Perioperative Airway Management for Midface Surgery in Children With Syndromic Craniosynostosis; a Single Center Experience With Immediate Extubation
ABSTRACT
Background
Midface advancements in children with syndromic craniosynostosis present challenges for anesthesiologists and intensive care teams.
Aims
This study reviewed the perioperative airway management protocol for immediate tracheal extubation after midface surgery at our tertiary center over the past 10 years.
Methods
A retrospective cohort study was performed to obtain information on respiratory disorders, surgical and anesthetic management, airway support, and respiratory complications following le Fort III (LF3) and monobloc (MB) with distraction. Patients with a tracheostomy were excluded.
Results
Thirty-two patients (12 LF3, 20 MB) were included. All were immediately extubated with a median of 25 min after surgery. Immediate extubation was performed in young patients (n = 8/32, < 5 years old), in patients with severe OSA (n = 6/32, median oAHI 23/h), with difficult airways (n = 5/32, Cormack-Lehane airway grade ≥ 3), with significant intraoperative blood loss (n = 32, median 46 mL/kg), and with long operative times (n = 32, median 223 min). The majority of patients received no or only oxygen support in the first hours after extubation (n = 29/32) and could be discharged from the pediatric intensive care unit to the surgical ward after 1 day (n = 30/32). A 5-month-old patient with MB required intermittent oxygen and Guedel airway throughout his hospitalization due to airway obstruction at the tongue base combined with supine positioning to allow external traction.
Conclusions
Despite the pre-existing airway disorder, the extent of the procedure and the effect of anesthesia on airway tone, all patients were extubated immediately after midface advancement, with only one young patient needing prolonged postoperative support. Immediate extubation is feasible following midface advancement in patients with syndromic craniosynostosis. Further prospective randomized trials are needed to demonstrate superiority to delayed extubation.
1 Introduction
Apert and Crouzon/Pfeiffer syndromes are two of the more severe craniosynostosis syndromes. Patients with these syndromes share the craniofacial characteristics of multisuture craniosynostosis, exorbitism, (severe) midface hypoplasia, and upper airway abnormalities. Because of their narrowed upper airway, two thirds of these patients are affected by obstructive sleep apnea (OSA) [1]. Upper airway endoscopy has shown that their airway obstruction is frequently multilevel, and even children without OSA can have partial obstruction [2, 3]. Obstruction is often situated at the level of the nose, nasopharynx, and/or hypopharynx and is associated with midface hypoplasia. Other levels of airway obstruction that may be present include choanal atresia, nasal septal deviation, hypertrophic adenoids or tonsils, mandibular hypoplasia with glossoptosis, palatal deformities, and tracheal cartilage abnormalities [4-7]. The different types of midface advancements that can be used to correct midface hypoplasia are Le Fort III (LF3), monobloc (MB) or facial bipartition (FB), and are routinely combined with distraction. These procedures, especially in these patients, present complex challenges to the anesthesiologists and critical care teams due to the potential for difficult airway, high prevalence of OSA, significant intraoperative blood loss with high transfusion rates, long operative time, potential postoperative swelling of the upper airway, and compromised airway access when external frames are used [8-13].
In a recent multicenter study conducted at seven European craniofacial centers, we observed variability in the Standard Operating Procedures (SOP) regarding perioperative airway management for midface surgery between hospitals and that delayed extubation was the standard practice in the majority of centers [14]. Only in two centers, one of which was ours, was immediate extubation the routine management. The previous study did not include some case-specific factors that are important in the decision to extubate or not to extubate (e.g., airway grade, presence of multilevel of airway obstruction). Therefore, the purpose of this study is to further evaluate our current perioperative airway management SOP for immediate extubation following midface surgery in patients with Apert and Crouzon/Pfeiffer syndromes.
