a previous whole-transcriptome analyses of drought stress on wheat, the transcript of TraesCS2D02G075800 was significantly repressed by drought treatment of 4 h compared to 0 h (Figure S1a). Sequence alignment analysis revealed that it encodes a SIR2 histone deacetylase, a putative ortholog of SRT1 in Arabidopsis, maize, rice, barley and Brachypodium, thus named as TaSRT1 (Figure S1b). The SIR2 histone deacetylase domain was predicted in all three TaSRT1 homoeologs, and this domain appears to be conserved in different species, suggesting TaSRT1 plays a fundamental role in the lysine deacetylation catalytic process (Figure S1c–e).
The TaSRT1 is expressed in roots, stem, leaves, rachis and glume (Figure 1a). We found that the expression of three homoeologs of TaSRT1 in leaves was decreased first and then increased, and finally was significantly lower than 0 h under drought stress. Intriguingly, the expression levels of both TaSRT1-A and TaSRT1-B were consistently lower than that of TaSRT1-D under drought stress, so we selected TaSRT1-D and referred to it as TaSRT1 for the subsequent experiments (Figure 1b). To determine the subcellular localization of TaSRT1, we transfected wheat protoplasts and Nicotiana benthamiana with a construct encoding a TaSRT1-GFP fusion protein, respectively. The obtained data indicated that TaSRT1 is mainly localized in the nucleus, suggesting that TaSRT1 is a nuclear protein (Figure 1c and Figure S2a). To test whether TaSRT1 has lysine deacetylase activity, we produced recombinant TaSRT1-GFP fusion protein from Nicotiana benthamiana, together with GFP alone, using Hela extract as a positive control. As shown in Figure 1d, TaSRT1-GFP showed deacetylase activity (Figure 1d). These results suggest that TaSRT1 has deacetylase activity and responds to drought stress in wheat.
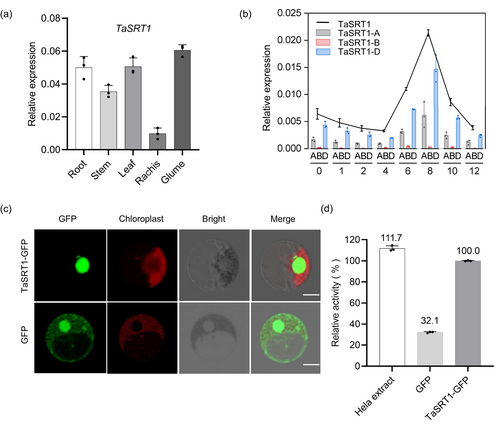
TaSRT1 functions as a negative regulator of drought resistance in wheat
To determine the functions of TaSRT1 in the regulation of wheat drought resistance, we generated two independent tasrt1 knockout (KO) mutant lines, SKO3 and SKO5, using the CRISPR/Cas9 system, for our investigations (Figure S2b). Under normal conditions, no obvious phenotypic change or developmental abnormalities are observed between KO lines and wild type (Figure 2a). To assess the performance of the tasrt1 mutants under water-deficient conditions, 3-week-old seedlings were exposed to drought stress by withholding water and then re-watered. Drought stress caused by water deficiency led to difficulties in restoring wheat growth to normal levels, with mutants exhibiting superior growth compared to wild types (Figure 2a). The shoot fresh weights and relative water contents were significantly higher than those of WT (Figure 2b,c), and the dry weight also showed a slight increase (Figure S2d,e), indicating that the mutants possess stronger drought resistance through enhanced leaf moisture retention.
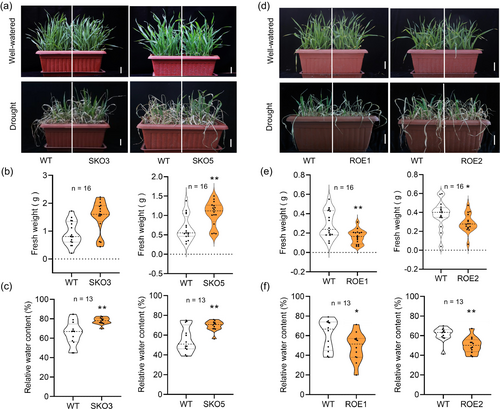
To further validate the functions of TaSRT1 in drought resistance, we also generated transgenic wheat plants overexpressing TaSRT1 (TaSRT1-OE). We selected two TaSRT1-OE lines (ROE1 and ROE2) with significantly elevated expression of TaSRT1 for further analysis (Figure S2c). Consistent with the above finding, there was no obvious phenotypic change or developmental abnormalities observed between TaSRT1 OE lines and wild type under normal conditions (Figure 2d). When subjected to drought stress, in contrast to the wild type, both ROE1 and ROE2 lines exhibited a hypersensitive phenotype (Figure 2d). Accordingly, the analysis under drought stress conditions showed that the TaSRT1-OE lines had a significant reduction in shoot fresh weight, dry weight and relative water contents compared to the wild-type plants (Figure 2e,f; Figure S2f,g). Survival rates (SR) were scored after 7 d recovery, and the tasrt1 mutants exhibited a higher SR than the wild type. Meanwhile, in contrast to the wild type, both ROE1 and ROE2 lines exhibited a hypersensitive phenotype with lower SR (Figure S2h–k). Collectively, these results indicate that TaSRT1 acts as a negative regulator of drought resistance in wheat.
TaSRT1 affects drought tolerance of wheat via regulating stomatal aperture
To further investigate how TaSRT1 modulates drought resistance in wheat, the water loss of detached leaves was examined in different transgenic lines. Results indicated that both ROE1 and ROE2 had faster water loss than those in wild-type plants (Figure 3b), while water loss in KO lines was significantly slower than that in wild-type plants (Figure 3a). In addition, an infrared camera was used to determine the transpiration levels of seedlings by measuring the leaf temperature, with higher temperatures, indicating lower levels of transpiration. There was no significant difference in leaf temperature between mutants and wild-type plants under normal conditions (Figure 3c–e). However, leaf temperatures were significantly higher in SKO-3 and SKO-5 than in wild-type plants under drought stress conditions (Figure 3c–e), suggesting that tasrt1 mutants displayed lower transpiration levels and less water loss than wild-type plants. Similar to the KO lines, under normal growth conditions, there was no significant difference in leaf temperature between both OE lines and wild-type plants (Figure 3c–e). After drought treatment, the leaf temperature of both OE lines was lower than that of the wild type (Figure 3c–e).
