Unexpectedly low oxygen saturation in a child with a variant hemoglobin
Section Editor: David M Polaner
Abstract
We report a child with an unexpected low saturation reading despite normal values having been recorded soon after birth. A family history of Rothschild hemoglobin variant affecting the father was obtained. Saturation values improved with oxygen and anesthesia was uneventful. The development of low saturation values as the child matures is explained, and management for future health presentations outlined.
1 INTRODUCTION
Variant hemoglobins are a rare cause of low oxygen saturation (SpO2) readings from standard pulse oximeters. Pulse oximeters provide a non-invasive method of measuring oxygen transport by hemoglobin. The algorithm assumes that only oxyhemoglobin and reduced hemoglobin species are present.
Some unusual hemoglobin (Hb) species such as Methemoglobin can lead to alterations in oximeter detected SpO2; however, most of the more than one thousand hemoglobin (Hb) variants recorded have neither altered light absorption or altered oxygen affinity and will not present with abnormal SpO2 readings. Rarely variant hemoglobins have altered oxygen affinity and the low SpO2 reflects a concordantly low arterial oxygen (SaO2) value.
Hb Rothschild (Hb R) is an extremely rare hemoglobin variant with low oxygen affinity, where the low SpO2 reflects a similarly low SaO2.1 A PubMed search for hemoglobin Rothschild returned only thirteen publications.
2 CASE REPORT
A six-month-old Caucasian boy presented for anesthesia for elongation of his Achilles tendon for mild talipes. His father was known to have low SpO2, usually in the mid eighty percent range. Despite this, he had an excellent exercise tolerance. He was aware of his history of heterozygous Hb R, and antenatal hemoglobinopathy testing of the child's mother was unremarkable. Because of this history, the patient had been reviewed by Hematology at birth, where SpO2 of 98% in air were documented. A review in 3 months was planned but not completed. The child was otherwise well apart from inguinal hernias and was developing normally. There was no history of cyanosis. On examination, he was extremely vigorous. There was no indication of either cardiac or respiratory abnormality, but the SpO2 obtained was 85% in air with an excellent trace displayed on the oximeter. With preoxygenation the SpO2 increased to 98%, and in the absence of any other explanation, it was assumed that this was due to the same hemoglobin variant as SpO2 will reflect SaO2 accurately for Hb R. Inhalational induction was followed by uneventful volatile anesthesia with SpO2 levels of 96% in 50% oxygen. In recovery, SpO2 ranged between 83 and 97% on air. Arterial blood gas measurement was not performed.
In order to rule out other more common physiological causes of low SpO2 a pediatric review was requested, an echocardiogram was performed, and a full blood count, ferritin and hemoglobinopathy screening investigations requested. Further hematology consultation was arranged. Echocardiography was normal for age, with no structural abnormalities, which might cause low SpO2 found. The full blood count revealed a hemoglobin of 106 g/dL.
Hemoglobinopathy investigations including HPLC (High Performance Liquid Chromatography) and capillary electrophoresis were performed. The Hb EPG showed Hb A 54.6%, Hb A2 3.1%, Hb F 5.9%, and a variant hemoglobin of 36.4% running in the Hb D zone on Capillary Electrophoresis. Given numerous variant hemoglobinopathies can be identified in the Hb D zone, further genetic testing is generally recommended for these Hb EPG findings Figure 1.
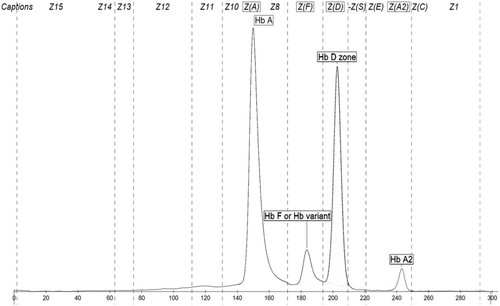
DNA sequencing confirmed a TGG (Trp) > CGG (Arg) mutation at codon 37, confirming that the patient is heterozygous for Hemoglobin Rothschild.
3 DISCUSSION
Pulse oximetry has been reported as a useful tool for detection and screening for variant hemoglobin types where there is a family history and other causes of low SpO2 have been excluded.1-3 At initial presentation, clinical evaluation for other more common causes of low SpO2 should be completed including exclusion of congenital heart disease and absence of respiratory disease. An arterial blood gas might also be helpful if the variant is unknown or is known to be associated with discordant SpO2/SaO2 values.
In Hb R, the globin subunits are destabilized due to a replacement of amino acid tryptophan by arginine at position 3 of the C helix of the chain encoded by the mutated allele. This is a contact point for the α1β2 and α2 β1 interfaces and the resulting destabilization results in a hemoglobin tetramer with low oxygen affinity, while the dissociated dimers have an increased affinities.1 The p50 of Hb Rothschild is higher than Hb A (~35 cf 25 mmHg for Hb A).3 Absence of symptoms may be due to delivery of increased amounts of oxygen to the tissues.2 There are laboratory studies suggesting that low affinity hemoglobins may actually confer some advantages in terms of muscle function and oxygen consumption.2 This may also result in a mild anemia due to reduced erythropoiesis secondary to reduced erythropoietin release from the kidney.3
The normal saturations recorded at birth for our patient presumably reflect the presence of high levels of neonatal hemoglobin (Hb F). Hb F contains four polypeptide chains but the β chains found in Hb A are replaced by γ chains (α2γ2 compared to α2β2). As the mutation for Hb R affects the β chains it will not be seen until the Hb F is replaced by the adult species, a process which is accelerating by 6 months of age, and usually complete by 2–3 years of age.
The absorbance of the 660 and 940 nm wavelengths used by the pulse oximeter for Hb R is unknown. Other case reports suggest that the PaO2 in patients with Hb R is similar to that which would be predicted by the SpO2, and co-oximetry may be useful if available.
Whilst no specific treatments for low affinity hemoglobin variants generally or for Hb R specifically, are required, diagnosis and documentation for patients with abnormal Hb variants and their families is important to prevent unnecessary procedures and investigations in future presentations to the health system and to prevent anxiety.
4 LEARNING POINTS
- Abnormal hemoglobin variants may produce unexpected saturation readings from standard pulse oximeters.
- Diagnosis and documentation for patients with abnormal Hb variants and their families is important to prevent unnecessary procedures and investigations in future presentations to the health system and to prevent anxiety.
- Further genetic testing is often required to identify variant hemoglobinopathies, particularly those of clinical and reproductive significance.
ACKNOWLEDGMENT
Open access publishing facilitated by The University of Newcastle, as part of the Wiley - The University of Newcastle agreement via the Council of Australian University Librarians.
CONFLICT OF INTEREST STATEMENT
No conflict of interest declared.
PARENTAL CONSENT
The parental consent was obtained.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