2 Materials and Methods
This retrospective cohort study was approved by the medical ethics committee of the Erasmus MC (MEC-2024-0024), and written informed consent was obtained from all subjects, a legal surrogate, the parents, or legal guardians for minor subjects. All Apert and Crouzon/Pfeiffer patients treated at our tertiary care center (Sophia Children's Hospital—Erasmus Medical Center, Rotterdam, the Netherlands) between 2014 and 2024 with LF3, MB, or FB were included. Patients with a tracheostomy were excluded. Details of perioperative outcomes for our patients and those from other craniofacial centers with delayed extubation or tracheostomy have been reported elsewhere [14].
The choice of midface advancement depended on several factors, including facial anomalies and the presence of increased intracranial pressure. Due to the severity of midface hypoplasia in both syndromes, distraction was typically indicated. Figures 1 and 2 show the osteotomies performed in LF3 and MB/FB, respectively. Very young children with syndromic craniosynostosis (< 2 years of age) whose severe respiratory problems could not be successfully managed with non-invasive ventilation received a tracheostomy [15]. MB was then planned from the age of 2.5 years. Elective tracheostomy to bridge major craniofacial surgery in patients with craniosynostosis has never been performed at our center [14]. MB at a younger age was considered in cases of severe exorbitism with corneal exposure. In young children whose respiratory problems were successfully managed with non-invasive ventilation, the timing of midface advancement was determined in consultation with the parents. If there was a relative indication for midface surgery in the absence of (severe) functional problems, the option of surgery was offered between the ages of 7 and 11 years. We tried to avoid midface surgery during puberty, as children of this age are often concerned about their appearance and a change may cause additional psychological problems. Alternatively, surgery was performed at skeletal maturity (from 17 to 18 years onwards).
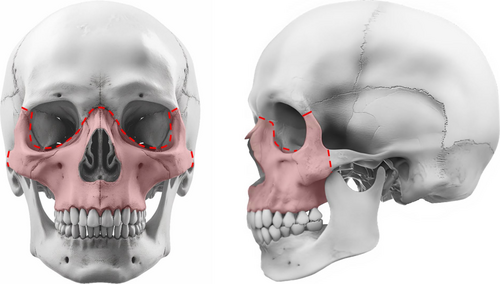
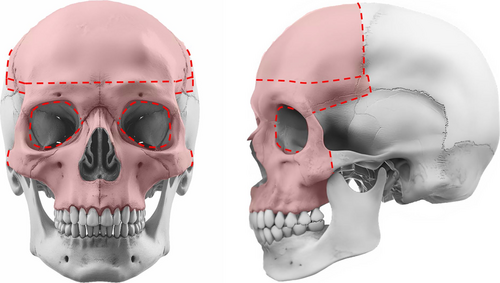
2.1 Historical Development of Current Extubation Strategy
Before 2010, there was a certain reluctance to extubate the patients immediately, and most children remained intubated in the pediatric intensive care unit (PICU) for 1–3 days [14]. It was at the discretion of the pediatric intensivist to decide when to attempt extubation. With time and experience, the aim shifted towards extubation at the end of the surgery, and prolonged intubation was used mainly in patients with suspected severe airway swelling or after a failed extubation attempt. In the last decade, the protocol has been changed to extubate the patient immediately after midface advancement in the operating room (OR).
2.2 Anesthesia and Perioperative Care
Preoperative anxiolytic medication was administered when indicated. Standard intraoperative monitoring was used: invasive blood pressure, electrocardiogram, oxygen saturation, end-tidal partial pressure of carbon dioxide, end-tidal concentration of sevoflurane, rectal temperature, and urine output.
Both inhalational and intravenous (iv) induction have been used. Maintenance of spontaneous ventilation in the presence of anticipated difficult intubation is useful but not mandatory. In the case of iv induction, anesthesia was induced with propofol 3 mg/kg. For inhalation induction, anesthesia was induced with 80% oxygen and either incremental increases or a direct 8% sevoflurane concentration. General anesthesia was maintained with propofol 10 mg/kg/h or an age-corrected median end-tidal concentration equal to 1 minimum alveolar concentration (MAC) sevoflurane. Both pressure-limited assist control (PAC) ventilation and volume-limited assist control (VAC) ventilation have been used. In the case of PAC ventilation, the target ventilation pressures were < 30 mmHg. In the case of VAC ventilation, the target tidal volumes were 6–8 mL/kg. Patients received an inspired oxygen fraction (FiO2) of approximately 30% during anesthesia.