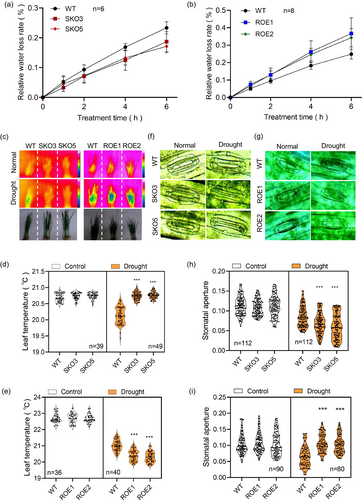
Furthermore, a stomatal aperture assay was performed to validate the function of TaSRT1 in drought-induced stomatal closure. The KO lines exhibited higher stomatal closure than that of wild-type plants (Figure 3f,h,i). Conversely, the OE plants exhibited lower stomatal closure than those of wild-type plants (Figure 3g,h,i). We also examined the stomatal density and root phenotypes of the tasrt1 mutants, TaSRT1-OE lines and wild-type plants, and there was no substantial difference in these traits between different transgenic lines and wild type (Figure S3). Together, these results indicated that TaSRT1 is mainly involved in modulating stomatal closure under drought stress of wheat.
TaSRT1 physically interacts with the transcription factor TaDT-A
To explore the regulatory mechanisms of TaSRT1 in drought resistance, we performed yeast-two-hybrid screening and identified a WRKY-like transcription factor TaDT-A (Liu et al., 2024) as a candidate protein that interacts with TaSRT1 (Data Set S1). We corroborated this TaSRT1-TaDT-A interaction using LCI assays, where the LUC activity signal was produced after TaSRT1-cLUC and TaDT-A-nLUC had been co-infiltrated into Nicotiana benthamiana leaves (Figure 4a). Furthermore, we performed pull-down assays and observed TaSRT1-His can directly interact with TaDT-A-GST (Figure 4b). We also generated transgenic tobacco plants expressing TaSRT1-GFP and TaDT-A-MYC plants to perform coimmunoprecipitation (Co-IP), which showed that TaSRT1-GFP, but not GFP, can interact with TaDT-A-MYC in plants (Figure 4c). These results indicated that TaSRT1 and TaDT-A interacted in vivo. Moreover, to investigate the subcellular locations of TaSRT1-TaDT-A interaction inside plant cells, we conducted BiFC assays. Co-expression of cYFP-TaSRT1 and nYFP-TaDT-A in the pavement cells of Nicotiana benthamiana yielded strong green fluorescence in the nucleus; however, no fluorescence signal was detected in the pavement cells transfected with control constructs (Figure 4d). Collectively, these results demonstrate the TaSRT1–TaDT-A interaction in vitro and in vivo, suggesting that TaSRT1 might participate in the TaDT-A pathway to regulate drought tolerance in wheat.
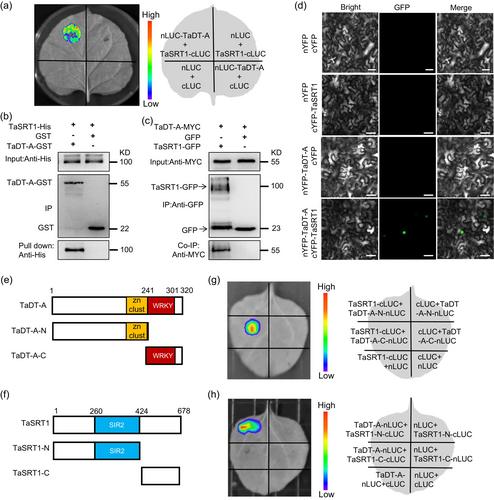
To determine the TaSRT1 and TaDT-A interaction region, we separated both TaSRT1 and TaDT-A into N-terminal (N ter), and C-terminal (C ter) regions (Figure 4e). We used LCI experiments to identify the interaction region of TaSRT1 and TaDT-A, which revealed that the C-terminal of TaDT-A interacted with TaSRT1, and the N-terminal of TaSRT1 interacted with TaDT-A (Figure 4e–h). Our results revealed that both full-length and the C-terminal fragment of TaDT-A interacted with the SIR2 domain of TaSRT1, whereas the N-terminal part of TaDT-A was unable to interact with this domain of TaSRT1 (Figure 4e–h). These results suggested that the C-terminus of TaDT-A could be a target for the lysine deacetylase activity of TaSRT1.
TaSRT1 reduces the acetylation level of TaDT-A
In addition to histones, many proteins, including transcription factors, also show lysine acetylation that modulates their function (Shen et al., 2015). The above findings that TaSRT1 physically interacts with TaDT-A, promoted us to investigate whether TaDT-A is a substrate for the deacetylase activity of TaSRT1. To address this possibility, we first expressed the TaDT-A-MYC in Nicotiana benthamiana, and compared the acetylation of TaDT-A either in the absence or presence of TaSRT1. After immunoprecipitation, the acetylation of TaDT-A was monitored with anti-acetyl antibodies. The results indicated that both in PEG and drought stress treatments, the acetylation level of TaDT-A was elevated compared with normal conditions. Intriguingly, in the presence of TaSRT1, the acetylation level of TaDT-A was reduced both in normal and drought treatment conditions (Figure 5a). Furthermore, in wheat protoplast tests, the acetylation level of TaDT-A protein in wild type was lower than that in tasrt1 mutants, but higher than that of TaSRT1-OE lines (Figure 5b,c). These results demonstrate that the acetylation level of TaDT-A is regulated by TaSRT1.
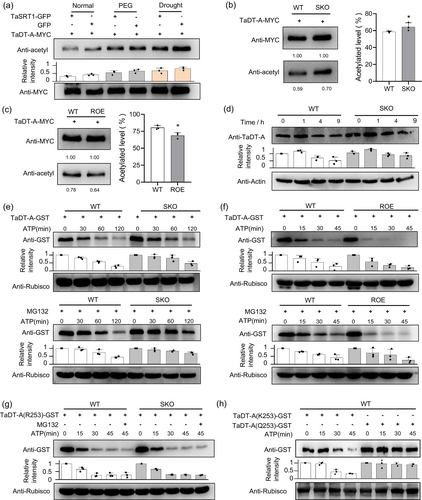
TaSRT1 promotes ubiquitination-dependent degradation of TaDT-A
A recent study showed that TaDT-A positively regulates drought resistance in wheat (Liu et al., 2024). We examined whether the abundance of TaDT-A is regulated by drought stress. The results demonstrated that under normal conditions, the TaDT-A protein abundance was comparable at each time point (Figure S4a); however, the TaDT-A protein increased rapidly within 1 h of drought treatment and decreased with the extension of drought treatment (Figure 5d). We then detected TaDT-A protein in tasrt1 mutants and found that with the extension of drought stress, the variation trend of TaDT-A protein abundance in the mutants was similar to that in wild type, but the abundance of TaDT-A protein in the mutants was higher than that in wild type at each time point (Figure 5d). Moreover, we co-transferred the vectors expressing TaSRT1-GFP and TaDT-A-MYC into tobacco and then detected the abundance of TaDT-A protein. The result indicated that TaDT-A protein level was increased without TaSRT1 after 1 h of drought treatment, whereas it was decreased when TaSRT1 was present (Figure S4b). These findings indicate that TaDT-A protein levels are induced by drought stress, and the presence of TaSRT1 affects the abundance of TaDT-A protein.
