Intermuscular Two-Incision Technique for Subcutaneous Implantable Cardioverter Defibrillator Implantation: Results from a Multicenter Registry
Disclosures: Dr. Federico Migliore and Dr. Emanuele Bertaglia are consultants for Boston Scientific.
Abstract
Background
The traditional technique for subcutaneous implantable cardioverter defibrillator (S-ICD) implantation, which involves three incisions and a subcutaneous pocket, is associated with possible complications, including inappropriate interventions. The aim of this prospective multicenter study was to evaluate the efficacy and safety of an alternative intermuscular two-incision technique for S-ICD implantation.
Methods
The study population included 36 consecutive patients (75% male, mean age 44 ± 12 years [range 20–69]) who underwent S-ICD implantation using the intermuscular two-incision technique. This technique avoids the superior parasternal incision for the lead placement and consists of creating an intermuscular pocket between the anterior surface of the serratus anterior and the posterior surface of the latissimus dorsi muscles instead of a subcutaneous pocket.
Results
All patients were successfully implanted in the absence of any procedure-related complications with a successful 65-J standard polarity defibrillation threshold testing, except in one, who received a second successful shock after pocket revision. During a mean follow-up of 10 months (range 3–30), no complications requiring surgical revision were observed. At device interrogation, stable sensing without interferences was observed in all patients. Two patients (5.5%) experienced appropriate and successful shock on ventricular fibrillation and in four patients (11%), a total of seven nonsustained self-terminated ventricular tachycardias were correctly detected. No inappropriate interventions were observed.
Conclusions
Our experience suggests that the two-incision intermuscular technique is a safe and efficacious alternative to the current technique for S-ICD implantation that may help reducing complications including inappropriate interventions and offer a better cosmetic outcome, especially in thin individuals.
Introduction
The subcutaneous implantable cardioverter defibrillator (S-ICD; Boston Scientific, Marlborough, MA, USA) was developed as an alternative therapy to transvenous ICD (TV-ICD) system, as it is a fully subcutaneous system without any transvenous or epicardial leads.1-4 Thus, the S-ICD system has the potential to decrease periprocedural implantation risks, eliminate the problem of difficult venous access, reduce endovascular mechanical stress on leads, and decrease the risk of systemic device-related infection.1-5 However, single case reports6 and the IDE Study and EFFORTLESS Registry reported that the total complication rate was as high as 11%, with the majority being pocket infection, erosion, or discomfort. Nevertheless, this registry included the initial worldwide experience with the S-ICD, and implant complications rate are expected to decrease with increasing operator experience and better surgical techniques.2, 4
The conventional S-ICD implantation technique consists of electrode and device implantation by making three incisions: one lateral pocket incision and two parasternal incisions. However, particularly the superior parasternal incision is a potential source of skin erosion and infection.2, 4, 5 Thus, a two-incision technique eliminating the superior parasternal incision has been developed.7 This less invasive and simplified technique is safe and may help reducing complications in S-ICD patients. Moreover, an intermuscular pocket has been proposed as an alternative to the standard subcutaneous pocket with the aim to achieve not only a better cosmetic result, especially in thin patients, but also to reduce pocket complications.8, 9 However, this technique needs further clinical validation.
The aim of this prospective multicenter study was to evaluate the efficacy and safety of the alternative intermuscular two-incision technique for S-ICD implantation.
Methods
Patients with indications for ICD implantation according to the European Society of Cardiology guidelines10 and who received an S-ICD (SQ-RX 1010, Cameron Health [now Boston Scientific] or Emblem S-ICD, Model 209, Boston Scientific) were included in the study. Patients <18 years old or having indications for pacing were excluded. The local Ethics Committee approved the study protocol and all patients provided written consent to be enrolled in the registry.