Given the risk of significant blood loss during these procedures, at least two iv cannulas were inserted and a unit of red blood cells (RBC) was available in the OR. Intraoperative fluid management was based on the 4-2-1 rule; 4 mL/kg/h is administered for the first 10 kg of body weight, 2 mL/kg/h for the second 10 kg of body weight, and 1 mL/kg/h for each additional kg of body weight. To minimize blood loss, normotension and normothermia were maintained, and tranexamic acid was administered (as infusion and/or bolus). Transfusion of blood products was initiated based on intraoperative blood loss with the goal of maintaining Hb above 7.0 g/dL. Vasoactive drugs were administered to maintain hemodynamic stability in the event of hypotension.
Intraoperative analgesia was most commonly provided by remifentanil 0.2mcg/kg/min infusion. Prior to incision, the surgeon administers a local anesthetic, which is usually combined with adrenaline (e.g., bupivacaine/adrenaline or lidocaine/adrenaline). At the end of surgery, patients receive a loading dose of 20 mg/kg paracetamol, 1 mg/kg diclofenac, and 0.1 mg/kg morphine. Neuromuscular blocking agents have been routinely administered to facilitate intubation. These procedures do not require continuous neuromuscular blockade, but if neuromuscular blocking agents are administered, train-of-four (TOF) monitors are always used to check for residual neuromuscular blockade, and the TOF ratio must be ≥ 90% before extubation. Airway suctioning was performed to remove respiratory secretions, blood, and gastric contents from the upper airway prior to extubation. Drugs to reduce cough or cardiovascular changes during extubation (e.g., low-dose boluses propofol or ketamine, dexmedetomidine, lidocaine (iv, intracuff, topical, or tracheal routes)) were not routinely used.
2.3 Outcome Variables and Data Collection
Data were collected on patient demographics (sex, diagnosis, age at surgery, BMI z-scores, congenital heart disease), respiratory disorders and airway obstruction (severity of OSA, preoperative drug-induced sleep endoscopy (DISE) findings), surgical procedures (previous midface surgery, type of midface advancement, concomitant airway-related surgery, intraoperative blood loss), anesthetic management (Cormack-Lehane (CL) airway grade, method of induction, general anesthesia, intraoperative transfused blood products (i.e., erythrocytes, fresh frozen plasma, platelet concentrate) and other agents (i.e., colloids and crystalloids)), respiratory support after extubation, postoperative sedation, analgesia, and (respiratory) complications from the patient records. Records were reviewed for complications up to 1 month postoperatively.
The airway/laryngoscopy was considered difficult if the CL airway grade was ≥ 3. Operating times were considered long if they were > 120 min because a previous meta-analysis showed that the likelihood of postoperative complications approximately doubled for operating time thresholds > 120 min [16]. The obstructive apnea-hypopnea index (oAHI) and the oxygen desaturation index (ODI) were used to assess respiratory disorders. The oAHI derived from polysomnography (PSG) was used to classify OSA; no = oAHI < 1/h, mild = 1/h ≤ oAHI < 5/h, moderate = 5/h ≤ oAHI < 10/h, severe = oAHI ≥ 10/h. Preoperative DISE is usually only done in patients with moderate or severe OSA. DISE findings were scored according to the Sleep Endoscopy Rating Scale (SERS). Briefly, the pattern of obstruction was scored on a three-point scale (0 = no obstruction, 1 = partial obstruction, 2 = complete obstruction) at six anatomical levels (nasal airway, nasopharynx, velopharynx, oropharynx, base of tongue (including epiglottis), and arytenoids), resulting in a maximum score of 12 [17]. Complete obstruction at two or more sites and a total SERS score ≥ 6 are associated with severe OSA [17].