In mammals, it is reported that for some proteins, the deacetylation of specific lysine residues is able to facilitate ubiquitination of the same residues, thereby promoting protein degradation (Simonsson et al., 2005). The results described above suggest a mechanism for the lysine deacetylase TaSRT1 in the protein stability of TaDT-A. We next investigated if TaDT-A itself is regulated through ubiquitination. We set up a cell-free degradation assay using recombined GST-TaDT-A purified from E. coli. The purified GST-tagged TaDT-A was incubated with crude proteins of wild-type seedlings. Following incubation at room temperature for the indicated time, the reaction was stopped and TaDT-A levels were examined by protein gel blot analysis. As shown in Figure 5e, TaDT-A was rapidly degraded in wild types (Figure 5e). Treatment with MG132, a 26S proteasome inhibitor, significantly repressed the degradation of TaDT-A (Figure 5e), indicating that TaDT-A was degraded through the 26S proteasome.
To dissect the biological significance of the deacetylation of TaDT-A by TaSRT1, we further investigated whether TaSRT1 promotes ubiquitination-mediated degradation of TaDT-A. Our results indicated that TaDT-A is degraded over time when incubated with the wild-type crude proteins, and importantly, the degradation rate of TaDT-A incubated in the tasrt1 mutants was slower than in wild type (Figure 5e). In addition, when MG132 was present, the degradation rate of TaDT-A incubated with both crude proteins was significantly slowed down, and the degradation rate of TaDT-A in wild type supplemented with MG132 was gradually consistent with that in mutant without MG132 (Figure 5e). We further took another cell-free degradation assay using recombined TaDT-A-GST incubated with TaSRT1-OE line crude proteins. We found that the degradation rate of TaDT-A incubated with the TaSRT1-OE line was faster than that of the wild type (Figure 5f). These results suggest that the degradation of TaDT-A is regulated by TaSRT1. Taken together, these data indicate that TaDT-A is a substrate for endogenous TaSRT1 and that TaSRT1-mediated deacetylation affects the stability of TaDT-A.
Lys253 is a key deacetylation site in TaDT-A
According to the above results, the SIR2 domain of TaSRT1 interacted with the WRKY domain of TaDT-A. In order to explore the specific deacetylation sites of TaDT-A, we used mass spectrometry combined with web-based tools to predict the potential acetylation site within the WRKY domain of TaDT-A. In total, three possible acetylation sites (Lys253, Lys256, Lys266) were annotated (Figure S4c–e; hereafter we used K253, K256, and K266 to illustrate these amino acids, respectively).
To identify the exact residues responsible for deacetylation, we introduced amino acid substitutions to replace the predicted deacetylation Lys residues in the WRKY domain of TaDT-A with arginine (Arg; R for short). Then we validated these three sites through cell-free experiments. We first mutated amino acid K at positions 253, 256 and 266 of TaDT-A to R, sequentially (hereafter we used R253, R256, and R266 to illustrate these mutant amino acids, respectively), and then incubated with crude protein extracts of wild type and tasrt1 mutant, respectively, to test their degradation rate by protein gel blot analysis. The result showed that the degradation rate of R256 and R266 incubated with tasrt1 mutant protein was lower than that of the wild type, and the changing trend of their protein abundance was consistent with that of the unmutated TaDT-A (Figure S4f,g). However, the degradation rate of R253 incubated with wild type was decreased and comparable with that of the tasrt1 mutant, and the addition of MG132 could not inhibit the degradation of R253 protein (Figure 5g), indicating that K253 mutation decelerated TaDT-A protein degradation. We change the K253 to Q253, which consistently maintains a high acetylation level in TaDT-A, to assess their degradation rates through cell-free assays (Figure 5h). The results demonstrated that the degradation rate of Q253 was slower compared to the unmutated K253, indicating that the acetylated TaDT-A exhibits enhanced stability. Together, these results support the notion that K253 is a major residue in TaSRT1 responsible for deacetylation of TaDT-A.
TaSRT1 inhibits the activation of downstream genes regulated by TaDT-A
Our recent study has shown that under drought conditions, TaDT-A can mediate the autophagic pathway by activating the expression of downstream genes TaATG8 and TaATG18, thereby enhancing drought resistance in wheat (Liu et al., 2024). In order to examine whether the interaction between TaSRT1 and TaDT-A affects the expression of TaDT-A downstream genes, we assessed the relative expression of TaATG8 and TaATG18 in tasrt1 mutants and TaSRT1 overexpression lines under drought stress. The expression of TaATG8 and TaATG18 was upregulated in SKO3 and SKO5 but downregulated in ROE1 and ROE2 lines compared to the wild type (Figure 6a,b). Furthermore, we conducted transcriptional activation assays in tobacco. The results showed that TaSRT1 could inhibit the transcriptional activation activity of TaDT-A to downstream genes TaATG8 and TaATG18. When NAM (Nicotinamide), a derivative of vitamin B3 and belongs to the SIR2 class of HDAC deacetylase inhibitors, was added, the effect of TaSRT1 was inhibited, the transcriptional activity of TaDT-A was restored (Figure 6c–e). These results illustrate that TaSRT1 could regulate the drought resistance of wheat by inhibiting the effect of TaDT-A on downstream genes.