Two-Incision Intermuscular Technique
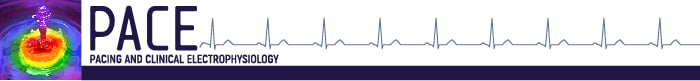
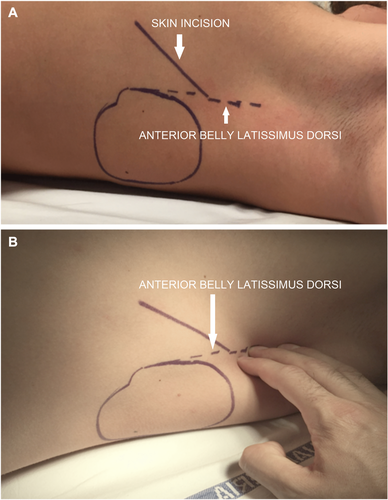
Before implantation, all patients were screened for eligibility for S-ICD using the Boston Scientific electrocardiogram (ECG) screening tool based on the surface ECG limb lead recording over the left and/or right parasternal regions to simulate the three S-ICD sensing vectors. The procedure was performed in an electrophysiology laboratory under standard sterile conditions and general or local anesthesia. Antibiotic prophylaxis was administered 1 hour before the procedure. In patients taking warfarin, the procedure was performed with a therapeutic international normalized ratio (INR) on the day of the procedure. Non-vitamin K oral anticoagulants treatments were interrupted 24–48 hours before the procedure depending on creatinine clearance. The conventional implantation technique suggested by the manufacturer is described in the S-ICD User's Manual.11 The two-incision intermuscular technique abandons the superior parasternal incision as previously reported in detail7 and consists of creating an intermuscular pocket for the pulse generator instead of a subcutaneous pocket using anatomical landmarks (Fig. 1). The implantation procedure consists of six steps: (1) the position of the lead and pulse generator relative to the heart silhouette is checked by fluoroscopy; (2) an incision is made along the inframammary crease at the anterior edge of the latissimus dorsi. When the latissimus dorsi anterior edge is exposed, the pocket is created by blunt dissection between the superior surface of the serratus anterior muscle and the posterior surface of the latissimus dorsi muscle so that the pulse generator can be placed into the virtual anatomical space between the two muscles (Fig. 2). When the serratus anterior is reached, it is important to recognize the change in the fiber pathway, horizontal versus vertical, so that the muscular fascia may be preserved in order to minimize bleeding; (3) a small 2-cm horizontal incision at the level of the xiphoid process (xiphoid incision) is made in the direction of the pocket incision. The distal tip of the electrode insertion tool (EIT), used to create the subcutaneous tunnels in which the electrode is placed, is inserted at the xiphoid incision and tunneled laterally until the distal tip emerges at the device pocket. Conventional suture material is used to tie the anchoring hole of the electrode to the EIT. With the electrode attached, the EIT is pulled back through the tunnel to the xiphoid incision until the proximal sensing electrode emerges. A suture sleeve is placed over the electrode shaft 1 cm below the proximal sensing electrode. The preformed grooves are used to bind the suture sleeve to the electrode shaft using nonabsorbable suture material. The suture that connects the tip of the lead to the EIT is cut and removed; (4) a peel-away sheath is placed over the shaft of the EIT, which is then tunneled approximately 14 cm superior to the xiphoid incision and approximately 1–2 cm to the left or right of the sternal midline. The peel-away sheath is advanced over the EIT until it is fully inserted. The EIT is removed, and the peel-away sheath is left in its subcutaneous position. The electrode is inserted into the subcutaneous sheath until the suture sleeve reaches the opening of the sheath. The sheath is peeled away, leaving the electrode in place. Attention is paid that the tip of the lead is at the required sterno-manubrial location, confirmed digitally. The suture sleeve is secured to the fascia; (5) the proximal end of the lead is inserted into the connector port in the device header of the S-ICD and the screw set tightened. Thus, the device is located into the intermuscular pocket, and anchored to the fascia to prevent possible migration using conventional nonabsorbable suture material. Particular attention is paid that the generator is placed posterior and inferior to the incision; (6) the two muscles (serratus anterior and latissimus dorsi) are sutured using conventional absorbable suture. Finally, after device setup, the two incisions (xiphoid and pocket incisions) are closed using intradermal suture. The best sensing vector is chosen automatically by the device. A chest x-ray was obtained the first postoperative day and 2–6 months after implantation to confirm stable lead and generator position. All implantations were performed by experienced operators with the traditional technique.