2.4 Statistical Analysis
Data were imported into R statistical software (version 4.1.2, R Foundation for Statistical Computing) for analysis. Histograms and QQ plots were used to assess the distribution of continuous variables. Normally distributed continuous data were presented as means with standard deviations and skewed data as medians with interquartile ranges (IQR). Categorical data were presented as counts and proportions. Descriptive statistics were used only.
3 Results
Between January 2014 and March 2024, 39 midface advancements were performed in 38 patients. Patients were immediately extubated in 32 of 39 procedures (86%) and had a tracheostomy in 7 of 39 procedures (14%). Finally, 32 procedures with immediate extubation in 32 patients were included, including 12 LF3 and 20 MB.
3.1 Preoperative Patient Characteristics
Patient characteristics can be found in Table 1. Eight out of thirty-two patients (25%) received midface surgery at a young age (< 5 years old); one patient at 5 months, two at 2 years, one at 3 years, and four at 4 years. Six of the thirty-two patients (19%) had severe OSA. In three patients with severe OSA, the oAHI could not be accurately determined due to poor PSG quality; they had a median ODI > 3% of 54/h (IQR 42–59) and a median ODI > 4% of 29/h (IQR 23–34). The other three patients with severe OSA had a median oAHI of 23/h (IQR 20–49). Preoperatively, 2/6 patients were treated with bilevel positive airway pressure (BiPAP), 1/6 with continuous positive airway pressure (CPAP), 2/6 with nasal steroids, and 1/6 received no treatment. Sixteen patients (50%) had a preoperative DISE available in which the SERS could be determined. Seven patients without OSA, four with mild OSA, and one patient with severe OSA did not receive a preoperative DISE, and in four patients, the SERS could not be determined because the DISE was not recorded. The median SERS was 6 (IQR 4–6) and half of the patients (8/16) had complete airway obstruction at two or more sites.
| Total (n = 32) | Apert | Crouzon/Pfeiffer | |||
|---|---|---|---|---|---|
| LF3 (n = 2) | MB (n = 10) | LF3 (n = 10) | MB (n = 10) | ||
| Males: females | 19: 13 (59: 41) | 2: 0 | 4: 6 | 7: 3 | 6: 4 |
| Median age (IQR), years | 8.7 (4.9 to 11.4) | 8.0 (7.8 to 8.1) | 9.2 (7.8 to 9.8) | 16.0 (11.4 to 18.6) | 4.1 (3.0 to 6.5) |
| Median BMI Z-score (IQR) | −0.66 (−1.46 to 0.48) | −0.95 (−1.05 to −0.85) | −0.97 (−1.51 to 0.56) | −0.82 (−1.42 to 0.36) | −0.08 (−1.44 to 0.38) |
| Previous midface surgery | 2 (6) | 0/2 | 0/10 | 2/10 | 0/10 |
| Congenital heart diseasea | 2 (6) | 0/2 | 0/10 | 1/10 | 1/10 |
| OSA classification | |||||
| No | 16 (50) | 2/2 | 8/10 | 3/10 | 3/10 |
| Mild | 10 (31) | 0/2 | 1/10 | 5/10 | 4/10 |
| Moderate | 0 (0) | 0/2 | 0/10 | 0/10 | 0/10 |
| Severe | 6 (19) | 0/2 | 1/10 | 2/10 | 3/10 |
| No. patients with preoperative DISE | 16 (50) | 1/2 | 7/10 | 3/10 | 5/10 |
| Median SERS (IQR)‡ | 6 (4–6) | 7 | 4 (4–5) | 5 (5–6) | 6 (6–7) |
| No complete obstruction | 2/16 | 0/1 | 1/7 | 1/3 | 0/5 |
| Complete obstruction 1 site | 6/16 | 0/1 | 5/7 | 0/3 | 1/5 |
| Complete obstruction ≥ 2 sites | 8/16 | 1/1 | 1/7 | 2/3 | 4/5 |
- Note: Values represent number of patients (percentages).