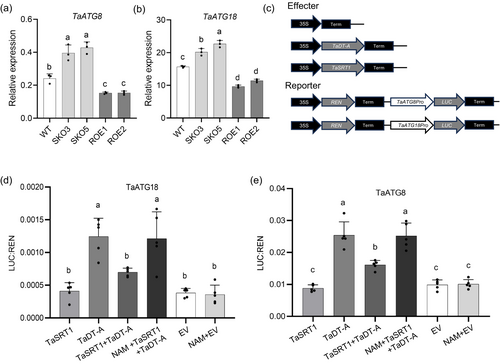
Genetic interaction of TaSRT1 with TaDT-A
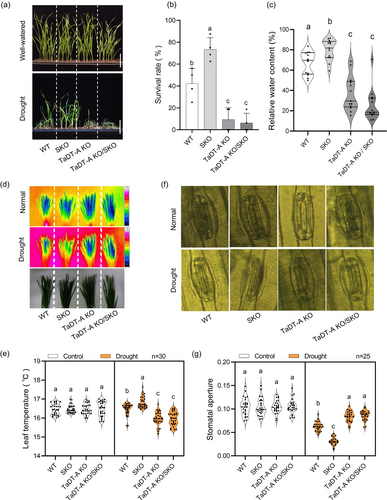
We next examined the genetic interaction between TaSRT1 and TaDT-A. For this purpose, we crossed a tasrt1 mutant line (SKO) to a tadt-a mutant line (TaDT-A KO) and identified TaDT-A KO/SKO double mutant plants (Figure S5a). The growth of WT, SKO, TaDT-A KO and TaDT-A KO/SKO double mutant plants showed no significant differences under normal conditions. However, both TaDT-A KO and the double mutant demonstrated extreme sensitivity to drought stress, in contrast to SKO, which exhibited enhanced drought resistance (Figure 7a). Additionally, the survival rates (SR) of these four materials after rehydration from drought stress were assessed, revealing that the SR of the double mutant and TaDT-A KO were similar but significantly lower than those of WT and the mutant of TaSRT1 (SKO) (Figure 7b). Under drought stress, the TaDT-A KO/SKO double mutant plants displayed the same decreased relative water content and reduced biomass, including both fresh and dry weight, as observed in TaDT-A KO plants (Figure 7c, Figure S5b,c). These phenotypic similarities suggest that TaDT-A operates downstream of TaSRT1 in response to drought stress.
In addition, we tested the leaf temperature and stomatal aperture of TaDT-A KO/SKO lines. Similar to TaDT-A KO lines, the leaf temperature of TaDT-A KO/SKO lines was significantly lower than wild type and SKO lines, and the stomatal aperture was significantly greater than wild type and SKO lines under drought stress (Figure 7d–g). However, there was no significant difference in leaf temperature and stomatal aperture between wild type, SKO, TaDT-A KO and TaDT-A KO/SKO lines under normal conditions (Figure 7d–g). Collectively, these results indicate that TaDT-A functions downstream of TaSRT1 and modulates drought resistance, suggesting that TaSRT1 most likely functions in a common genetic pathway and acts upstream of TaDT-A to response to drought stress.
Mutation of TaSRT1 holds the potential for improving drought resistance
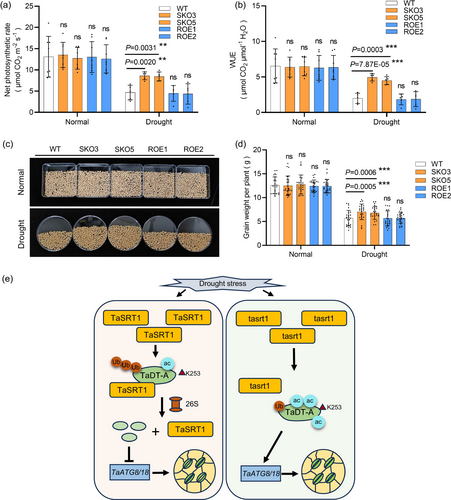
To assess whether decreased stomatal aperture could be translated to an increased grain yield of the TaSRT1 knockout lines under drought stress, we carried out field trials with the tasrt1 mutants and wild-type plants under water-limited and well-water conditions. We first measured the photosynthetic capacity of wheat and found that the net photosynthetic rate, transpiration rate and WUE of the mutants under normal growth conditions were not substantially different from that of wild type (Figure 8a,b; Figure S6). However, under drought stress treatment, the net photosynthetic rate and WUE of the tasrt1 mutants were significantly higher than those of wild type, but the transpiration rate was slightly lower than wild-type plants (Figure 8a,b; Figure S6). Meanwhile, the agronomic traits of the field during the harvest period were measured. Under normal conditions, the spike length, spikelet number and seeds per spike of the tasrt1 mutants were comparable to the wild type, but under drought stress, tasrt1 mutants produced larger panicles with a longer spike, higher seed number per spike, and higher spikelet number than wild type (Figures S7a–c, S8a and S9a,c–e). While the seed width, seed length and plant height were similar between tasrt1 mutants and wild-type plants under both conditions (Figures S7e,f,h, S8b–d and S9b,h–j). At the same time, we measured grain weight per plant and per panicle, and found that after drought treatment, the tasrt1 mutants had higher grain weight per plant (increased by 34.3% and 30.2%, respectively) and grain weight per panicle (increased by 15.2% and 14.8%, respectively) than wild type (Figure 8c,d; Figures S7d and S9f,g), but no substantial change in thousand seeds weight (Figures S7g and S9k). There were no differences in grain weight per panicle, grain weight per plant and thousand seeds weight under normal conditions (Figure 8c,d; Figures S7d,g and S9f,g,k). Two years of field data analysis showed similar results that the tasrt1 mutants performed much better than wild-type plants at the harvest stage under water-limited conditions (Figure S9), indicating enhanced drought resistance in tasrt1 mutants. The above data indicated that mutation of TaSRT1 in wheat confers a yield advantage under water deficit, without a growth and development penalty on wheat under well-watered conditions.
Discussion
Drought stress is the most prevalent environmental factor limiting crop production and productivity. Consequently, understanding the mechanisms and improving crop resistance to drought has been a high priority for research activities and a variety of breeding programs. Genetic and molecular analyses have revealed major quantitative trait loci (QTLs) and genes that affect drought resistance in crops (Dolferus et al., 2019; Kadam et al., 2012; Kirigwi et al., 2007; Li et al., 2019; Mathews et al., 2008; Rabbi et al., 2021; Sallam et al., 2022). However, the underlying mechanisms and regulatory pathways in drought stress response remain to be fully dissected. This question is particularly challenging in common wheat, which carries a large hexaploid genome with limited gene editing efficiency.