Defibrillator Threshold Testing and Device Programming
After the procedure, defibrillator threshold testing (DFT) was performed after induction of ventricular fibrillation (VF) by 50-Hz stimulation. The DFT was considered successful if the device detected and terminated VF using a 65-J shock. In all patients, the device programming features included two tachyarrhythmia detection zones: (1) the shock-only zone, in which detection and therapy were based on rate only; and (2) an additional “conditional zone,” in which a morphology analysis algorithm was applied in addition to rate. Rate cutoffs were individualized for each patient based on clinical indications. The sensing vector (primary, secondary, or alternate) was automatically selected by the device at the time of implantation and optimized during supine and upright positions prior to discharge.
Follow-Up
All patients were followed-up at 1 month, 3 months, and every 6 months thereafter. At these visits, patient's clinical conditions and complications (including device-related complications and inappropriate shocks) were assessed. Acute complications were defined as complications that occurred during or within 24 hours of S-ICD implantation and were classified as (1) procedure-related complications, including pneumothorax, pleural effusion, hematoma ≥2 cm, drop in hemoglobin>2 g/dL, bleeding requiring wound exploration or transfusion or generator/lead dislocation at the chest x-ray obtained 24 hours after the procedure; (2) technical complications, such as failure of the device to communicate with the programmer. Late complications were defined as those occurring more than 24 hours after the procedure and included: pocket discomfort, incomplete wound healing, skin erosion of pulse generator or electrode, local and systemic device-related infections, and migration of pulse generator or electrode and technical complications such as failure of the device to communicate with the programmer or premature battery depletion. Interventions were considered inappropriate when triggered by anything other than ventricular tachycardia (VT) or VF, including sinus tachycardia, supraventricular arrhythmias, or device or lead malfunction.
Statistical Analysis
Continuous variables are expressed as mean ± standard deviation (SD). Categorical variables are presented with actual numbers and frequencies. All analyses were performed using the SPSS statistic software (version 21.0; IBM Corp., Armonk, NY, USA).
Results
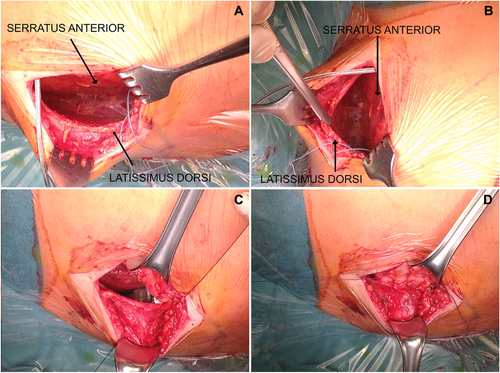
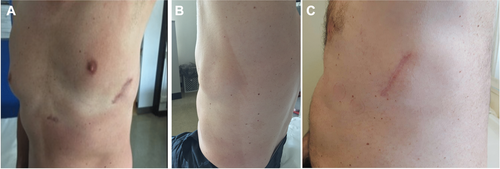
The study population included 36 consecutive patients (75% male, mean age 44 ± 12 years [range 20–69 years]) who were implanted with an S-ICD using the alternative two-incision intermuscular implantation technique. Baseline clinical and technical device characteristics are reported in Table I. The reason for subcutaneous ICD placement was the presence of previous TV-ICD (patients underwent lead extraction for infection or lead failure) in seven (19.5%) patients and the absence of transvenous access for ventricular lead placement in two (5.5%) patients. In the remaining patients, the choice of implanting an S-ICD instead of a TV-ICD was at the discretion of the physician based on clinical indications. The procedure was performed under general anesthesia in 13 patients (36%) and local anesthesia with sedation in the remaining 23 patients (64%). Implantation was performed in two patients (5.5%) taking warfarin with a therapeutic INR on the day of the procedure, in one patient (2.7%) taking rivaroxaban stopped 24 hours before the procedure and resumed the day after, and in four patients (11%) taking dual-antiplatelet treatment (such as aspirin plus clopidogrel, ticagrelor, or prasugrel). The average procedure time (“skin to skin”) was 69 ± 17 minutes. DFT was performed in all patients except in two, one with left ventricular thrombus and one with labile hemodynamic status. All patients had successful DFT testing with 65-J standard polarity after implantation except one, who received a successful DFT testing after pocket revision because of the suspected presence of air in the pocket. Mean time from VF induction to shock delivery was 14 ± 1.8 seconds (range 12–19 seconds). No early complications occurred. A postoperative chest radiography confirmed stable device and lead location in all patients (Fig. 3).