- Abbreviations: BMI, body mass index; DISE, drug-induced sleep endoscopy; LF3, Le Fort III; MB, monobloc; OSA, obstructive sleep apnea; SERS, Sleep Endoscopy Rating Scale.
- a 1 Crouzon patient treated with LF3 had a tetralogy of Fallot that was treated with a Gore-Tex valve; 1 Crouzon patient treated with MB had a small membranous VSD and a type II ASD that had closed spontaneously.
3.2 Surgical Characteristics
Surgical characteristics can be found in Table 2. Five out of thirty-two patients (15%) underwent concomitant procedures during midface advancement; the two patients who received concomitant bilateral sagittal split osteotomy (BSSO) were 14 and 19 years old, while the other three patients with adenotonsillectomy (ATE) or mandibular distraction osteogenesis (MDO) were ≤ 4 years old. The median intraoperative advancement for patients without distractors was 10 mm (8.5–10.0) and for patients with distractors, 3.3 mm (IQR 2.9–4.3).
| Total (n = 32) | LF3 (n = 12) | MB (n = 20) | |
|---|---|---|---|
| Type of distraction | |||
| Perioperative distraction only | 4 (13) | 3 (25) | 1 (5) |
| Internal | 19 (59) | 1 (9) | 18 (90) |
| External | 5 (15) | 4 (33) | 1 (5) |
| Internal + external | 4 (13) | 4 (33) | 0 (0) |
| Concomitant surgery | |||
| ATE | 2 (6) | 0 (0) | 2 (10) |
| BSSO | 2 (6) | 2 (17) | 0 (0) |
| MDO | 1 (3) | 0 (0) | 1 (5) |
| Median operative time (IQR), min | 223 (188–257) | 196 (170–226) | 245 (212–274) |
| Median LOS (IQR), days | 6 (5–8) | 5 (4–7) | 6 (6–8) |
- Note: Values represent number of patients (percentages).
- Abbreviations: ATE, adenotonsillectomy; BSSO, bilateral sagittal split osteotomy; LF3, Le Fort III; LOS, length of hospital stay; MB, monobloc; MDO, mandibular distraction osteogenesis.
3.3 Anesthesiologic Characteristics
Details on anesthesiologic management can be found in Table 3. Inhalational induction was performed in younger patients compared to iv induction (median age 7.9 (IQR 4.5–9.4) and 11.8 (IQR 8.2–18) years, respectively). Five out of 32 patients (15%) were classified as having a difficult airway/laryngoscopy (CL airway grade ≥ 3); most of them (4/5) were Crouzon/Pfeiffer patients. In 8/18 patients with inhalation induction, the anesthetic was switched to propofol to maintain anesthesia. In 6/14 patients with iv induction, the anesthetic was switched to sevoflurane to maintain anesthesia. In 5/32 patients (15%) the blood loss exceeded the circulating blood volume (> 80 mL/kg). Nearly all patients (n = 31/32, 97%) received intraoperative tranexamic acid. All patients except one were extubated in the OR, with a median time of 25 min (IQR 17–33) after surgery. One patient was extubated in the recovery room 206 min after surgery due to postoperative drowsiness.