In this study, we demonstrated that mutation of TaSRT1 contributes to drought resistance of wheat by affecting stomatal aperture (Figures 2 and 3). We further reveal that TaSRT1 directly interacts with the WRKY transcription factor TaDT-A, and regulates its protein stability and transcription activity via lysine deacetylation, thus affecting the drought resistance (Figures 4-6). Furthermore, the TaDT-A deacetylation/acetylation site is conserved and plays an essential role in modulating TaDT-A protein stability (Figure 5g,h). These results provide a novel mechanism of plant response to drought stress, which provides effective molecular targets and a feasible strategy for breeding drought-resistant wheat varieties.
Lysine acetylation is an evolutionarily conserved PTM occurring in both prokaryotes and eukaryotes. Lysine acetylation was discovered as a PTM of histones and has been extensively studied in the context of transcription regulation (Lin et al., 2022; Song et al., 2022; Zheng et al., 2021). In the past decades, proteomic analyses have revealed that non-histone proteins are also frequently acetylated and constitute a major portion of the acetylome in mammalian cells (Chen et al., 2012; Choudhary et al., 2009; Kim et al., 2006; Zhao et al., 2010). Evidence for the regulation of transcription factor activity by HDAC-mediated lysine deacetylation is increasing in plants (Choudhary et al., 2014; Hartl et al., 2017; Narita et al., 2019; Sun et al., 2024). For instance, the Arabidopsis sirtuin-like histone deacetylase AtSRT1 deacetylates and enhances the stability of AtMBP-1, a transcriptional repressor of stress-responsive and glycolytic genes (Liu et al., 2017). Similarly, OsSRT1 could deacetylate glyceraldehyde-3-phosphate dehydrogenase and inhibit its nuclear localization and function as a transcriptional activator of glycolytic genes (Zhang et al., 2017). In the present study, we provide evidence that TaSRT1 interacts with and promotes protein degradation of TaDT-A to inhibit the activation of its downstream genes (such as TaATG8 and TaATG18), suggesting that TaSRT1 suppresses the gene regulatory network controlled by TaDT-A. Furthermore, TaSRT1-mediated lysine deacetylation was involved in the inhibition of TaDT-A transcription activity. When the inhibitor NAM was added, the inhibitory effect of TaSRT1 could be alleviated in the LUC assays in tobacco (Figure 6d,e), affecting TaSRT1 inhibition of TaDT-A binding to its target sequence in vivo. In line with this, the application of NAM could improve the drought tolerance of wheat seedlings under drought stress (Figure S10a–d). In addition, the expression levels of TaDT-A downstream genes TaATG8 and TaATG18 in tasrt1 mutants were significantly higher, but lower in TaSRT1 overexpression lines than those of the wild type under drought stress (Figure 6a,b). These results imply that the acetylated form of TaDT-A functions as an activator of downstream genes in response to drought stress. Importantly, the drought resistance assessment demonstrated that the TaDT-A KO/SKO plants were more sensitive to drought stress (Figure 7a–c; Figure S5), which was similar to those of TaDT-A KO, in the opposite of SKO (Figure 2a–c). Thus, TaSRT1 most likely functions in a common genetic pathway as and acts upstream of TaDT-A to respond to drought stress. TaDT-A positively regulates the drought tolerance in wheat by regulating stomata aperture via the autophagy pathway under water deficit conditions (Liu et al., 2024). In line with this, tasrt1 mutants have smaller stomatal apertures and lower water loss rates under water deficit conditions than the wild type, which contributes to higher WUE and drought resistance, suggesting TaSRT1 is an upstream regulator of TaDT-A and modulates the stomatal aperture via autophagy activity. Further investigations are needed to explore and decipher the accurate molecular mechanisms of the TaSRT1-mediated stomata function via autophagy pathway response to drought stress.
Most mammalian proteins are modified by multiple PTMs, which can reciprocally influence each other, and are often referred to as PTM crosstalk. PTM crosstalk can integrate diverse signals and vastly increase their regulatory potential. Because the amino group of lysine can be diversely modified, including by acetylation, methylation, ubiquitylation and sumoylation, this can result in competitive PTM crosstalk whereby different PTMs compete for modifying the same lysine residue (Ali et al., 2018; Wang and Cole, 2020). A common mechanism of acetylation-dependent protein stability is the prevention of protein ubiquitylation. This could be due to direct competition between acetylation and ubiquitylation for modification of the same lysine residues. In this study, the cell-free assays showed that TaSRT1 mediated TaDT-A protein degradation (Figure 5e,f). After adding MG132, the degradation of TaDT-A protein was inhibited (Figure 5e,f). The prediction showed that three lysine residues (K253, K256, K266) in TaDT-A might be potential deacetylation sites (Figure S4c). However, after the mutation of Lys at position 253 into Arg, the effect of TaSRT1-mediated accelerated degradation of TaDT-A protein disappeared, and the MG132 treatment could not inhibit the degradation of TaDT-A (R253) protein (Figure 5g); while the changing trend of their protein abundance of mutation of R256 and R266 was consistent with that of the unmutated TaDT-A (Figure S4f,g). This indicates that K253 is the key deacetylation/acetylation modification site of TaSRT1 on TaDT-A and may also be the site of ubiquitination. The key deacetylation/acetylation modification site was also identified in the WRKY domain of RRS1 and WRKY53 (Sarris et al., 2015; Zheng et al., 2020), which implies that lysine deacetylases act conservatively on the acetylation/deacetylation site of the WRKY TF family. These results indicate that the stability of TaDT-A protein is weakened by lysine deacetylation of TaSRT1 and suggest that competition between ubiquitination and acetylation of the common lysine residue controls the stability of TaDT-A. Moreover, we explored the potential E3 ubiquitin ligases interacting with TaDT-A via Y2H assay. However, no potential E3 ligases were identified. We also tested the interaction between TaDT-A and TaAIRP3/LOG2, TaDHS, TaGW2, TaDSG1, TaSARD1, TaPUB11 and TaPUB19, which play important roles in response to abiotic stresses in plants (Chen et al., 2021; Kim and Kim, 2013; Li et al., 2024; Liu et al., 2011; Park et al., 2010, 2018; Wang et al., 2018), but the results did not meet our expectations. The potential E3 ubiquitin ligase that interacts with TaDT-A is worth investigating in future work.