| Male | 27 (75%) |
| Age at implant (years) | 44 ± 12 (range 20–69) |
| BMI (kg/m2) | 24 ± 4 |
| Indication | |
| Primary prevention | 29 (80.5%) |
| Secondary prevention | 7 (19.5%) |
| Primary cardiac disease | |
| Ischemic cardiomyopathy | 9 (25%) |
| Nonischemic dilated cardiomyopathy | 7 (19.5%) |
| Hypertrophic cardiomyopathy | 4 (11.1%) |
| Arrhythmogenic right ventricular cardiomyopathy | 2 (5.5%) |
| Brugada syndrome | 3 (8.3%) |
| Idiopathic VF | 5 (13.8%) |
| Noncompaction cardiomyopathy | 1 (2.8%) |
| Other | 5 (13.8%) |
| Left ventricular ejection fraction | 47 ± 19 (range 16–67) |
| Medical history | |
| NYHA function classification II–III | 9 (25%) |
| Atrial fibrillation | 2 (5.5%) |
| Hypertension | 6 (16.6%) |
| Diabetes | 3 (8.3%) |
| Chronic kidney disease | 4 (11.1%) |
| Previous transvenous ICD (patients underwent lead extraction) | 7 (19.5%) |
| Left parasternal led position | 33 (91.7%) |
| Right parasternal lead position | 3 (8.3%) |
| Sensing vector | |
| Primary | 26 (72%) |
| Secondary | 9 (25%) |
| Alternate | 1 (2.8%) |
| Shock impedance (ohm) | 69 ± 8 |
| Dual-zone programming | |
| Conditional zone, mean rate (beats/min) | 211 ± 11 |
| Shock zone, mean rate (beats/min) | 245 ± 7 |
- Values are number of patients n (percentage, %) or mean ± standard deviation.
- BMI = body mass index; ICD = implantable cardioverter defibrillator; NYHA = New York Heart Association; VF = ventricular fibrillation.
During a mean follow-up of 10 months (range 3–30 months), no late complications requiring surgical revision were observed (Fig. 4). Two patients (5.5%) reported discomfort from the pulse generator pocket during the first weeks after implantation that spontaneously resolved without the need for pocket revision. In two female patients (5.5%), delayed wound healing was reported at the xiphoid incision without signs of infection. These resolved spontaneously and did not lead to device explantation. At device interrogation, we observed stable sensing without interferences in all patients. Two patients (5.5%) experienced appropriate and successful shock on VF and in four patients (11%), a total of seven nonsustained self-terminated VTs were correctly detected. No inappropriate interventions were observed. In one patient, a short interval of T-wave oversensing was detected. After the S-ICD was reprogrammed to a different vector, no other oversensing episodes were recorded.