| Total (n = 32) | LF3 (n = 12) | MB (n = 20) | |
|---|---|---|---|
| Median age (IQR), years | 8.7 (4.9–11.4) | 13.3 (10.1–18.2) | 7.3 (4.1–9.6) |
| Laryngoscope | |||
| Direct/Macintosh | 28 (88) | 9 (75) | 19 (95) |
| Video | 4 (12) | 3 (25) | 1 (5) |
| CL airway grade | |||
| 1 | 19 (59) | 5 (42) | 14 (70) |
| 2 | 8 (26) | 4 (33) | 4 (20) |
| 3 | 2 (6) | 1 (8) | 1 (5) |
| 4 | 3 (9) | 2 (17) | 1 (5) |
| Method of induction | |||
| Intravenous | 14 (44) | 8 (67) | 6 (30) |
| Inhalational | 18 (56) | 4 (33) | 14 (70) |
| General anesthesia | |||
| Propofol | 18 (56) | 10 (84) | 8 (40) |
| Sevoflurane | 13 (41) | 1 (8) | 12 (60) |
| Propofol + sevoflurane | 1 (3) | 1 (8) | 0 (0) |
| Opioid infusion | |||
| Remifentanil | 30 (94) | 12 (0) | 18 (90) |
| Remifentanil + sufentanil | 2 (6) | 0 (0) | 2 (10) |
| Opioid bolus | |||
| None | 5 (16) | 1 (8) | 4 (20) |
| Fentanyl | 5 (16) | 3 (25) | 2 (10) |
| Sufentanil | 20 (62) | 7 (59) | 13 (65) |
| Piritramide | 1 (3) | 1 (8) | 0 (0) |
| Fentanyl + sufentanil | 1 (3) | 0 (0) | 1 (5) |
| Median blood loss (IQR), mL/kg | 46 (31–60) | 34 (21–39) | 55 (43–73) |
| Median transfused blood agents (IQR), mL/kg | 28 (12–40) | 11 (0–15) | 35 (29–48) |
| Median transfused other agents (IQR), mL/kg | 41 (21–57) | 27 (7–42) | 47 (29–69) |
| Median tranexamic acid infusion (IQR), mL/kg | 20 (15–35) | 20 (16–28) | 20 (15–35) |
| Median extubation timea (IQR), min | 25 (17–33) | 30 (23–39) | 21 (16–30) |
- Note: Values represent number of patients (percentages).
- Abbreviations: CL, Cormack-Lehane; LF3, Le Fort III; MB, monobloc.
- a Median extubation time was missing for 1 Crouzon patient treated with a LF3, but he left the operating room extubated within 1 h after surgery.
3.4 Postoperative Period
Almost all patients were discharged from the PICU to the surgical ward after 1 (n = 28/32, 88%) or 2 (n = 1/32, 3%) days. Two older patients who had received an LF3 went to the surgical ward immediately after surgery. There was one patient who remained in the PICU throughout his hospitalization; this was a severe Crouzon/Pfeiffer patient who was operated on at 5 months of age. He had a difficult airway with a SERS of 7 and a CL airway grade of 4, and he was treated with MB with a facial pin and external traction [18]. He initially had mild OSA, but MB was indicated to correct severe exorbitism with recurrent globe luxation. Postoperatively, he developed obstructive breathing due to his multi-level severe airway obstruction, particularly at the base of the tongue, combined with the supine position associated with external traction, requiring a Guedel airway. He required the Guedel airway continuously for the first two postoperative days and then alternately during sleep throughout his hospital stay. A repeat PSG before discharge showed frequent obstructions but no desaturations. He was discharged after 21 days with side positioning, nasal steroids, and a pulse oximeter, and eventually received a tracheostomy a few months after midface advancement due to worsening severe OSA.
The majority of patients recovered uneventfully and required no respiratory support or only oxygen for the first few hours after extubation (n = 29/32, 91%) (Table 4). None of the three patients who received BiPAP/CPAP preoperatively required it postoperatively. Midface advancement had reduced the degree of OSA in all six patients with severe OSA; their postoperative PSG within 6 months of surgery showed no OSA in 2/6 and mild OSA in 4/6. In addition to the young patient of 5 months with a MB we described earlier in this section, two other patients received a Guedel airway for obstructive breathing; this was caused by short-term postoperative drowsiness due to residual effects of anesthesia, and in both cases, the Guedel airway could be removed in the first hours after surgery. Intermittent postoperative sedation was used in only 2/32 (6%) patients; the young patient mentioned in the previous paragraph due to discomfort associated with the use of the Guedel airway and one patient with high anxiety and developmental delay to increase comfort during distractor activation. Twenty-three patients (72%) received opioids postoperatively; they received morphine for a median of 2 days (IQR 1–3), after which adequate analgesia was usually achieved with a combination of paracetamol and diclofenac alone (n = 20/23, 87%). The other nine patients (28%) only received a combination of non-opioid analgesics.