In addition, it should be noted that TaSRT1 may also inhibit downstream gene expression by interacting with TaDT-A via histone deacetylation. This is understandable since TaSRT1 also modulates histone deacetylation. Previous studies have shown that OsSRT1 is an important histone deacetylase required for both vegetative and reproductive development, and mediates H3 deacetylation of the genes in rice seedlings (Huang et al., 2007; Zhang et al., 2016; Zhong et al., 2013). OsSRT1 also negatively regulates leaf senescence by repressing the expression of the biosynthetic genes of the MeOH-jasmonate cascade (Fang et al., 2016). Studies show that AtSRT1 is also involved in the deacetylation of H3K9ac in Arabidopsis (Zhang et al., 2018). In this study, we found lysine deacetylation sites in histones, including H3K23ac and H3K27ac were also affected in tasrt1 mutant plants (Figure S10e,f). These results suggested besides lysine deacetylation in non-histones, TaSRT1 may associate with and regulate target genes via histone deacetylation, an area that deserves further study.
Depending on the duration and severity of water deficit, plants must carefully coordinate growth and stress responses. An overactive response may impose a fitness and yield penalty. Therefore, identifying the target genes and elucidating the underlying mechanisms that coordinate growth and stress responses are crucial for engineering crops with optimized stress responses. The field experiment data reveals that the net photosynthesis and WUE of tasrt1 mutants are significantly higher than those of wild-type plants under water-deficit conditions (Figure 8a,b). Moreover, the increase in yield of tasrt1 mutants under water-limited conditions may be attributed to the enhancement in spike length, spikelet number and seeds per spike, rather than the change in thousand seeds weight or seed size (Figures S7 and S9). These results suggest that manipulation of TaSRT1 did not result in clear pleiotropic phenotypes or yield penalty under field conditions but increased drought resistance compared with the wild type under water-limited conditions. These findings suggest that TaSRT1 could serve as a target for both genetic engineering and selection for the improvement of wheat drought resistance. The regulatory factors or genes involved in drought resistance identified in this study may also be of great value for the genetic improvement of other crops.
Methods
Plant materials
Wheat plants of different genotypes were cultivated in the experimental field in Beijing (lat. 39°57′ N, long. 116°17′ E) under natural growing seasons or in a greenhouse at a relative humidity of 70% and 26/20 °C day/night temperatures, with a 16 h:8 h/light:dark photoperiod and a light intensity of 3000 lux (Master GreenPower CG T 400W E40; Philips). The surface-sterilized seeds were incubated at 4 °C for 3 d in the dark and then exposed to white light at room temperature. Germinated seeds were transplanted into pots. The materials were subject to different treatments. Nicotiana benthamiana plants, used as host plants, were grown in a glasshouse at 24–25 °C under a 16 h:8 h, light:dark cycle for 4–5 weeks before agroinfection.
Plasmid construction and plant transformation
In order to make the candidate gene knockout constructs, the single guide RNAs (sgRNAs) target sequences were designed according to the conserved region using the E-Crispr Design Website (http://www.e-crisp.org/E-CRISP/). Then corresponding sgRNA sequences were synthesized (Tsingke Biotechnology Co., Ltd.), digested and inserted into the terminal vector pBUE411 via T4 DNA ligase (M0202, New England Biolabs). The open reading frame (ORF) of TaSRT1 was amplified from the cDNA of Fielder seedling leaves and inserted into the pMWB110 vector using BamHI sites to achieve the Ubipro:TaSRT1 construct. All binary vectors harbouring the desired constructs were transferred into Agrobacterium (Agrobacterium tumefaciens) strain EHA105 and transformed into wheat cultivar Fielder by Agrobacterium-mediated transformation. Primers used to identify the knockout and overexpression transgenic plants are listed in Table S1.
Water-deficit drought assays in the seedling stage
For phenotypic analysis in the seedling stage, the seeds of different genotypes and wild-type plants with similar vitality and germination rates were transplanted on both sides of the same pot (30 × 15 × 15 cm or 55 × 15 × 6 cm, length × width × depth) which was filled with a certain amount of uniformly mixed soil (nutrient soil to vermiculite in a ratio of 2:1), respectively, and grown in a greenhouse (70% relative humidity, 26/20 °C day/night temperature, 16 h:8 h/light:dark light cycle). Before sowing wheat seeds, water the soil in the pot thoroughly to allow the seedlings to grow normally in the early stage. All drought treatments were achieved by stopping irrigation to reach ~3.5% VWC for 5 days to ensure that the different genotypes being compared were all exposed to the same severity of soil drying, and then watering was resumed to allow plants to recover and the number of surviving plants was recorded 7 days later. Evaluated phenotype based on survival rate, relative water content, fresh weight, and dry weight. At least 15 plants of each line were compared in each test, and statistical analyses were based on data obtained from three independent experiments.
For the phenotypic analysis of NAM treatment in drought tolerance of wheat, following the drought treatment aforementioned, spray 100 mL of different concentrations (5, 20, 100 and 500 mM) NAM (S1761, Beyotime, China) onto the leaves regularly every day during drought treatment for 5 days. For the mock treatment, the same volume of ddH2O was sprayed and treated for the same time. At least 25 plants of each treatment were compared in each test, and statistical analyses were based on data obtained from three independent experiments.
For the root phenotyping, germinated seeds were transplanted into pots (7 × 7 × 10 cm, length × width × depth), which were fully filled with 2–3 mm white vermiculite granules. The control group and treatment group were set up with three replicates, respectively. Drought treatment was achieved by stopping watering to reach ~3.5% RSWC for 3 days, and then, the roots were statistically analysed and recorded phenotypes.
Phylogenetic analysis
To perform phylogenetic analysis, the amino acid sequences of TaSRT1 from different plant species were downloaded from Ensembl Plants (http://plants.ensembl.org/index.html). Mega X (Kumar et al., 2018) was employed to construct the phylogenetic tree with the following programs: first, all protein sequences were aligned in Clustal W with default parameters; second, the maximum likelihood (ML) method was used to build a phylogenetic tree with the Poisson model and 1000 bootstrap replicates.
Wheat protoplast isolation and transfection
The protoplasts were prepared from the wheat variety Fielder. Wheat protoplast isolation and transfection were performed as described (Wang et al., 2014). The EndoFree Maxi Plasmid Kit (DP117, TIIANGEN, China) was used to extract plasmid DNA for protoplast transfection. Each plasmid (5–10 μg per construct) was introduced by polyethylene glycol (PEG)/Ca2+–mediated transfection. The transfected protoplasts were incubated at room temperature (22–24 °C) for 14–16 h in the dark.