Discussion
The traditional technique for S-ICD implantation is associated with possible complications, including skin erosion, infection, discomfort, and inappropriate intervention, particularly in patients lacking sufficient subcutaneous tissue to cover the device adequately.1-6 Knops et al. reported that the two-incision technique, consisting in the omission of the superior parasternal incision, is a potential alternative approach for S-ICD implantation to simplify the procedure and reduce complications.7 Recently, Migliore et al. reported an intermuscular approach for S-ICD generator placement in a single case report.8 Subsequently, a single-center experience including 14 patients was reported by Ferrari et al.9 Our multicenter registry with a larger study population confirmed and extended such previous observations demonstrating that the two-incision intermuscular technique is a safe and feasible alternative to the standard S-ICD implantation technique that involves three incisions and a subcutaneous pocket, potentially reducing pocket-related complications and providing a better cosmetic outcome, especially in thin individuals. Although creating the intermuscular pocket for the pulse generator may seem a complex surgical procedure, the simple knowledge of anatomical landmarks and the presence of an anatomical virtual pocket between the muscles make it an easy procedure even for physicians performing it for the first time as those involved in this study. However, as recent studies showed a learning curve involved in implanting and programming the S-ICD,4, 6 we recommend to first acquire great comfort with anatomical landmarks and technical issues of the traditional implantation technique.
Compared to the traditional technique, the intermuscular technique provides a wider pocket in a more posterior left axillary region, an extra layering, a virtual space between device and chest, resulting in potential reduction of pocket-related complications, especially skin erosions and infections (see Fig. 3). An important implication for using the intermuscular approach is that anatomical landmarks ensure the right position of the pulse generator in obese patients. Furthermore, the more posterior placement of the device may provide an improved vector toward the shocking coil capturing more of the left ventricle compared with conventional subcutaneous approach. In our study, no procedure-related complications, such as neurovascular damage or bleeding, during implantation and follow-up were observed. In particular, there were no late complications requiring surgical revision, including skin erosion of pulse generator or electrode, hematoma, local and systemic device-related infections, migration of pulse generator or electrode, and device lead malfunction. Additionally, in contrast with previous studies,2, 5 we did not observe any inappropriate shock therapy during follow-up. This difference can be explained by different characteristics of the study populations and technical reasons such as device programming (single- vs dual-zone programing) and software upgrade. However, whether the new technique for S-ICD implantation may contribute to these excellent results, remains to be proved by studies comparing the two techniques (subcutaneous vs intermuscular).
Recently, it has been reported that in young patients with cardiomyopathies and channelopathies, TV-ICD-related adverse events are particularly common in thin subjects.12 Hence, we strongly believe that physicians should consider implantation of an S-ICD with the intermuscular two-incision technique in these patients to limit complications also during physical activities. To this regard, four patients enrolled in the present registry were underweight and none of them experienced complications.
Recent studies showed that there is a learning curve involved in implanting and programming the S-ICD.4, 5 Thus, we recommend to first become confident with the anatomical landmarks and technical issues of the traditional implantation technique.
A potential limitation of this novel technique could be a potential greater complexity for battery replacement. However, we believe that both the long battery duration of the current Emblem S-ICD system and reduction of acute and late complications may overcome this limitation. Another limitation is that our study population with a mean age of 44 years is younger than those previously reported in prospective TV-ICD studies. However, the mean age and clinical characteristics of our study population are comparable with that reported in the IDE Study and EFFORTLESS Registry.4
Conclusions
In conclusion, our experience suggests that the two-incision intermuscular technique is a safe and effective alternative to the current technique for S-ICD implantation and may help to reduce complications, including inappropriate interventions, and to offer a better cosmetic outcome, especially in thin individuals. Long-term data on larger patient populations are needed to establish the superiority of this novel technique compared to the standard approach.
Acknowledgment
We thank the engineers Andrea Galvagni, Mirco Ponzin, and Carlo Rojatti of the Boston Scientific S.P.A. for important technical support.