| Total (n = 32) | LF3 (n = 12) | MBa (n = 20) | |
|---|---|---|---|
| Respiratory support after extubation | |||
| None | 23 (72) | 7 (58) | 16 (80) |
| Oxygen | 7 (22) | 4 (25) | 3 (15) |
| Guedel airway | 3 (9) | 1 (8) | 2 (10) |
| CPAP | 0 (0) | 0 (0) | 0 (0) |
| Endotracheal reintubation | 0 (0) | 0 (0) | 0 (0) |
| New tracheostomy | 1 (3) | 0 (0) | 1 (5) |
| Complications | |||
| Pneumonia | 0 (0) | 0 (0) | 0 (0) |
| Deathb | 0 (0) | 0 (0) | 0 (0) |
- Note: Values represent number of patients (percentages). Patients could have had multiple types of respiratory support.
- Abbreviations: CPAP, continuous positive airway pressure; LF3, Le Fort III; MB, monobloc.
- a One patient had oxygen and oropharyngeal airway support and ultimately received a tracheostomy postoperatively.
- b There were no mortalities (airway-related or otherwise).
4 Discussion
This study demonstrates the feasibility of immediate extubation after midface advancement in Apert and Crouzon/Pfeiffer syndrome patients with a wide range of upper airway pathology and perioperative conditions. Immediate extubation was performed in young patients, patients with multilevel airway obstruction, severe OSA, difficult airway/laryngoscopy, diffuse intraoperative blood loss and transfusion, long operative times, concomitant airway-related surgery, and external distractor devices. Despite the pre-existing airway anomaly, the extent of the procedures and the effect of anesthesia on airway tone, patients were promptly extubated, and all but one required no additional long-term respiratory support.
Patients were extubated in the OR a median of 30 min after surgery, regardless of excessive blood loss, the use of vasoactive drugs, or a difficult airway. We prefer continuous remifentanil infusion for intraoperative analgesia since its pharmacokinetics are predictable. Relatively large doses can be administered, ensuring deep intraoperative analgesia and stable hemodynamics while enabling rapid extubation and awakening at the end of surgery irrespective of the duration of the infusion [19]. An important consideration in the decision to extubate in the OR or PICU is the transport time between the two units. In hospitals with long transport times, it is understandable that the patient may remain intubated during transport. In our hospital, the OR is located next to the PICU, which limits the transport time (< 5 min) and contributes to safe extubation in the OR. The anesthesia and intensive care teams also work closely together due to the complexity of these procedures and the patient population. The PICU is informed in advance of the type of patient they will be receiving at the end of the day. Prior to transport, the PICU is notified that the patient is en route, and the anesthesiologist personally hands the patient over to the intensivist postoperatively. In case of any questions regarding airway, hemodynamics, or pain management, there is direct communication between the intensivist and anesthesiologist.
In the few patients with some residual effect of anesthesia postoperatively, a safe airway was easily maintained with a Guedel airway, which could be safely removed after a few hours. Only one patient required long-term respiratory assistance in the postoperative period. We have seen similar postoperative outcomes in other patients who had mild OSA on the initial PSG after birth, but deteriorated over several months. Due to the pre-existing multilevel narrow upper airway anatomy, delayed upper airway growth and increased respiratory demands as these infants grow, they ‘grow into respiratory deficit’. These infants often deteriorate further during a respiratory infection. The young patient in our cohort had to remain in the supine position after surgery due to his specific external traction system, which exacerbated the obstruction of the tongue. This case demonstrates the need for preoperative DISE in patients undergoing early MB, initially for exorbitism and not moderate/severe OSA, to identify obstruction at the level of the base of the tongue.