Subcellular localization
To detect the subcellular localization of TaSRT1 protein, the full-length coding sequence of TaSRT1-D was cloned into pCAMBIA1300-GFP vector using a Seamless Cloning and Assembly Kit (Biomed, China) to generate a fusion construct pCAMBIA1300-GFP-TaSRT1-D named TaSRT1-GFP (GFP, green fluorescent protein). The fusion construct and empty vector (as a control) were then separately transfected into wheat protoplasts according to the above method. After incubation at room temperature for 14–16 h in the dark, the chlorophyll and GFP fluorescence were observed by confocal microscopy (LSM880; Carl Zeiss) and quantified using the image analysis software provided by the manufacturer.
Total RNA extraction and RT-qPCR
The 2-week-old seedlings were grown under normal conditions in the greenhouse. Samples from the control group were collected when the soil moisture content was 10%, while samples from the treatment group were collected 1, 2, 4, 6, 8, 10 and 12 h after the soil moisture content reached 3.5%. Total RNA was extracted using TRIzol Reagent (15596018, Thermo Fisher Scientific). The reverse transcription of cDNAs was performed using HiScript II Q RT SuperMix (R223, Vazyme Biotech, China) according to the manufacturer's recommendations. AceQ qPCR SYBR Green Master Mix (Q121, Vazyme Biotech, China) was used for RT-qPCR analysis. The expression of specific genes was detected on the C1000 Touch Real-Time PCR system (Bio-Rad). All primers are shown in Data Set S2, and TaACTIN was used as an internal control.
Yeast library screening
Yeast library screening was performed using the Matchmaker GAL4 Gold Two-Hybrid System 3 (PT3024-1, Clontech Laboratories, Inc.), according to the manufacturer's instructions. Briefly, the full-length coding sequences of TaSRT1 without autoactivation activity were amplified and cloned into the pGBKT7 vector (PT3024-1, Clontech Laboratories, Inc.), which was used as a bait to identify the candidate TaSRT1-interacting proteins. The Y2H Gold yeast strain containing TaSRT1-pGBKT7 was incubated with the Y187 yeast strain containing a wheat cDNA library derived from Fielder seedlings constructed in pGADT7 for mating, and synthetic defined (SD) medium/-Ade/-His/-Leu/-Trp + X-α-gal was used for screening.
Co-immunoprecipitation (Co-IP) assay
For the Co-IP assay in Nicotiana benthamiana leaf tissues, the TaSRT1-GFP and TaDT-A-MYC fusion constructs were prepared. The full-length coding sequences of TaSRT1 and TaDT-A were individually cloned into pCAMBIA1300-GFP or pCAMBIA1300-MYC vectors, respectively. The fusion construct TaSRT1-GFP and TaDT-A-MYC were transferred into Agrobacterium strain GV3101 and co-infiltrated into the leaves of Nicotiana benthamiana by Agrobacterium-mediated infiltration. After incubated at 22 °C for 48 h, total proteins were isolated from different infiltrated leaves with lysis buffer (50 mM Tris–HCl [pH 7.5], 150 mM NaCl, 5 mM EDTA [pH 8.0], 0.1% Triton X-100, and 0.2% NP-40 supplemented with 5 mM DTT [dithiothreitol], 0.6 mM PMSF, 20 μM MG132, and 1 × complete protease inhibitor cocktail), and incubated with anti-GFP magnetic beads (F043, Forscience, China) at 4 °C for 2–4 h with gently shaking. The immunoprecipitated samples were washed six times with washing buffer (50 mM Tris–HCl [pH 7.5], 150 mM NaCl, 0.1% NP-40). Total proteins were extracted from wheat seedlings using SDS loading buffer (200 mM Tris–HCl [pH 6.8], 40% glycerol, 8% SDS, and 20% β-mercaptoethanol) at 100 °C for 5 min. The proteins were separated on SDS-PAGE and subjected to immunoblot analysis with anti-GFP (mouse, 1:2000; Abclonal, AE012, China) or anti-c-MYC (mouse, 1:2000; TransGen Biotech, HT101-01, China) antibodies.
Bimolecular fluorescence complementation (BiFC) assay
For the BiFC assays, the full-length coding sequences of TaSRT1 and TaDT-A were amplified and individually cloned into cYFP and nYFP vectors, respectively. The fusion constructs and empty cYFP and nYFP vectors were transferred into Agrobacterium strain GV3101. Then the fusion constructs, or one of them combined with its corresponding empty cYFP or nYFP vector, were co-transformed into Nicotiana benthamiana leaves. After incubated at 22 °C for 48 h, the YFP fluorescence signals were imaged with a confocal microscope (LSM880; Carl Zeiss).
Luciferase complementation imaging (LCI) assay
The LCI assay was carried out between TaSRT1 and TaDT-A as described previously (Song et al., 2022). Briefly, the full-length coding sequences of the TaSRT1 and TaDT-A were cloned into the KpnI/BamHI-digested pCAMBIA1300-cLUC (cLUC) or KpnI/SalI-digested pCAMBIA1300-nLUC (nLUC) vectors separately. The resulting constructs and empty cLUC and nLUC vectors were transformed into Agrobacterium strain GV3101. The Agrobacterium cultures were then resuspended in infiltrating buffer (10 mM MgCl2, 10 mM MES, 100 μM AS) to OD600 nm equal to 3, mixed in a 1:1 ratio (v/v), and co-infiltrated into Nicotiana benthamiana leaves. Approximately 48 h after infiltration, the firefly LUC signal within the infiltration region was analysed using the cooled charge-coupled device camera NightSHADE LB 985 plant imaging system (Berthold Technologies, Bad Wildbad, Germany) after spraying 1 mM of D-luciferin onto the leaves (Coolaber; CL6928, China).
Pull-down assay
Recombinant proteins TaDT-A-GST and TaSRT1-His were purified from E. coli BL21 (DE3). TaDT-A-GST (50 μg) or GST (50 μg) were mixed with the Tris buffer (25 mM Tris–HCl [pH 7.5], 50 mM NaCl, and 3 mM DTT) and incubated with ProteinIso® GST Resin (DP201-01; TransGen) at 4 °C for 2 h, and subsequently incubated with TaSRT1-His (50 μg) for 2–3 h. The mixture was collected by centrifugation at 500 g for 5 min at 4 °C, then washed with the Tris buffer (supplemented with 0.1% Triton X-100 and 0.1% SDS) six times. After eluted from the beads, the proteins were separated on 8% (w/v) SDS-PAGE and subjected to immunoblot analysis with anti-His (mouse, 1:2000; TransGen Biotech, HT501-01, China) and anti-GST (mouse, 1:2000; TransGen Biotech, HT601-0, China) antibodies.