Interestingly, our study also showed that patients with difficult airways and severe OSA can be extubated immediately after midface surgery when combined with a few mm of distraction or concomitant airway-related procedures. OSA is known to be associated with an increased risk of perioperative morbidity and mortality as well as postoperative respiratory complications [20-23]. Therefore, the risk of postoperative residual OSA, especially when caused by tongue base obstruction, is important in the decision to extubate. One way to reduce residual OSA is to begin advancement in the OR at the end of surgery. Lateral rather than supine sleep positioning is also important in patients with base of tongue obstruction. In addition, concomitant airway-related procedures can be performed to improve oropharyngeal obstruction in carefully selected patients. We have performed ATE, BSSO, and MDO in the same procedure in 5/32 patients. However, at our center, it is more common to perform bimaxillary surgery at skeletal maturity and ATE before midface advancement or at the time of distractor removal, as these procedures, especially ATE-related complications, add to the airway challenge [15].
None of the children in our cohort required reintubation. The children who required reintubation in the previous multicenter study were slightly younger (median age 6 years), more likely to have moderate/severe OSA (64%), and more likely to receive delayed extubation and to be intubated for longer (median 6 days) than the overall group [14].
Postoperative sedation is not routinely used because sedatives may increase upper airway collapsibility and predispose patients to perioperative respiratory events, especially in patients with OSA or in combination with opioids [21, 23]. In our experience, most patients were comfortable with a combination of intermittent opioids and standing NSAIDs (paracetamol and diclofenac). If postoperative sedation is indicated, intermittent short-acting benzodiazepine agonists can be safely administered to allow the patient to maintain a safe airway.
Nearly all patients could be discharged from the PICU to the surgical ward after 1 day. Previously, patients remained intubated for 1–3 days [14]. Thus, immediate extubation allowed us to reduce the LOS in the PICU and improve our PICU bed turnover. The median LOS for our immediately extubated patients was 6 days. Another study, in both immediate and delayed extubation patients, showed a median LOS of 7 days for patients with LF3 and 10 days for patients with MB [24]. We did not perform a cost analysis in this study, but since LOS is the most important contributor to total PICU costs [25, 26], and since immediate extubation did not result in unusually long LOS in our cohort [24], it is likely that immediate extubation reduced total hospital costs.
Another benefit of immediate extubation is that it has previously been shown that patients who were immediately extubated were also less likely to develop postoperative pneumonia compared with delayed extubation [14]. At our center, delayed extubation would only be considered if unusual circumstances occurred during surgery. Examples of potential indications include newly developed arrhythmias during surgery, moderate oxygenation during surgery in a patient with pre-existing lung disease, a patient who is insufficiently responsive after anesthesia withdrawal (no cough or swallow reflex and no independent breathing), and patients with unusual residual neuromuscular blockade (e.g., due to unknown pseudocholinsterase deficiency). However, these scenarios are rare and were not encountered in this study.
This retrospective study has some limitations. We considered scoring respiratory events, defined as the loss of upper airway patency requiring intervention from induction of anesthesia to patient hospital discharge [13]. Given the study's retrospective design, there is a potential variability in clinician reporting of incidents and quality of documentation. Therefore, we chose to collect information only on the type of postoperative respiratory support required by patients. This may have led to an underreporting of minor events, but we are confident that major adverse events would always have been reported. It is also important to note that the most severely affected children with severe OSA receive a tracheostomy at a young age and are therefore not included in this study.
In conclusion, we have shown that in experienced hands, immediate extubation after midface surgery is feasible in children with Apert and Crouzon/Pfeiffer syndromes. In addition, immediate extubation may reduce PICU stay, improve PICU bed turnover, and have the potential to lower total hospital cost for midface advancements. Immediate extubation is feasible following midface advancement in patients with syndromic craniosynostosis. Further prospective randomized trials are needed to demonstrate superiority in outcomes compared to delayed extubation.
Acknowledgements
The authors would like to thank prof. Frank Weber for his help in refining the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