Transient dual luciferase reporter assay
The promoter sequences of TaATG8 and TaATG18 were cloned into pGreen II 0800-LUC vector to be used as reporter plasmids. The full-length coding sequences of TaSRT1 and TaDT-A were individually constructed into pCAMBIA1300 vector to generate effector constructs. The relative LUC activity was measured in tobacco, with different combinations of constructs co-transfected. After incubated at 22 °C for 48 h, the detection of dual luciferase (LUC) activity was performed as described previously (Zheng et al., 2021). Each sample had more than three independent transfections and primers were listed in Data Set S2.
Cell-free degradation assay
Cell-free degradation assay was performed as reported previously with some modifications (Wang et al., 2019). Briefly, total proteins were extracted from 7-day-old seedlings with lysis buffer as described above. Purified recombinant TaDT-A-GST was added into equal amounts of WT, SKO and ROE total proteins with 2 mM ATP, 150 μM CHX and incubated at 25 °C for different time periods with or without 10 μM MG132. TaDT-A-GST was separated on SDS-PAGE and detected with anti-GST (mouse, 1:2000; TransGen Biotech, HT601-01, China) and anti-Rubisco (rabbit, 1:2000; Sangon Biotech, D191102-0100, China) antibodies.
HDAC assays of TaSRT1 protein
The TaSRT1-GFP and empty GFP vector were transformed into N. benthamiana leaves, respectively, and total proteins were extracted with lysis buffer as described above. TaSRT1-GFP and GFP proteins were used to test HDAC activity. An Epigenase HDAC activity colorimetric kit (BioVision, Catalogue#K331-100) was used to measure the TaSRT1 activity according to the manufacturer's instructions. Briefly, The TaSRT1-GFP fusion protein was incubated with a fluorogenic peptidic HDAC substrate containing an ε-acetylated lysyl moiety. The HeLa (nuclear extract) containing a mixture of HDAC was used as a positive control. The GFP protein served as a negative control. Then it was treated with a lysine developer to produce chromophore. Finally, the OD value at 400 nm was measured by ELISA, and its relative activity was calculated.
Site-directed mutagenesis
Acetylation site mutations in TaDT-A (K253 to R253, K256 to R256, and K266 to R266) were generated by one-step site-directed mutagenesis as previously described (Zheng et al., 2004). In brief, the WT CDS of TaSRT1-D was cloned into the pEASY-Blunt (CB111-01, TransGen Biotech) vector. The resulting Blunt-TaSRT1 vector was used as a template for further PCR amplification. A 20 μL PCR mixture was prepared containing 40 ng Blunt-TaSRT1, 1 μM primer pairs harbouring certain mutagenized sites (Data Set S2), and 10 μL 2 × Phanta Max Master Mix (P515-01, Vazyme). PCR amplification was carried out by preheating the mixture to 95 °C for 5 min, followed by 35 cycles of 95 °C for 30 s, 58 °C for 30 s, and 72 °C for 1 min. The PCR ended with a final incubation step at 72 °C for 10 min. The resulting PCR products were evaluated by agarose gel electrophoresis and treated with the restriction enzyme DpnI (R0176L, New England Biolabs) for 1 h. Approximately 3 μL of the above PCR product was transformed into E. coli chemocompetent cells (DH5α) for sequencing analysis and plasmid isolation.
Immunoblotting analysis of TaDT-A and histone lysine acetylation
To detect lysine acetylation of the TaDT-A protein, the TaSRT1-GFP and TaDT-A-MYC fusion constructs were co-transformed into wheat protoplasts or N. benthamiana leaves. Total proteins were isolated with lysis buffer as described above. and incubated with anti-MYC magnetic beads (M047-11, MBL) at 4 °C for 5 h with gentle shaking. The immunoprecipitated samples were washed five times with washing buffer (50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 0.1% NP-40), separated on SDS-PAGE and subjected to immunoblot analysis with anti-MYC and anti-acetyl lysine (anti-acetyl) (Abcam ab80178) antibodies using the ECL SuperSignal system.
For histone acetylation analysis, the total proteins were extracted from tissues of wild type, SKO and ROE transgenic lines. Antibodies to anti-H3K9ac (ab32129, Abcam), anti-H3K23ac (ab177275, Abcam), anti-H3K27ac (ab177178, Abcam), anti-H4K8ac (ab45166, Abcam), and anti-H3(Abcam, ab1791) were used. All immunoblots were developed using the ECL SuperSignal system.
Water-deficit drought assays in the field
For reproductive stages, the plots were designed for the well-watered planting conditions and drought treatment conditions according to methods described previously (Li et al., 2024). In brief, the drought treatment plot was separated by a 1-m deep waterproof layer to prevent plants from being affected by irrigation water. Well-watered plots were irrigated throughout the growth period, while the drought plot was subjected to drought treatment after the first irrigation and only accepted natural rainfall for growth. The plants that were subjected to drought treatment received approximately 40% of the water that the plants in the sufficiently irrigated plots received.
The photosynthetic parameters were analysed on the flag leaves at a predetermined date around 14:00 using a LICOR-6400 CO2 gas exchange analyser (LICOR-6400, Lincoln, NE). Then, the photosynthesis rates and transpiration rates in all of the tested genotypes under different conditions were obtained. For the estimation of WUE, the calculation was conducted by photosynthesis rates in relation to transpiration rates (Wang et al., 2016). Agronomic traits related to yield were analysed following the maturation of the grains and the harvest.
Statistical analysis
In total, at least 3 independent biological replicates were employed in each experiment. The numbers of samples (n) used for statistical analysis are listed in the figures or figure legends. Error bars represent the SD of each set of data. The statistical significance of differences was evaluated by one-way analysis of variance (ANOVA) followed by a two-tailed Student's t-test or Tukey's test. The asterisks indicate significant differences (*P < 0.05, **P < 0.01, ***P < 0.001). Different lowercase letters indicate a significant difference (P < 0.05).
Acknowledgements
This work was funded by the Biological Breeding-National Science and Technology Major Project (2023ZD0407103), the National Natural Science Foundation of China (32130078, 32441061, 32072001), the National Key Research and Development Program of China (2022YFF1001604), Frontiers Science Center for Molecular Design Breeding (2024TC188) and Young-scholar program of China Agricultural University-Bayannur Research Institute (2024BYNECAU009).
Conflicts of interest
The authors declare no conflict of interest.
Open Research
Data availability statement
The data that supports the findings of this study are available in the supplementary material of this article.




